Sulfamic Acid in Environmental Impact Reduction Measures
JUL 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulfamic Acid Background and Objectives
Sulfamic acid, also known as amidosulfonic acid, has a rich history in industrial and domestic applications. Its discovery in the late 19th century marked the beginning of its journey as a versatile chemical compound. Over the years, sulfamic acid has gained prominence in various sectors due to its unique properties and environmental advantages.
The evolution of sulfamic acid usage has been closely tied to the growing awareness of environmental concerns. Initially employed primarily as a descaling agent in industrial settings, its applications have expanded significantly. The chemical's ability to effectively remove mineral deposits and its relatively low environmental impact compared to alternative acids have driven its adoption across diverse industries.
In recent decades, the focus on sustainable practices and environmental protection has intensified the interest in sulfamic acid. Its biodegradability and lower corrosivity compared to traditional acids like hydrochloric acid have positioned it as a more eco-friendly option. This shift in perception has led to increased research and development efforts aimed at optimizing sulfamic acid's performance while minimizing its environmental footprint.
The primary objective of researching sulfamic acid in environmental impact reduction measures is to explore and enhance its potential as a green alternative in various applications. This includes investigating its efficacy in water treatment processes, industrial cleaning, and as a replacement for more harmful chemicals in manufacturing processes. Researchers aim to develop innovative formulations and application methods that maximize sulfamic acid's benefits while addressing any potential drawbacks.
Another crucial goal is to quantify and compare the environmental impact of sulfamic acid with that of conventional acids and cleaning agents. This involves comprehensive life cycle assessments, toxicity studies, and long-term environmental monitoring. By establishing a clear understanding of sulfamic acid's ecological footprint, industries can make informed decisions about its adoption and implementation in their processes.
Furthermore, the research seeks to identify new areas where sulfamic acid can contribute to environmental sustainability. This includes exploring its potential in emerging technologies such as advanced materials production, renewable energy systems, and circular economy initiatives. By broadening the scope of sulfamic acid applications, researchers aim to unlock new pathways for reducing the overall environmental impact of industrial and commercial activities.
The evolution of sulfamic acid usage has been closely tied to the growing awareness of environmental concerns. Initially employed primarily as a descaling agent in industrial settings, its applications have expanded significantly. The chemical's ability to effectively remove mineral deposits and its relatively low environmental impact compared to alternative acids have driven its adoption across diverse industries.
In recent decades, the focus on sustainable practices and environmental protection has intensified the interest in sulfamic acid. Its biodegradability and lower corrosivity compared to traditional acids like hydrochloric acid have positioned it as a more eco-friendly option. This shift in perception has led to increased research and development efforts aimed at optimizing sulfamic acid's performance while minimizing its environmental footprint.
The primary objective of researching sulfamic acid in environmental impact reduction measures is to explore and enhance its potential as a green alternative in various applications. This includes investigating its efficacy in water treatment processes, industrial cleaning, and as a replacement for more harmful chemicals in manufacturing processes. Researchers aim to develop innovative formulations and application methods that maximize sulfamic acid's benefits while addressing any potential drawbacks.
Another crucial goal is to quantify and compare the environmental impact of sulfamic acid with that of conventional acids and cleaning agents. This involves comprehensive life cycle assessments, toxicity studies, and long-term environmental monitoring. By establishing a clear understanding of sulfamic acid's ecological footprint, industries can make informed decisions about its adoption and implementation in their processes.
Furthermore, the research seeks to identify new areas where sulfamic acid can contribute to environmental sustainability. This includes exploring its potential in emerging technologies such as advanced materials production, renewable energy systems, and circular economy initiatives. By broadening the scope of sulfamic acid applications, researchers aim to unlock new pathways for reducing the overall environmental impact of industrial and commercial activities.
Market Demand Analysis for Eco-Friendly Chemicals
The market demand for eco-friendly chemicals, particularly in the context of sulfamic acid for environmental impact reduction, has been steadily increasing in recent years. This growth is driven by several factors, including stricter environmental regulations, growing consumer awareness, and corporate sustainability initiatives.
Sulfamic acid, known for its versatility and relatively low environmental impact compared to some alternatives, has found applications in various industries. In the cleaning sector, there's a notable shift towards sulfamic acid-based products due to their effectiveness in removing limescale and rust without the harsh environmental effects associated with hydrochloric acid. This trend is particularly evident in regions with hard water, where limescale buildup is a common issue.
The water treatment industry has also shown increased interest in sulfamic acid. Its use in water softening and pH adjustment processes aligns well with the growing emphasis on sustainable water management practices. Municipal water treatment facilities and industrial water systems are key drivers of this demand, as they seek to optimize their operations while minimizing environmental impact.
In the manufacturing sector, sulfamic acid is gaining traction as a more environmentally friendly alternative for metal cleaning and descaling processes. Industries such as automotive, aerospace, and electronics are exploring sulfamic acid-based solutions to replace more hazardous chemicals in their production lines. This shift is partly driven by occupational health and safety concerns, as well as environmental compliance requirements.
The agricultural sector presents another growing market for sulfamic acid. Its use in fertilizer production and soil pH adjustment has increased as farmers look for more sustainable farming practices. The ability of sulfamic acid to improve nutrient uptake in plants while minimizing soil and water pollution has made it an attractive option in precision agriculture.
Geographically, the demand for eco-friendly chemicals like sulfamic acid is particularly strong in regions with stringent environmental regulations. The European Union, North America, and parts of Asia-Pacific, especially Japan and South Korea, are leading this trend. Emerging economies are also showing increased interest as they balance industrial growth with environmental protection.
Market analysts project a compound annual growth rate (CAGR) for the eco-friendly chemicals market, including sulfamic acid, to be in the high single digits over the next five years. This growth is expected to be fueled by ongoing research and development in green chemistry, as well as increasing adoption across various industries seeking to reduce their environmental footprint.
Sulfamic acid, known for its versatility and relatively low environmental impact compared to some alternatives, has found applications in various industries. In the cleaning sector, there's a notable shift towards sulfamic acid-based products due to their effectiveness in removing limescale and rust without the harsh environmental effects associated with hydrochloric acid. This trend is particularly evident in regions with hard water, where limescale buildup is a common issue.
The water treatment industry has also shown increased interest in sulfamic acid. Its use in water softening and pH adjustment processes aligns well with the growing emphasis on sustainable water management practices. Municipal water treatment facilities and industrial water systems are key drivers of this demand, as they seek to optimize their operations while minimizing environmental impact.
In the manufacturing sector, sulfamic acid is gaining traction as a more environmentally friendly alternative for metal cleaning and descaling processes. Industries such as automotive, aerospace, and electronics are exploring sulfamic acid-based solutions to replace more hazardous chemicals in their production lines. This shift is partly driven by occupational health and safety concerns, as well as environmental compliance requirements.
The agricultural sector presents another growing market for sulfamic acid. Its use in fertilizer production and soil pH adjustment has increased as farmers look for more sustainable farming practices. The ability of sulfamic acid to improve nutrient uptake in plants while minimizing soil and water pollution has made it an attractive option in precision agriculture.
Geographically, the demand for eco-friendly chemicals like sulfamic acid is particularly strong in regions with stringent environmental regulations. The European Union, North America, and parts of Asia-Pacific, especially Japan and South Korea, are leading this trend. Emerging economies are also showing increased interest as they balance industrial growth with environmental protection.
Market analysts project a compound annual growth rate (CAGR) for the eco-friendly chemicals market, including sulfamic acid, to be in the high single digits over the next five years. This growth is expected to be fueled by ongoing research and development in green chemistry, as well as increasing adoption across various industries seeking to reduce their environmental footprint.
Current State and Challenges in Sulfamic Acid Usage
Sulfamic acid has gained significant attention in recent years as an environmentally friendly alternative to traditional cleaning agents and descaling solutions. The current state of sulfamic acid usage in environmental impact reduction measures is characterized by a growing adoption across various industries, particularly in water treatment, industrial cleaning, and household applications.
In the water treatment sector, sulfamic acid has emerged as a promising solution for scale removal and pH adjustment in cooling towers and boiler systems. Its effectiveness in dissolving mineral deposits without causing corrosion to metal surfaces has made it a preferred choice over hydrochloric acid. However, the challenge lies in optimizing dosage levels to ensure maximum efficiency while minimizing environmental impact.
The industrial cleaning industry has witnessed a shift towards sulfamic acid-based products due to their biodegradability and lower toxicity compared to traditional cleaning agents. This transition aligns with increasingly stringent environmental regulations and corporate sustainability goals. Nevertheless, the industry faces challenges in developing formulations that maintain cleaning efficacy while further reducing environmental footprint.
In household applications, sulfamic acid has found its way into bathroom cleaners and descaling products. Its ability to effectively remove limescale and soap scum without harsh fumes has contributed to its popularity. However, consumer awareness and education regarding proper usage and disposal remain significant challenges in maximizing its environmental benefits.
Despite its advantages, the widespread adoption of sulfamic acid faces several technical and practical challenges. One major hurdle is the limited production capacity and higher cost compared to conventional acids, which can hinder its adoption in price-sensitive markets. Additionally, the transportation and storage of sulfamic acid require special considerations due to its hygroscopic nature, presenting logistical challenges for manufacturers and end-users alike.
Research efforts are currently focused on enhancing the performance of sulfamic acid-based solutions while addressing these challenges. Scientists are exploring novel formulations that combine sulfamic acid with other eco-friendly compounds to improve its efficacy and reduce overall environmental impact. Furthermore, studies are underway to develop more efficient production methods that could potentially lower costs and increase availability.
The regulatory landscape surrounding sulfamic acid usage is evolving, with environmental agencies worldwide recognizing its potential as a greener alternative. However, the development of comprehensive guidelines for its application across different industries remains a work in progress. This regulatory uncertainty poses challenges for businesses looking to incorporate sulfamic acid into their processes, as they navigate varying standards and compliance requirements.
In the water treatment sector, sulfamic acid has emerged as a promising solution for scale removal and pH adjustment in cooling towers and boiler systems. Its effectiveness in dissolving mineral deposits without causing corrosion to metal surfaces has made it a preferred choice over hydrochloric acid. However, the challenge lies in optimizing dosage levels to ensure maximum efficiency while minimizing environmental impact.
The industrial cleaning industry has witnessed a shift towards sulfamic acid-based products due to their biodegradability and lower toxicity compared to traditional cleaning agents. This transition aligns with increasingly stringent environmental regulations and corporate sustainability goals. Nevertheless, the industry faces challenges in developing formulations that maintain cleaning efficacy while further reducing environmental footprint.
In household applications, sulfamic acid has found its way into bathroom cleaners and descaling products. Its ability to effectively remove limescale and soap scum without harsh fumes has contributed to its popularity. However, consumer awareness and education regarding proper usage and disposal remain significant challenges in maximizing its environmental benefits.
Despite its advantages, the widespread adoption of sulfamic acid faces several technical and practical challenges. One major hurdle is the limited production capacity and higher cost compared to conventional acids, which can hinder its adoption in price-sensitive markets. Additionally, the transportation and storage of sulfamic acid require special considerations due to its hygroscopic nature, presenting logistical challenges for manufacturers and end-users alike.
Research efforts are currently focused on enhancing the performance of sulfamic acid-based solutions while addressing these challenges. Scientists are exploring novel formulations that combine sulfamic acid with other eco-friendly compounds to improve its efficacy and reduce overall environmental impact. Furthermore, studies are underway to develop more efficient production methods that could potentially lower costs and increase availability.
The regulatory landscape surrounding sulfamic acid usage is evolving, with environmental agencies worldwide recognizing its potential as a greener alternative. However, the development of comprehensive guidelines for its application across different industries remains a work in progress. This regulatory uncertainty poses challenges for businesses looking to incorporate sulfamic acid into their processes, as they navigate varying standards and compliance requirements.
Existing Environmental Impact Reduction Solutions
01 Biodegradability and environmental fate
Sulfamic acid's environmental impact is influenced by its biodegradability and fate in various ecosystems. Studies have been conducted to assess its persistence in water, soil, and air, as well as its potential for bioaccumulation. The acid's relatively rapid degradation in natural environments and low potential for bioaccumulation contribute to its generally favorable environmental profile.- Biodegradability and environmental fate: Sulfamic acid's environmental impact is influenced by its biodegradability and fate in ecosystems. Studies have shown that it can be broken down by certain microorganisms, reducing its long-term presence in the environment. However, its persistence and potential accumulation in aquatic systems may still pose concerns for aquatic life and water quality.
- Aquatic toxicity and ecosystem effects: The impact of sulfamic acid on aquatic ecosystems is a significant environmental concern. Research has indicated potential toxicity to various aquatic organisms, including fish, invertebrates, and algae. The acid's effects on pH levels in water bodies can also disrupt ecosystem balance and affect sensitive species.
- Soil and plant impacts: Sulfamic acid's effects on soil chemistry and plant life are important aspects of its environmental impact. Studies have explored its influence on soil pH, nutrient availability, and microbial communities. The acid's potential to alter soil properties may affect plant growth and agricultural productivity in contaminated areas.
- Air quality and atmospheric effects: While sulfamic acid is not highly volatile, its potential atmospheric impacts should be considered. Research has investigated its role in air pollution, particularly in industrial settings where it may be released as aerosols or vapors. The acid's contribution to acid rain formation and its effects on air quality in surrounding areas have been studied.
- Waste management and environmental remediation: Proper handling and disposal of sulfamic acid waste are crucial for minimizing its environmental impact. Studies have focused on developing effective treatment methods for contaminated water and soil, including chemical neutralization, adsorption techniques, and biological remediation approaches. Sustainable waste management practices aim to reduce the acid's release into the environment.
02 Aquatic toxicity and ecosystem effects
The impact of sulfamic acid on aquatic ecosystems has been a focus of environmental research. Studies have examined its toxicity to various aquatic organisms, including fish, invertebrates, and algae. While sulfamic acid can have acute effects at high concentrations, its rapid degradation and dilution in water bodies often mitigate long-term impacts on aquatic ecosystems.Expand Specific Solutions03 Use in environmentally friendly cleaning products
Sulfamic acid has found applications in eco-friendly cleaning formulations due to its effectiveness and relatively low environmental impact. When used in appropriate concentrations and formulations, it can serve as an alternative to more harmful cleaning agents. Research has focused on developing cleaning products that balance efficacy with minimal environmental footprint.Expand Specific Solutions04 Waste treatment and environmental remediation
Sulfamic acid has been investigated for its potential use in environmental remediation and waste treatment processes. Its ability to react with certain pollutants and its role in pH adjustment have been explored in various treatment applications. Research has focused on optimizing its use in these contexts while minimizing any potential negative environmental impacts.Expand Specific Solutions05 Industrial applications and emission control
The use of sulfamic acid in industrial processes has led to research on emission control and environmental management strategies. Studies have focused on minimizing releases to the environment, developing closed-loop systems, and implementing best practices for handling and disposal. These efforts aim to reduce the potential environmental impact associated with industrial applications of sulfamic acid.Expand Specific Solutions
Key Players in Sulfamic Acid Industry
The research on sulfamic acid in environmental impact reduction measures is in a developing stage, with growing market potential as industries seek sustainable solutions. The technology's maturity varies across sectors, with established players like BASF Corp. and China Petroleum & Chemical Corp. leading in chemical applications. Emerging companies such as New Sky Energy, Inc. and Verdesian Life Sciences LLC are exploring innovative uses in biogas treatment and agriculture. The competitive landscape is diverse, including major petrochemical firms, specialized environmental technology companies, and academic institutions like Zhejiang University, indicating a multifaceted approach to advancing this technology for environmental benefits.
BASF Corp.
Technical Solution: BASF has developed innovative sulfamic acid-based solutions for environmental impact reduction. Their approach involves using sulfamic acid as a key component in eco-friendly cleaning formulations and water treatment processes. BASF's sulfamic acid products are designed to be biodegradable and less corrosive than traditional alternatives[1]. The company has also invested in research to optimize sulfamic acid production methods, reducing energy consumption and waste generation[2]. BASF's sulfamic acid applications extend to descaling operations in industrial settings, where it effectively removes mineral deposits while minimizing environmental impact[3].
Strengths: Extensive R&D capabilities, global market presence, and diverse product portfolio. Weaknesses: Potential higher production costs compared to less environmentally friendly alternatives.
Qingdao Huicheng Environmental Technology Group Co., Ltd.
Technical Solution: Qingdao Huicheng has focused on developing sulfamic acid-based solutions for water treatment and environmental remediation. Their approach integrates sulfamic acid into advanced oxidation processes for the degradation of persistent organic pollutants in wastewater[4]. The company has patented a sulfamic acid-enhanced electrochemical treatment system that significantly improves the removal efficiency of heavy metals and organic contaminants from industrial effluents[5]. Qingdao Huicheng has also explored the use of sulfamic acid in soil remediation techniques, particularly for the treatment of contaminated sites in industrial areas[6].
Strengths: Specialized focus on environmental applications, strong presence in the Chinese market. Weaknesses: Limited global reach compared to multinational corporations.
Core Innovations in Sulfamic Acid Technology
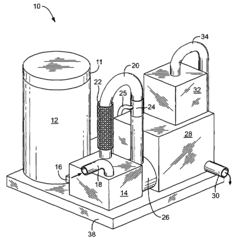
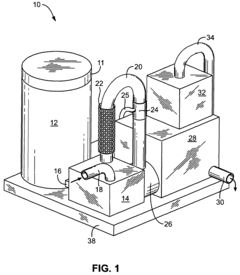
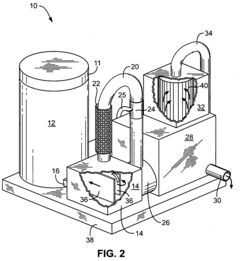
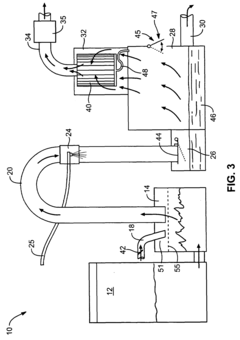
Sulfurous acid mist and sulfur dioxide gas recovery system
PatentInactiveUS20080044341A1
Innovation
- A recovery system is integrated with the sulfurous acid generator to separate and capture the mist and residual sulfur dioxide gas from the exhaust using apertures sized to remove particles smaller than 30 microns, employing filters like fiber beds or textile fabrics, and a motive source to direct the exhaust through these apertures, allowing the sulfur dioxide to be absorbed into liquid and converted into sulfurous acid.
Process for the regeneration of residual sulfuric acids contaminated with organic material
PatentInactiveEP0087346A1
Innovation
- A method combining conventional concentration under reduced pressure and electrochemical oxidation in an undiaphragmed electrolyser with a platinum anode and graphite cathode, optimizing energy efficiency by pre-concentrating the acid to 75-85% sulfuric acid content before electrolysis at controlled temperatures and current densities.
Regulatory Framework for Chemical Industry
The regulatory framework for the chemical industry plays a crucial role in governing the use, production, and disposal of sulfamic acid in environmental impact reduction measures. This framework encompasses a complex web of international, national, and local regulations designed to protect human health and the environment while promoting sustainable industrial practices.
At the international level, organizations such as the United Nations Environment Programme (UNEP) and the Organisation for Economic Co-operation and Development (OECD) provide guidelines and recommendations for the safe handling and use of chemicals, including sulfamic acid. These guidelines often serve as a foundation for national regulatory bodies to develop their own specific regulations.
In the United States, the Environmental Protection Agency (EPA) is the primary regulatory body overseeing the use of sulfamic acid and other chemicals in industrial processes. The EPA's Toxic Substances Control Act (TSCA) and the Clean Water Act (CWA) are key pieces of legislation that regulate the manufacture, import, and use of chemicals, as well as their discharge into water bodies. These regulations set specific limits on the concentration of sulfamic acid and its byproducts in wastewater and establish reporting requirements for manufacturers and users.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which requires companies to register chemical substances and provide safety data. This comprehensive regulatory framework ensures that sulfamic acid and other chemicals are used safely and that their environmental impacts are minimized.
In addition to these overarching regulations, many countries have specific laws and standards governing the use of sulfamic acid in various industries. For example, in the food industry, regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) set guidelines for the use of sulfamic acid as a food additive and cleaning agent.
The regulatory landscape also includes industry-specific standards and best practices developed by professional organizations and trade associations. These standards often go beyond legal requirements and promote the adoption of more sustainable and environmentally friendly practices in the use of sulfamic acid and other chemicals.
As environmental concerns continue to grow, regulatory frameworks are evolving to address emerging challenges. This includes the development of more stringent emissions standards, increased focus on lifecycle assessments of chemicals, and the promotion of green chemistry principles. These evolving regulations are driving innovation in the chemical industry, encouraging the development of more environmentally friendly alternatives and improved processes for using sulfamic acid in environmental impact reduction measures.
At the international level, organizations such as the United Nations Environment Programme (UNEP) and the Organisation for Economic Co-operation and Development (OECD) provide guidelines and recommendations for the safe handling and use of chemicals, including sulfamic acid. These guidelines often serve as a foundation for national regulatory bodies to develop their own specific regulations.
In the United States, the Environmental Protection Agency (EPA) is the primary regulatory body overseeing the use of sulfamic acid and other chemicals in industrial processes. The EPA's Toxic Substances Control Act (TSCA) and the Clean Water Act (CWA) are key pieces of legislation that regulate the manufacture, import, and use of chemicals, as well as their discharge into water bodies. These regulations set specific limits on the concentration of sulfamic acid and its byproducts in wastewater and establish reporting requirements for manufacturers and users.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which requires companies to register chemical substances and provide safety data. This comprehensive regulatory framework ensures that sulfamic acid and other chemicals are used safely and that their environmental impacts are minimized.
In addition to these overarching regulations, many countries have specific laws and standards governing the use of sulfamic acid in various industries. For example, in the food industry, regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) set guidelines for the use of sulfamic acid as a food additive and cleaning agent.
The regulatory landscape also includes industry-specific standards and best practices developed by professional organizations and trade associations. These standards often go beyond legal requirements and promote the adoption of more sustainable and environmentally friendly practices in the use of sulfamic acid and other chemicals.
As environmental concerns continue to grow, regulatory frameworks are evolving to address emerging challenges. This includes the development of more stringent emissions standards, increased focus on lifecycle assessments of chemicals, and the promotion of green chemistry principles. These evolving regulations are driving innovation in the chemical industry, encouraging the development of more environmentally friendly alternatives and improved processes for using sulfamic acid in environmental impact reduction measures.
Life Cycle Assessment of Sulfamic Acid
Life Cycle Assessment (LCA) of sulfamic acid provides a comprehensive evaluation of its environmental impacts throughout its entire lifecycle, from raw material extraction to disposal. This assessment is crucial for understanding the overall environmental footprint of sulfamic acid and identifying potential areas for improvement in its production and use.
The production of sulfamic acid begins with the extraction of raw materials, primarily urea and sulfuric acid. The manufacturing process involves the reaction of these components under controlled conditions. This stage of the lifecycle typically accounts for a significant portion of the overall environmental impact, due to energy consumption and potential emissions associated with chemical synthesis.
Transportation and distribution of sulfamic acid contribute to its carbon footprint, with the impact varying based on the distance and mode of transport. Packaging materials used for sulfamic acid storage and shipping also factor into the overall environmental assessment.
In its application phase, sulfamic acid is widely used in various industries, including cleaning products, water treatment, and metal finishing. The environmental impact during this stage depends on the specific application and concentration used. For instance, in cleaning products, the release of sulfamic acid into wastewater systems may have implications for aquatic ecosystems.
The end-of-life stage of sulfamic acid involves its disposal or potential degradation in the environment. Sulfamic acid is biodegradable, which can be advantageous from an environmental perspective. However, the rate and products of degradation may vary depending on environmental conditions.
LCA studies on sulfamic acid typically consider multiple impact categories, such as global warming potential, acidification, eutrophication, and resource depletion. These assessments often reveal that the production phase contributes significantly to the overall environmental impact, primarily due to energy consumption and chemical processes involved.
Comparative LCAs between sulfamic acid and alternative chemicals used for similar purposes can provide valuable insights into its relative environmental performance. Such studies may consider factors like persistence in the environment, toxicity to aquatic organisms, and potential for bioaccumulation.
Efforts to reduce the environmental impact of sulfamic acid often focus on improving production efficiency, optimizing transportation logistics, and developing more environmentally friendly formulations. Additionally, research into recovery and recycling methods for sulfamic acid could potentially reduce its overall lifecycle impact.
By conducting thorough Life Cycle Assessments, manufacturers and users of sulfamic acid can identify opportunities for environmental improvement and make informed decisions about its use in various applications. This approach aligns with broader sustainability goals and supports the development of more environmentally responsible chemical products and processes.
The production of sulfamic acid begins with the extraction of raw materials, primarily urea and sulfuric acid. The manufacturing process involves the reaction of these components under controlled conditions. This stage of the lifecycle typically accounts for a significant portion of the overall environmental impact, due to energy consumption and potential emissions associated with chemical synthesis.
Transportation and distribution of sulfamic acid contribute to its carbon footprint, with the impact varying based on the distance and mode of transport. Packaging materials used for sulfamic acid storage and shipping also factor into the overall environmental assessment.
In its application phase, sulfamic acid is widely used in various industries, including cleaning products, water treatment, and metal finishing. The environmental impact during this stage depends on the specific application and concentration used. For instance, in cleaning products, the release of sulfamic acid into wastewater systems may have implications for aquatic ecosystems.
The end-of-life stage of sulfamic acid involves its disposal or potential degradation in the environment. Sulfamic acid is biodegradable, which can be advantageous from an environmental perspective. However, the rate and products of degradation may vary depending on environmental conditions.
LCA studies on sulfamic acid typically consider multiple impact categories, such as global warming potential, acidification, eutrophication, and resource depletion. These assessments often reveal that the production phase contributes significantly to the overall environmental impact, primarily due to energy consumption and chemical processes involved.
Comparative LCAs between sulfamic acid and alternative chemicals used for similar purposes can provide valuable insights into its relative environmental performance. Such studies may consider factors like persistence in the environment, toxicity to aquatic organisms, and potential for bioaccumulation.
Efforts to reduce the environmental impact of sulfamic acid often focus on improving production efficiency, optimizing transportation logistics, and developing more environmentally friendly formulations. Additionally, research into recovery and recycling methods for sulfamic acid could potentially reduce its overall lifecycle impact.
By conducting thorough Life Cycle Assessments, manufacturers and users of sulfamic acid can identify opportunities for environmental improvement and make informed decisions about its use in various applications. This approach aligns with broader sustainability goals and supports the development of more environmentally responsible chemical products and processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!