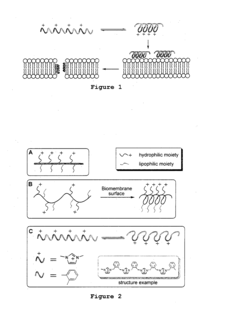
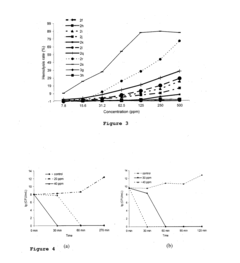
Ammonium hydroxide in the synthesis of amphiphilic polymers
AUG 14, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ammonium Hydroxide in Polymer Synthesis: Background and Objectives
Amphiphilic polymers have gained significant attention in recent years due to their unique properties and diverse applications. The synthesis of these polymers often involves complex processes and careful control of reaction conditions. Ammonium hydroxide, a common inorganic compound, has emerged as a valuable tool in the synthesis of amphiphilic polymers, offering several advantages in terms of reaction control and product characteristics.
The use of ammonium hydroxide in polymer synthesis dates back to the mid-20th century, with early applications primarily focused on pH control and neutralization. However, as the field of polymer chemistry advanced, researchers began to explore the potential of ammonium hydroxide as a reactive component in polymer synthesis, particularly for amphiphilic polymers.
Amphiphilic polymers are characterized by their dual nature, containing both hydrophilic and hydrophobic segments. This unique structure allows them to interact with both polar and non-polar environments, making them ideal for applications such as drug delivery, surfactants, and self-assembling materials. The synthesis of these polymers requires precise control over the balance between hydrophilic and hydrophobic components, which is where ammonium hydroxide plays a crucial role.
The primary objective of research into the use of ammonium hydroxide in amphiphilic polymer synthesis is to develop more efficient and controllable synthesis methods. Ammonium hydroxide offers several advantages in this context, including its ability to act as both a base and a nucleophile, its pH-dependent reactivity, and its potential to introduce amine functionalities into the polymer structure.
One of the key areas of focus is the use of ammonium hydroxide in the synthesis of block copolymers, where it can facilitate the controlled growth of hydrophilic segments. Additionally, researchers are exploring its potential in the modification of existing polymers to introduce amphiphilic properties, as well as its role in controlling the self-assembly behavior of amphiphilic polymers.
The evolution of this technology has been driven by advancements in polymer chemistry, characterization techniques, and the growing demand for functional materials in various industries. As research progresses, the aim is to develop more sustainable and scalable synthesis methods, expand the range of achievable polymer architectures, and enhance the performance of amphiphilic polymers in specific applications.
Understanding the fundamental principles and potential applications of ammonium hydroxide in amphiphilic polymer synthesis is crucial for advancing this field. This research not only contributes to the development of novel materials but also has implications for various industries, including healthcare, environmental remediation, and advanced manufacturing.
The use of ammonium hydroxide in polymer synthesis dates back to the mid-20th century, with early applications primarily focused on pH control and neutralization. However, as the field of polymer chemistry advanced, researchers began to explore the potential of ammonium hydroxide as a reactive component in polymer synthesis, particularly for amphiphilic polymers.
Amphiphilic polymers are characterized by their dual nature, containing both hydrophilic and hydrophobic segments. This unique structure allows them to interact with both polar and non-polar environments, making them ideal for applications such as drug delivery, surfactants, and self-assembling materials. The synthesis of these polymers requires precise control over the balance between hydrophilic and hydrophobic components, which is where ammonium hydroxide plays a crucial role.
The primary objective of research into the use of ammonium hydroxide in amphiphilic polymer synthesis is to develop more efficient and controllable synthesis methods. Ammonium hydroxide offers several advantages in this context, including its ability to act as both a base and a nucleophile, its pH-dependent reactivity, and its potential to introduce amine functionalities into the polymer structure.
One of the key areas of focus is the use of ammonium hydroxide in the synthesis of block copolymers, where it can facilitate the controlled growth of hydrophilic segments. Additionally, researchers are exploring its potential in the modification of existing polymers to introduce amphiphilic properties, as well as its role in controlling the self-assembly behavior of amphiphilic polymers.
The evolution of this technology has been driven by advancements in polymer chemistry, characterization techniques, and the growing demand for functional materials in various industries. As research progresses, the aim is to develop more sustainable and scalable synthesis methods, expand the range of achievable polymer architectures, and enhance the performance of amphiphilic polymers in specific applications.
Understanding the fundamental principles and potential applications of ammonium hydroxide in amphiphilic polymer synthesis is crucial for advancing this field. This research not only contributes to the development of novel materials but also has implications for various industries, including healthcare, environmental remediation, and advanced manufacturing.
Market Analysis for Amphiphilic Polymers
The market for amphiphilic polymers has been experiencing significant growth in recent years, driven by their unique properties and diverse applications across various industries. These polymers, characterized by their ability to interact with both hydrophilic and hydrophobic environments, have found extensive use in sectors such as pharmaceuticals, personal care, and advanced materials.
In the pharmaceutical industry, amphiphilic polymers play a crucial role in drug delivery systems, enhancing the solubility and bioavailability of poorly water-soluble drugs. This application has been particularly important in the development of targeted therapies and controlled-release formulations, contributing to the overall growth of the pharmaceutical market.
The personal care and cosmetics sector has also embraced amphiphilic polymers, utilizing them in the formulation of skincare products, hair care solutions, and color cosmetics. These polymers offer improved stability, texture, and performance in various personal care applications, meeting the increasing consumer demand for high-quality and multifunctional products.
In the field of advanced materials, amphiphilic polymers have gained traction in the development of smart materials, self-healing coatings, and responsive surfaces. Their ability to change properties in response to environmental stimuli has opened up new possibilities in areas such as sensors, actuators, and adaptive materials.
The global market for amphiphilic polymers is expected to continue its upward trajectory, with a compound annual growth rate (CAGR) projected to remain strong over the next five years. This growth is fueled by ongoing research and development efforts, as well as the expansion of application areas in emerging technologies such as nanotechnology and biomaterials.
Regionally, North America and Europe currently dominate the amphiphilic polymer market, owing to their well-established pharmaceutical and personal care industries. However, the Asia-Pacific region is anticipated to witness the fastest growth, driven by rapid industrialization, increasing healthcare expenditure, and growing consumer awareness in countries like China and India.
The use of ammonium hydroxide in the synthesis of amphiphilic polymers represents an important area of research within this market. This approach offers potential advantages in terms of cost-effectiveness, scalability, and environmental sustainability. As the demand for more efficient and eco-friendly synthesis methods grows, research in this area is likely to contribute significantly to market expansion and product innovation.
In the pharmaceutical industry, amphiphilic polymers play a crucial role in drug delivery systems, enhancing the solubility and bioavailability of poorly water-soluble drugs. This application has been particularly important in the development of targeted therapies and controlled-release formulations, contributing to the overall growth of the pharmaceutical market.
The personal care and cosmetics sector has also embraced amphiphilic polymers, utilizing them in the formulation of skincare products, hair care solutions, and color cosmetics. These polymers offer improved stability, texture, and performance in various personal care applications, meeting the increasing consumer demand for high-quality and multifunctional products.
In the field of advanced materials, amphiphilic polymers have gained traction in the development of smart materials, self-healing coatings, and responsive surfaces. Their ability to change properties in response to environmental stimuli has opened up new possibilities in areas such as sensors, actuators, and adaptive materials.
The global market for amphiphilic polymers is expected to continue its upward trajectory, with a compound annual growth rate (CAGR) projected to remain strong over the next five years. This growth is fueled by ongoing research and development efforts, as well as the expansion of application areas in emerging technologies such as nanotechnology and biomaterials.
Regionally, North America and Europe currently dominate the amphiphilic polymer market, owing to their well-established pharmaceutical and personal care industries. However, the Asia-Pacific region is anticipated to witness the fastest growth, driven by rapid industrialization, increasing healthcare expenditure, and growing consumer awareness in countries like China and India.
The use of ammonium hydroxide in the synthesis of amphiphilic polymers represents an important area of research within this market. This approach offers potential advantages in terms of cost-effectiveness, scalability, and environmental sustainability. As the demand for more efficient and eco-friendly synthesis methods grows, research in this area is likely to contribute significantly to market expansion and product innovation.
Current Challenges in Amphiphilic Polymer Synthesis
The synthesis of amphiphilic polymers using ammonium hydroxide faces several significant challenges that researchers and industry professionals are actively working to overcome. One of the primary issues is achieving precise control over the polymer architecture and composition. The amphiphilic nature of these polymers requires a delicate balance between hydrophilic and hydrophobic segments, which can be difficult to maintain consistently during synthesis.
The use of ammonium hydroxide as a reagent introduces additional complexities. While it offers advantages such as its ability to act as both a base and a nucleophile, its volatile nature can lead to inconsistencies in reaction conditions. This volatility can result in variations in pH levels throughout the synthesis process, potentially affecting the polymerization kinetics and final product characteristics.
Another challenge lies in the scalability of the synthesis process. Laboratory-scale production often yields promising results, but translating these methods to industrial-scale manufacturing presents hurdles. The heat management and mixing efficiency required for larger batch sizes can significantly impact the reaction outcomes, potentially leading to inconsistent product quality or reduced yields.
The purification of amphiphilic polymers synthesized using ammonium hydroxide also poses difficulties. The presence of residual ammonium ions can affect the final properties of the polymer, necessitating thorough purification steps. However, traditional purification methods may not be as effective due to the unique solubility characteristics of amphiphilic polymers, requiring the development of specialized techniques.
Environmental and safety concerns associated with the use of ammonium hydroxide in polymer synthesis are also noteworthy challenges. The compound's corrosive nature and potential for releasing ammonia gas require stringent safety protocols and specialized handling equipment, which can increase production costs and complexity.
Furthermore, achieving high molecular weight polymers while maintaining narrow polydispersity indices remains a significant challenge. The presence of ammonium hydroxide can influence chain termination and transfer reactions, potentially leading to broader molecular weight distributions or limiting the achievable molecular weights.
Lastly, the long-term stability of amphiphilic polymers synthesized using this method is an area of ongoing research. The potential for hydrolysis or degradation of certain polymer linkages in the presence of residual ammonium ions or under varying pH conditions needs to be thoroughly investigated to ensure the durability and shelf-life of the final products.
The use of ammonium hydroxide as a reagent introduces additional complexities. While it offers advantages such as its ability to act as both a base and a nucleophile, its volatile nature can lead to inconsistencies in reaction conditions. This volatility can result in variations in pH levels throughout the synthesis process, potentially affecting the polymerization kinetics and final product characteristics.
Another challenge lies in the scalability of the synthesis process. Laboratory-scale production often yields promising results, but translating these methods to industrial-scale manufacturing presents hurdles. The heat management and mixing efficiency required for larger batch sizes can significantly impact the reaction outcomes, potentially leading to inconsistent product quality or reduced yields.
The purification of amphiphilic polymers synthesized using ammonium hydroxide also poses difficulties. The presence of residual ammonium ions can affect the final properties of the polymer, necessitating thorough purification steps. However, traditional purification methods may not be as effective due to the unique solubility characteristics of amphiphilic polymers, requiring the development of specialized techniques.
Environmental and safety concerns associated with the use of ammonium hydroxide in polymer synthesis are also noteworthy challenges. The compound's corrosive nature and potential for releasing ammonia gas require stringent safety protocols and specialized handling equipment, which can increase production costs and complexity.
Furthermore, achieving high molecular weight polymers while maintaining narrow polydispersity indices remains a significant challenge. The presence of ammonium hydroxide can influence chain termination and transfer reactions, potentially leading to broader molecular weight distributions or limiting the achievable molecular weights.
Lastly, the long-term stability of amphiphilic polymers synthesized using this method is an area of ongoing research. The potential for hydrolysis or degradation of certain polymer linkages in the presence of residual ammonium ions or under varying pH conditions needs to be thoroughly investigated to ensure the durability and shelf-life of the final products.
Existing Methodologies for Amphiphilic Polymer Synthesis
01 Use in chemical processes
Ammonium hydroxide is widely used in various chemical processes as a reactant, catalyst, or pH regulator. It plays a crucial role in the synthesis of organic compounds, production of fertilizers, and treatment of industrial waste. Its alkaline properties make it suitable for neutralizing acidic solutions and controlling pH levels in different applications.- Use of ammonium hydroxide in chemical processes: Ammonium hydroxide is widely used in various chemical processes as a reactant, catalyst, or pH regulator. It plays a crucial role in the synthesis of organic compounds, production of fertilizers, and treatment of industrial waste. Its alkaline properties make it suitable for neutralizing acids and controlling pH levels in different applications.
- Application in cleaning and surface treatment: Ammonium hydroxide is utilized in cleaning formulations and surface treatment processes. It is effective in removing grease, oils, and other contaminants from various surfaces. In the semiconductor industry, it is used for etching and cleaning silicon wafers. Its ability to dissolve certain metals and oxides makes it valuable in metal surface treatment and electroplating applications.
- Role in textile and leather processing: Ammonium hydroxide finds applications in the textile and leather industries. It is used in dyeing processes to adjust pH levels and improve color fastness. In leather processing, it helps in dehairing and softening hides. Its alkaline nature aids in breaking down proteins and fats, facilitating various stages of textile and leather manufacturing.
- Environmental and agricultural applications: Ammonium hydroxide is employed in environmental and agricultural sectors. It is used in air pollution control systems to neutralize acidic gases. In agriculture, it serves as a source of nitrogen for fertilizers and soil amendments. Its ability to capture and store carbon dioxide makes it valuable in carbon capture technologies and greenhouse gas reduction efforts.
- Use in personal care and cosmetic products: Ammonium hydroxide is utilized in various personal care and cosmetic formulations. It acts as a pH adjuster in hair dyes, helping to open the hair cuticle for better color penetration. In some skincare products, it can help balance pH levels. Its alkaline properties also make it useful in certain depilatory creams and hair relaxers.
02 Application in cleaning and surface treatment
Ammonium hydroxide is utilized in cleaning formulations and surface treatment processes. It is effective in removing grease, oils, and other contaminants from various surfaces. In the semiconductor industry, it is used for etching and cleaning silicon wafers. Additionally, it finds applications in the textile industry for fabric treatment and in the leather industry for dehairing hides.Expand Specific Solutions03 Role in environmental and waste management
Ammonium hydroxide is employed in environmental and waste management applications. It is used in flue gas treatment to reduce nitrogen oxide emissions, in wastewater treatment for pH adjustment and ammonia recovery, and in soil remediation processes. Its ability to neutralize acidic compounds makes it valuable in managing industrial effluents and controlling air pollution.Expand Specific Solutions04 Use in personal care and cosmetic products
Ammonium hydroxide finds applications in personal care and cosmetic products. It is used as a pH adjuster in hair dyes, permanent wave solutions, and other hair care products. In some cosmetic formulations, it acts as a buffering agent or helps in the emulsification process. Its alkaline nature assists in opening hair cuticles for better dye penetration in hair coloring products.Expand Specific Solutions05 Application in food processing
Ammonium hydroxide has specific applications in food processing. It is used as a leavening agent in baked goods, helping to create a lighter texture. In some countries, it is approved as a food additive for pH control and as an antimicrobial agent. It also finds use in the production of caramel coloring and in the processing of cocoa powder to adjust its color and flavor profile.Expand Specific Solutions
Key Players in Amphiphilic Polymer Industry
The research on ammonium hydroxide in amphiphilic polymer synthesis is in a developing stage, with growing market potential due to increasing demand for advanced materials in various industries. The global market for amphiphilic polymers is expanding, driven by applications in drug delivery, nanotechnology, and environmental remediation. Technologically, the field is progressing rapidly, with key players like BASF Corp., Massachusetts Institute of Technology, and L'Oréal SA leading innovation. These organizations are investing in R&D to improve synthesis methods and explore novel applications. While the technology is not yet fully mature, significant advancements are being made, particularly in optimizing the use of ammonium hydroxide to enhance polymer properties and performance.
BASF Corp.
Technical Solution: BASF has developed a novel approach for synthesizing amphiphilic polymers using ammonium hydroxide as a key reagent. Their method involves a controlled free radical polymerization process, where ammonium hydroxide acts as both a pH regulator and a chain transfer agent[1]. This allows for precise control over the hydrophilic-lipophilic balance of the resulting polymers. The company has also implemented a green chemistry approach, using supercritical CO2 as a reaction medium, which enhances the efficiency of the ammonium hydroxide-mediated synthesis[3]. BASF's technology enables the production of amphiphilic block copolymers with tailored properties for applications in personal care, pharmaceuticals, and industrial formulations[5].
Strengths: Precise control over polymer properties, environmentally friendly process, versatile applications. Weaknesses: Potential scalability challenges, higher production costs compared to conventional methods.
Massachusetts Institute of Technology
Technical Solution: MIT researchers have pioneered a novel approach to synthesizing amphiphilic polymers using ammonium hydroxide in conjunction with reversible addition-fragmentation chain transfer (RAFT) polymerization[2]. This method allows for the creation of well-defined block copolymers with controlled molecular weight and narrow polydispersity. The team has developed a unique ammonium hydroxide-mediated RAFT agent that facilitates the synthesis of polymers with tunable hydrophilic and hydrophobic segments[4]. Additionally, MIT has explored the use of ammonium hydroxide in post-polymerization modifications, enabling the introduction of functional groups to enhance the amphiphilic properties of the polymers[6]. Their research has led to the development of smart materials with stimuli-responsive behavior, particularly useful in drug delivery systems and self-assembling nanostructures[8].
Strengths: High precision in polymer architecture control, versatility in functional group incorporation, potential for smart material development. Weaknesses: Complex synthesis procedures, potential limitations in large-scale production.
Innovations in Ammonium Hydroxide-Based Synthesis
Antimicrobial composition
PatentInactiveUS20130210881A1
Innovation
- Development of antimicrobial compositions comprising hydrophilic heterocyclic amine monomers coupled by hydrophobic linkers, which assume a facially amphiphilic conformation to disrupt bacterial cytoplasmic membranes, reducing hemolytic effects and maintaining safety for mammalian cells.
Environmental Impact of Ammonium Hydroxide in Synthesis
The use of ammonium hydroxide in the synthesis of amphiphilic polymers has significant environmental implications that warrant careful consideration. While this compound plays a crucial role in various industrial processes, its impact on the environment cannot be overlooked. Ammonium hydroxide, when released into aquatic ecosystems, can lead to eutrophication, a process that causes excessive algal growth and subsequent oxygen depletion in water bodies. This can have detrimental effects on aquatic life and overall ecosystem balance.
Furthermore, the production and use of ammonium hydroxide contribute to atmospheric pollution. When released into the air, it can form particulate matter and react with other pollutants, potentially leading to the formation of smog and acid rain. These atmospheric effects can have far-reaching consequences on both human health and the environment, impacting air quality in urban and industrial areas.
The synthesis process itself may also generate waste products that require proper disposal. Improper handling or disposal of these wastes can lead to soil contamination and groundwater pollution. This highlights the need for stringent waste management protocols in facilities utilizing ammonium hydroxide for polymer synthesis.
On a positive note, recent advancements in green chemistry have led to the development of more environmentally friendly alternatives and improved synthesis methods. These innovations aim to reduce the environmental footprint of amphiphilic polymer production by minimizing the use of harmful chemicals like ammonium hydroxide or finding safer substitutes.
Regulatory bodies worldwide have implemented strict guidelines for the use and disposal of ammonium hydroxide in industrial processes. These regulations often require companies to implement emission control technologies, conduct regular environmental impact assessments, and adhere to specific waste treatment protocols. Compliance with these regulations is crucial for mitigating the environmental risks associated with ammonium hydroxide use in polymer synthesis.
As the demand for amphiphilic polymers continues to grow across various industries, including pharmaceuticals, cosmetics, and materials science, the environmental impact of their production becomes increasingly significant. This necessitates ongoing research into more sustainable synthesis methods and the development of eco-friendly alternatives to traditional reagents like ammonium hydroxide.
Furthermore, the production and use of ammonium hydroxide contribute to atmospheric pollution. When released into the air, it can form particulate matter and react with other pollutants, potentially leading to the formation of smog and acid rain. These atmospheric effects can have far-reaching consequences on both human health and the environment, impacting air quality in urban and industrial areas.
The synthesis process itself may also generate waste products that require proper disposal. Improper handling or disposal of these wastes can lead to soil contamination and groundwater pollution. This highlights the need for stringent waste management protocols in facilities utilizing ammonium hydroxide for polymer synthesis.
On a positive note, recent advancements in green chemistry have led to the development of more environmentally friendly alternatives and improved synthesis methods. These innovations aim to reduce the environmental footprint of amphiphilic polymer production by minimizing the use of harmful chemicals like ammonium hydroxide or finding safer substitutes.
Regulatory bodies worldwide have implemented strict guidelines for the use and disposal of ammonium hydroxide in industrial processes. These regulations often require companies to implement emission control technologies, conduct regular environmental impact assessments, and adhere to specific waste treatment protocols. Compliance with these regulations is crucial for mitigating the environmental risks associated with ammonium hydroxide use in polymer synthesis.
As the demand for amphiphilic polymers continues to grow across various industries, including pharmaceuticals, cosmetics, and materials science, the environmental impact of their production becomes increasingly significant. This necessitates ongoing research into more sustainable synthesis methods and the development of eco-friendly alternatives to traditional reagents like ammonium hydroxide.
Scale-up Considerations for Industrial Production
When considering the scale-up of ammonium hydroxide use in the synthesis of amphiphilic polymers for industrial production, several key factors must be addressed. Firstly, the reactor design and size need to be carefully evaluated. Larger reactors may require modifications to ensure uniform mixing and temperature control, which are critical for maintaining product quality and consistency. The heat transfer capabilities of the scaled-up system should be assessed to manage the exothermic nature of the reaction effectively.
Material handling and storage become more complex at industrial scales. Ammonium hydroxide is corrosive and releases ammonia gas, necessitating appropriate safety measures and specialized equipment. Bulk storage tanks, transfer systems, and ventilation must be designed to handle larger quantities safely. Additionally, the increased volume of reactants and products requires efficient logistics and inventory management systems.
Process control and automation play a crucial role in maintaining consistent product quality during scale-up. Advanced monitoring systems for temperature, pH, and reaction progress should be implemented. Real-time data analysis and feedback loops can help optimize reaction conditions and ensure reproducibility across batches. The development of robust standard operating procedures (SOPs) is essential for maintaining consistency in the scaled-up process.
Waste management and environmental considerations become more significant at industrial scales. The increased use of ammonium hydroxide may require enhanced treatment systems for waste streams and off-gases. Recycling and recovery processes for excess ammonia should be explored to improve efficiency and reduce environmental impact. Compliance with local and national regulations regarding emissions and waste disposal must be ensured.
Economic factors also play a crucial role in scale-up decisions. A thorough cost-benefit analysis should be conducted, considering factors such as raw material costs, energy consumption, labor requirements, and potential economies of scale. The feasibility of continuous production methods versus batch processes should be evaluated, as continuous systems may offer advantages in terms of efficiency and product consistency for large-scale operations.
Quality control and product characterization methods may need to be adapted for larger batch sizes. Sampling techniques and analytical procedures should be reviewed to ensure they remain representative and efficient at industrial scales. The development of in-line or at-line monitoring techniques can provide real-time quality assurance and reduce the need for time-consuming offline analyses.
Material handling and storage become more complex at industrial scales. Ammonium hydroxide is corrosive and releases ammonia gas, necessitating appropriate safety measures and specialized equipment. Bulk storage tanks, transfer systems, and ventilation must be designed to handle larger quantities safely. Additionally, the increased volume of reactants and products requires efficient logistics and inventory management systems.
Process control and automation play a crucial role in maintaining consistent product quality during scale-up. Advanced monitoring systems for temperature, pH, and reaction progress should be implemented. Real-time data analysis and feedback loops can help optimize reaction conditions and ensure reproducibility across batches. The development of robust standard operating procedures (SOPs) is essential for maintaining consistency in the scaled-up process.
Waste management and environmental considerations become more significant at industrial scales. The increased use of ammonium hydroxide may require enhanced treatment systems for waste streams and off-gases. Recycling and recovery processes for excess ammonia should be explored to improve efficiency and reduce environmental impact. Compliance with local and national regulations regarding emissions and waste disposal must be ensured.
Economic factors also play a crucial role in scale-up decisions. A thorough cost-benefit analysis should be conducted, considering factors such as raw material costs, energy consumption, labor requirements, and potential economies of scale. The feasibility of continuous production methods versus batch processes should be evaluated, as continuous systems may offer advantages in terms of efficiency and product consistency for large-scale operations.
Quality control and product characterization methods may need to be adapted for larger batch sizes. Sampling techniques and analytical procedures should be reviewed to ensure they remain representative and efficient at industrial scales. The development of in-line or at-line monitoring techniques can provide real-time quality assurance and reduce the need for time-consuming offline analyses.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!