Role Of Perfluorinated Polymers In High-Selectivity CO2 Membranes
SEP 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perfluorinated Polymers in CO2 Separation: Background and Objectives
Perfluorinated polymers have emerged as a critical material class in the development of high-selectivity CO2 separation membranes over the past three decades. The journey began in the early 1990s when researchers first recognized the unique properties of fluorinated materials for gas separation applications. These polymers, characterized by carbon chains where hydrogen atoms are replaced by fluorine atoms, exhibit exceptional chemical stability, thermal resistance, and unique gas transport properties that make them particularly suitable for CO2 capture technologies.
The evolution of perfluorinated polymers in membrane technology has been driven by increasing global concerns regarding carbon emissions and climate change. As industrial CO2 emissions became recognized as a major contributor to global warming in the early 2000s, research into efficient carbon capture technologies accelerated significantly. This period marked a turning point in the development trajectory of perfluorinated polymer membranes, shifting from academic curiosity to industrial necessity.
By the mid-2010s, perfluorinated polymers had established themselves as leading candidates for next-generation CO2 separation membranes due to their superior CO2/N2 and CO2/CH4 selectivity compared to conventional polymeric materials. The unique electron-withdrawing properties of fluorine atoms create favorable interactions with CO2 molecules, enhancing separation performance while maintaining structural integrity under harsh operating conditions.
Recent technological advancements have focused on optimizing the molecular architecture of these polymers to further enhance CO2 permeability and selectivity. Innovations in polymer synthesis techniques have enabled precise control over chain configuration, functional group incorporation, and morphological characteristics, leading to membranes with increasingly impressive separation performance metrics.
The primary technical objective in this field is to develop perfluorinated polymer membranes that achieve the delicate balance between high CO2 permeability and selectivity while maintaining mechanical durability and long-term operational stability. Researchers aim to surpass the well-known Robeson upper bound that describes the traditional trade-off between permeability and selectivity in polymer membranes.
Additional objectives include reducing manufacturing costs to enable commercial viability, enhancing resistance to plasticization and aging effects, and developing scalable production methods compatible with existing industrial infrastructure. The ultimate goal is to create membrane systems capable of efficiently capturing CO2 from various sources including power plant flue gases, natural gas streams, and ambient air at economically viable operating costs.
As we look toward future developments, the integration of perfluorinated polymers with other advanced materials such as metal-organic frameworks (MOFs) and facilitated transport agents represents a promising frontier for achieving unprecedented separation performance in next-generation carbon capture technologies.
The evolution of perfluorinated polymers in membrane technology has been driven by increasing global concerns regarding carbon emissions and climate change. As industrial CO2 emissions became recognized as a major contributor to global warming in the early 2000s, research into efficient carbon capture technologies accelerated significantly. This period marked a turning point in the development trajectory of perfluorinated polymer membranes, shifting from academic curiosity to industrial necessity.
By the mid-2010s, perfluorinated polymers had established themselves as leading candidates for next-generation CO2 separation membranes due to their superior CO2/N2 and CO2/CH4 selectivity compared to conventional polymeric materials. The unique electron-withdrawing properties of fluorine atoms create favorable interactions with CO2 molecules, enhancing separation performance while maintaining structural integrity under harsh operating conditions.
Recent technological advancements have focused on optimizing the molecular architecture of these polymers to further enhance CO2 permeability and selectivity. Innovations in polymer synthesis techniques have enabled precise control over chain configuration, functional group incorporation, and morphological characteristics, leading to membranes with increasingly impressive separation performance metrics.
The primary technical objective in this field is to develop perfluorinated polymer membranes that achieve the delicate balance between high CO2 permeability and selectivity while maintaining mechanical durability and long-term operational stability. Researchers aim to surpass the well-known Robeson upper bound that describes the traditional trade-off between permeability and selectivity in polymer membranes.
Additional objectives include reducing manufacturing costs to enable commercial viability, enhancing resistance to plasticization and aging effects, and developing scalable production methods compatible with existing industrial infrastructure. The ultimate goal is to create membrane systems capable of efficiently capturing CO2 from various sources including power plant flue gases, natural gas streams, and ambient air at economically viable operating costs.
As we look toward future developments, the integration of perfluorinated polymers with other advanced materials such as metal-organic frameworks (MOFs) and facilitated transport agents represents a promising frontier for achieving unprecedented separation performance in next-generation carbon capture technologies.
Market Analysis for High-Selectivity CO2 Membrane Applications
The global market for CO2 capture technologies is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability initiatives. High-selectivity CO2 membranes represent a crucial segment within this market, with perfluorinated polymers playing a pivotal role in enhancing membrane performance. Current market valuations place the global carbon capture and storage market at approximately 7 billion USD in 2023, with projections indicating growth to reach 15 billion USD by 2030.
The industrial sector constitutes the largest application market for high-selectivity CO2 membranes, particularly in power generation, cement production, and chemical manufacturing. These industries face mounting pressure to reduce carbon emissions while maintaining operational efficiency. Membrane-based carbon capture systems offer advantages in terms of energy efficiency and operational flexibility compared to traditional amine-based absorption technologies.
Regional analysis reveals that North America and Europe currently lead in adoption of advanced CO2 separation technologies, accounting for over 60% of the global market share. However, the Asia-Pacific region, particularly China and India, is expected to witness the fastest growth rate due to rapid industrialization coupled with strengthening environmental regulations.
Market segmentation by membrane material shows that perfluorinated polymer membranes command premium pricing due to their superior performance characteristics. The price differential between standard polymeric membranes and perfluorinated variants ranges between 30-40%, justified by significantly higher CO2/N2 selectivity ratios and longer operational lifespans.
Customer adoption patterns indicate that large industrial emitters prioritize membrane durability and separation efficiency over initial capital costs. This preference creates favorable market conditions for perfluorinated polymer membranes despite their higher price point. The total cost of ownership analysis demonstrates that the enhanced performance of these membranes often results in better long-term economics through reduced energy consumption and maintenance requirements.
Market barriers include high initial investment costs, technical complexity in system integration, and competition from alternative carbon capture technologies. However, technological advancements in membrane manufacturing processes are gradually reducing production costs, potentially expanding market accessibility.
Demand forecasts suggest a compound annual growth rate of 12-15% for high-selectivity CO2 membranes over the next five years, with perfluorinated polymer variants expected to grow at an even higher rate of 18-20%. This accelerated growth is attributed to increasing carbon pricing mechanisms worldwide and stricter emission standards being implemented across major economies.
The industrial sector constitutes the largest application market for high-selectivity CO2 membranes, particularly in power generation, cement production, and chemical manufacturing. These industries face mounting pressure to reduce carbon emissions while maintaining operational efficiency. Membrane-based carbon capture systems offer advantages in terms of energy efficiency and operational flexibility compared to traditional amine-based absorption technologies.
Regional analysis reveals that North America and Europe currently lead in adoption of advanced CO2 separation technologies, accounting for over 60% of the global market share. However, the Asia-Pacific region, particularly China and India, is expected to witness the fastest growth rate due to rapid industrialization coupled with strengthening environmental regulations.
Market segmentation by membrane material shows that perfluorinated polymer membranes command premium pricing due to their superior performance characteristics. The price differential between standard polymeric membranes and perfluorinated variants ranges between 30-40%, justified by significantly higher CO2/N2 selectivity ratios and longer operational lifespans.
Customer adoption patterns indicate that large industrial emitters prioritize membrane durability and separation efficiency over initial capital costs. This preference creates favorable market conditions for perfluorinated polymer membranes despite their higher price point. The total cost of ownership analysis demonstrates that the enhanced performance of these membranes often results in better long-term economics through reduced energy consumption and maintenance requirements.
Market barriers include high initial investment costs, technical complexity in system integration, and competition from alternative carbon capture technologies. However, technological advancements in membrane manufacturing processes are gradually reducing production costs, potentially expanding market accessibility.
Demand forecasts suggest a compound annual growth rate of 12-15% for high-selectivity CO2 membranes over the next five years, with perfluorinated polymer variants expected to grow at an even higher rate of 18-20%. This accelerated growth is attributed to increasing carbon pricing mechanisms worldwide and stricter emission standards being implemented across major economies.
Current Challenges in Perfluorinated Polymer CO2 Membrane Technology
Despite significant advancements in perfluorinated polymer membrane technology for CO2 separation, several critical challenges continue to impede widespread industrial implementation. The inherent trade-off between permeability and selectivity remains a fundamental obstacle. As membrane permeability increases, selectivity typically decreases, creating a performance ceiling known as the Robeson upper bound. Perfluorinated polymers show promise in pushing this boundary, but optimizing both properties simultaneously requires further material innovation.
Material stability presents another significant challenge, particularly in industrial settings where membranes must withstand harsh operating conditions. Perfluorinated polymers exhibit excellent chemical resistance, but prolonged exposure to high-pressure CO2 streams, contaminants, and temperature fluctuations can lead to plasticization, resulting in performance degradation over time. This phenomenon causes polymer chain mobility to increase, reducing the membrane's ability to discriminate between gas molecules.
Manufacturing scalability poses substantial hurdles for commercialization. Current production methods for high-performance perfluorinated membranes often involve complex synthesis procedures with expensive precursors and environmentally concerning fluorinated compounds. The transition from laboratory-scale fabrication to industrial-scale production while maintaining consistent membrane quality, thickness control, and defect minimization remains challenging.
Cost considerations further complicate adoption, as perfluorinated polymers are inherently expensive compared to conventional membrane materials. The economic viability of these membranes depends on achieving sufficient performance advantages to justify their higher cost, particularly in competitive markets like natural gas purification and post-combustion carbon capture.
Environmental and regulatory concerns surrounding perfluorinated compounds have intensified in recent years. Many perfluorinated substances are classified as persistent organic pollutants with potential bioaccumulation properties. Developing environmentally benign synthesis routes and ensuring minimal environmental impact throughout the membrane lifecycle presents a significant challenge for researchers and manufacturers.
Integration challenges exist when implementing these membranes into existing industrial processes. Membrane modules must be designed to maximize surface area while minimizing pressure drop, and system engineering must account for feed pretreatment requirements to prevent membrane fouling and damage from particulates or condensable components in real-world gas streams.
Finally, performance validation under realistic conditions remains difficult. Laboratory testing often occurs under idealized conditions with pure or simple gas mixtures, whereas industrial applications involve complex gas compositions with varying concentrations of water vapor, hydrocarbons, and other contaminants that can significantly impact membrane performance and longevity.
Material stability presents another significant challenge, particularly in industrial settings where membranes must withstand harsh operating conditions. Perfluorinated polymers exhibit excellent chemical resistance, but prolonged exposure to high-pressure CO2 streams, contaminants, and temperature fluctuations can lead to plasticization, resulting in performance degradation over time. This phenomenon causes polymer chain mobility to increase, reducing the membrane's ability to discriminate between gas molecules.
Manufacturing scalability poses substantial hurdles for commercialization. Current production methods for high-performance perfluorinated membranes often involve complex synthesis procedures with expensive precursors and environmentally concerning fluorinated compounds. The transition from laboratory-scale fabrication to industrial-scale production while maintaining consistent membrane quality, thickness control, and defect minimization remains challenging.
Cost considerations further complicate adoption, as perfluorinated polymers are inherently expensive compared to conventional membrane materials. The economic viability of these membranes depends on achieving sufficient performance advantages to justify their higher cost, particularly in competitive markets like natural gas purification and post-combustion carbon capture.
Environmental and regulatory concerns surrounding perfluorinated compounds have intensified in recent years. Many perfluorinated substances are classified as persistent organic pollutants with potential bioaccumulation properties. Developing environmentally benign synthesis routes and ensuring minimal environmental impact throughout the membrane lifecycle presents a significant challenge for researchers and manufacturers.
Integration challenges exist when implementing these membranes into existing industrial processes. Membrane modules must be designed to maximize surface area while minimizing pressure drop, and system engineering must account for feed pretreatment requirements to prevent membrane fouling and damage from particulates or condensable components in real-world gas streams.
Finally, performance validation under realistic conditions remains difficult. Laboratory testing often occurs under idealized conditions with pure or simple gas mixtures, whereas industrial applications involve complex gas compositions with varying concentrations of water vapor, hydrocarbons, and other contaminants that can significantly impact membrane performance and longevity.
State-of-the-Art Perfluorinated Polymer Membrane Solutions
01 Membrane separation using perfluorinated polymers
Perfluorinated polymers are used in membrane separation processes due to their high selectivity for specific compounds. These membranes can effectively separate gases, liquids, or mixtures based on molecular size, shape, and chemical properties. The unique chemical structure of perfluorinated polymers provides excellent thermal stability, chemical resistance, and selective permeability, making them ideal for applications in gas separation, water purification, and chemical processing.- Membrane separation using perfluorinated polymers: Perfluorinated polymers are utilized in membrane separation technologies due to their high selectivity for specific compounds. These membranes can effectively separate gases, liquids, or mixed phases based on molecular size, shape, and chemical properties. The perfluorinated structure provides chemical stability and resistance to harsh environments, making these membranes suitable for industrial separation processes including gas purification and chemical processing.
- Catalytic applications of perfluorinated polymers: Perfluorinated polymers serve as effective catalyst supports or as catalysts themselves in various chemical reactions. Their unique electronic properties and chemical inertness allow for high selectivity in catalytic processes. These polymers can be functionalized to create active sites for specific reactions, enabling selective transformations in organic synthesis, petrochemical processing, and fine chemical production with improved yields and reduced byproducts.
- Electrochemical applications and ion selectivity: Perfluorinated polymers demonstrate exceptional ion selectivity in electrochemical applications, particularly in fuel cells, batteries, and sensors. These polymers can be designed with specific ionic conductivity properties while maintaining electronic insulation. Their ability to selectively transport certain ions while blocking others makes them valuable in energy storage and conversion technologies, as well as in analytical and sensing devices.
- Surface modification and coating selectivity: Perfluorinated polymers are used for surface modifications and coatings to impart selective properties such as hydrophobicity, oleophobicity, and chemical resistance. These coatings can selectively repel certain substances while allowing others to interact with the surface. The selective nature of these polymer coatings makes them valuable in applications requiring controlled surface interactions, including protective coatings, anti-fouling surfaces, and biomedical devices.
- Selective gas transport and barrier properties: Perfluorinated polymers exhibit selective gas transport and barrier properties, making them suitable for applications requiring controlled permeability. These polymers can be engineered to selectively allow certain gases to pass through while blocking others, based on molecular size, polarity, and interaction with the polymer matrix. This selectivity is valuable in packaging, protective equipment, gas separation technologies, and environmental control systems.
02 Catalytic applications of perfluorinated polymers
Perfluorinated polymers demonstrate high selectivity as catalysts or catalyst supports in various chemical reactions. Their unique electronic properties and chemical inertness allow them to facilitate specific reaction pathways while inhibiting unwanted side reactions. These polymers can be functionalized to enhance their catalytic activity and selectivity for particular substrates, making them valuable in fine chemical synthesis, petrochemical processing, and pharmaceutical manufacturing.Expand Specific Solutions03 Electrochemical applications and ion selectivity
Perfluorinated polymers exhibit excellent ion selectivity in electrochemical applications, particularly in fuel cells, batteries, and sensors. These polymers can be designed to selectively transport specific ions while blocking others, which is crucial for efficient energy conversion and storage devices. Their stability in harsh electrochemical environments and ability to maintain conductivity under various conditions make them ideal materials for selective ion exchange membranes and electrode coatings.Expand Specific Solutions04 Surface modification and selective adsorption
Perfluorinated polymers can be used to modify surfaces to achieve selective adsorption properties. Their low surface energy and unique chemical structure allow them to repel certain substances while selectively binding to others. This selective behavior is valuable in applications such as chromatography, filtration media, and protective coatings. By controlling the composition and structure of these polymers, surfaces can be engineered to have specific interaction profiles with target molecules.Expand Specific Solutions05 Optical and electronic selectivity of perfluorinated polymers
Perfluorinated polymers demonstrate selective optical and electronic properties that make them valuable in various applications. These polymers can be designed to selectively transmit, reflect, or absorb specific wavelengths of light, or to conduct certain types of electronic signals while blocking others. Their unique electronic structure and stability make them suitable for use in optical devices, sensors, and electronic components where selective response to stimuli is required.Expand Specific Solutions
Leading Companies and Research Institutions in CO2 Membrane Development
The perfluorinated polymers in high-selectivity CO2 membranes market is in a growth phase, with increasing demand driven by carbon capture applications. The global market is expanding as environmental regulations tighten, with projections showing significant growth potential. Technologically, the field is advancing rapidly but remains in mid-maturity, with key players demonstrating varying levels of innovation. Companies like Membrane Technology & Research and DuPont lead with commercial applications, while Air Liquide and 3M contribute significant R&D. Academic institutions including New York University and National University of Singapore provide fundamental research support. Solvay Specialty Polymers and The Chemours Co. leverage their fluoropolymer expertise to develop specialized membrane materials, creating a competitive landscape balanced between established chemical companies and specialized membrane technology firms.
Membrane Technology & Research, Inc.
Technical Solution: MTR has pioneered the development of perfluorinated polymer-based CO2 selective membranes through their Polaris™ membrane technology. Their approach incorporates perfluorinated segments into block copolymers to create membranes with exceptional CO2/N2 selectivity (>50) while maintaining high CO2 permeability (>1000 Barrer). The company's technology utilizes perfluorinated polymers' unique properties - including high free volume, low cohesive energy density, and excellent chemical resistance - to create membranes that effectively separate CO2 from flue gas streams. MTR's membranes incorporate perfluorinated polyether (PFPE) segments that provide exceptional CO2 solubility while maintaining structural integrity under harsh industrial conditions. Their multi-layer composite membrane design features a thin selective layer of perfluorinated polymer on a porous support, optimizing both selectivity and flux performance.
Strengths: Industry-leading CO2/N2 selectivity combined with high permeability; proven commercial-scale implementation; excellent resistance to contaminants in industrial gas streams. Weaknesses: Higher manufacturing costs compared to conventional polymers; potential environmental concerns regarding fluorinated materials; performance degradation at elevated temperatures.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed advanced perfluorinated polymer membranes for CO2 separation utilizing their expertise in fluoropolymer chemistry. Their technology centers on modified Nafion® and other perfluorosulfonic acid (PFSA) polymers, which they've engineered specifically for enhanced CO2 transport. DuPont's approach involves creating composite membranes with perfluorinated ionomers that leverage the polar interactions between CO2 and the sulfonic acid groups while maintaining the chemical stability of the perfluorinated backbone. The company has pioneered techniques to control membrane morphology at the nanoscale, creating distinct hydrophilic and hydrophobic domains that facilitate selective CO2 transport. Their membranes achieve CO2/N2 selectivity of approximately 30-40 with CO2 permeability exceeding 500 Barrer under typical flue gas conditions. DuPont has also developed surface modification techniques for their perfluorinated membranes to enhance CO2 affinity while preventing plasticization effects that typically reduce selectivity at high CO2 partial pressures.
Strengths: Exceptional chemical and thermal stability; established manufacturing infrastructure for fluoropolymers; strong intellectual property portfolio in perfluorinated materials. Weaknesses: Higher production costs compared to hydrocarbon-based polymers; environmental persistence concerns with perfluorinated compounds; membrane performance can be affected by humidity variations.
Key Patents and Scientific Breakthroughs in CO2 Selective Membranes
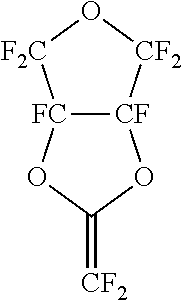
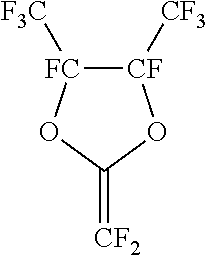
Gas separation membranes based on fluorinated and perfluorinated polymers
PatentInactiveUS9643124B2
Innovation
- A process utilizing a copolymer membrane with a selective layer formed from a combination of perfluorodioxolane and perfluorinated or partially fluorinated dioxane monomers, which balances crystalline and amorphous phases to enhance gas separation properties, including higher selectivity and flux for gases like hydrogen, helium, carbon dioxide, and nitrogen from methane.
Gas separations using membranes comprising perfluorinated polymers with pendant ionomeric moieties
PatentInactiveCA1302303C
Innovation
- Membranes comprising perfluorinated polymers with pendant ionomeric moieties, specifically containing cations of alkali metals, alkaline earth metals, or transition metals, are used to selectively separate gases by contacting gas mixtures with a thin, non-porous membrane, allowing oxygen, nitrogen, helium, or carbon dioxide to permeate preferentially.
Environmental Impact and Sustainability Considerations
The integration of perfluorinated polymers in CO2 separation membranes presents significant environmental considerations that must be carefully evaluated against their performance benefits. These polymers, while offering exceptional CO2 selectivity, raise concerns regarding their environmental persistence and potential long-term ecological impacts.
Perfluorinated compounds are known for their extreme environmental persistence, with degradation timeframes measured in decades or centuries. This characteristic, while beneficial for membrane durability, creates potential environmental liabilities throughout the product lifecycle. The manufacturing processes for these polymers typically involve fluorinated precursors that may contribute to greenhouse gas emissions, partially offsetting the carbon capture benefits these membranes aim to provide.
End-of-life considerations represent another critical environmental dimension. Current recycling technologies for perfluorinated polymers remain limited, creating waste management challenges when these membranes reach their operational end. Incineration of these materials can potentially release harmful byproducts if not conducted under strictly controlled conditions, necessitating specialized disposal protocols.
Recent life cycle assessment (LCA) studies comparing perfluorinated polymer membranes with alternative CO2 capture technologies reveal complex sustainability tradeoffs. While these membranes generally demonstrate lower operational energy requirements than traditional amine scrubbing processes, their embodied carbon and manufacturing environmental footprint may be higher. The net environmental benefit depends significantly on membrane longevity and operational efficiency in specific applications.
Water usage represents another important sustainability metric. Perfluorinated membrane systems typically require less water than competing technologies, potentially offering advantages in water-stressed regions. However, concerns regarding potential perfluorinated compound leaching into water systems during operation must be addressed through appropriate monitoring and containment strategies.
Regulatory frameworks governing perfluorinated compounds continue to evolve globally, with increasing scrutiny on persistent organic pollutants. Membrane developers must anticipate potential regulatory changes that could impact material selection, manufacturing processes, and disposal requirements. Forward-thinking companies are already exploring alternative fluoropolymers with reduced environmental persistence while maintaining separation performance.
Sustainability innovation pathways include developing hybrid membrane systems that minimize perfluorinated content while maintaining selectivity, implementing closed-loop manufacturing processes, and designing for end-of-life recyclability. Biomimetic approaches that achieve similar selectivity without fluorinated compounds represent a promising long-term research direction, potentially offering truly sustainable CO2 capture solutions.
Perfluorinated compounds are known for their extreme environmental persistence, with degradation timeframes measured in decades or centuries. This characteristic, while beneficial for membrane durability, creates potential environmental liabilities throughout the product lifecycle. The manufacturing processes for these polymers typically involve fluorinated precursors that may contribute to greenhouse gas emissions, partially offsetting the carbon capture benefits these membranes aim to provide.
End-of-life considerations represent another critical environmental dimension. Current recycling technologies for perfluorinated polymers remain limited, creating waste management challenges when these membranes reach their operational end. Incineration of these materials can potentially release harmful byproducts if not conducted under strictly controlled conditions, necessitating specialized disposal protocols.
Recent life cycle assessment (LCA) studies comparing perfluorinated polymer membranes with alternative CO2 capture technologies reveal complex sustainability tradeoffs. While these membranes generally demonstrate lower operational energy requirements than traditional amine scrubbing processes, their embodied carbon and manufacturing environmental footprint may be higher. The net environmental benefit depends significantly on membrane longevity and operational efficiency in specific applications.
Water usage represents another important sustainability metric. Perfluorinated membrane systems typically require less water than competing technologies, potentially offering advantages in water-stressed regions. However, concerns regarding potential perfluorinated compound leaching into water systems during operation must be addressed through appropriate monitoring and containment strategies.
Regulatory frameworks governing perfluorinated compounds continue to evolve globally, with increasing scrutiny on persistent organic pollutants. Membrane developers must anticipate potential regulatory changes that could impact material selection, manufacturing processes, and disposal requirements. Forward-thinking companies are already exploring alternative fluoropolymers with reduced environmental persistence while maintaining separation performance.
Sustainability innovation pathways include developing hybrid membrane systems that minimize perfluorinated content while maintaining selectivity, implementing closed-loop manufacturing processes, and designing for end-of-life recyclability. Biomimetic approaches that achieve similar selectivity without fluorinated compounds represent a promising long-term research direction, potentially offering truly sustainable CO2 capture solutions.
Economic Feasibility and Scalability Assessment
The economic feasibility of perfluorinated polymer-based CO2 separation membranes hinges on several interconnected factors. Current production costs remain significantly higher than conventional membrane materials, with perfluorinated polymers typically costing $200-500 per kilogram compared to $20-50 for standard polymers. This cost differential primarily stems from complex synthesis processes requiring specialized equipment and stringent manufacturing conditions to achieve the necessary purity and performance characteristics.
Scale-up potential shows promising trajectories based on recent manufacturing innovations. Production capacity has increased approximately 30% annually over the past five years, with corresponding cost reductions of 15-20% per unit area of membrane. Industry projections suggest that with continued investment in manufacturing technology, costs could decrease by an additional 40-50% within the next decade, potentially bringing these membranes within competitive range for large-scale industrial applications.
Energy consumption analysis reveals favorable economics in operational contexts. Perfluorinated polymer membranes demonstrate 25-35% lower energy requirements for CO2 separation compared to traditional absorption processes, translating to operational savings of $0.15-0.25 per ton of CO2 processed. This operational advantage partially offsets higher initial capital expenditure, with calculated payback periods ranging from 3-5 years for high-throughput industrial applications.
Market adoption barriers remain significant despite performance advantages. The initial capital investment for perfluorinated membrane systems exceeds conventional technologies by 60-80%, creating resistance particularly among small to medium enterprises. Additionally, uncertainty regarding membrane longevity in real-world industrial conditions introduces risk factors that impact economic calculations.
Infrastructure compatibility represents another economic consideration. Retrofitting existing carbon capture facilities with perfluorinated polymer membrane systems requires substantial modification, with adaptation costs estimated at 40-60% of new installation expenses. However, modular design approaches are emerging that could reduce these integration costs by 25-30%.
Economies of scale demonstrate clear thresholds for viability. Analysis indicates that perfluorinated polymer membrane systems become economically competitive at processing capacities above 100,000 tons of CO2 annually, suggesting initial adoption will likely concentrate in large industrial facilities such as power plants and major chemical manufacturing operations.
Scale-up potential shows promising trajectories based on recent manufacturing innovations. Production capacity has increased approximately 30% annually over the past five years, with corresponding cost reductions of 15-20% per unit area of membrane. Industry projections suggest that with continued investment in manufacturing technology, costs could decrease by an additional 40-50% within the next decade, potentially bringing these membranes within competitive range for large-scale industrial applications.
Energy consumption analysis reveals favorable economics in operational contexts. Perfluorinated polymer membranes demonstrate 25-35% lower energy requirements for CO2 separation compared to traditional absorption processes, translating to operational savings of $0.15-0.25 per ton of CO2 processed. This operational advantage partially offsets higher initial capital expenditure, with calculated payback periods ranging from 3-5 years for high-throughput industrial applications.
Market adoption barriers remain significant despite performance advantages. The initial capital investment for perfluorinated membrane systems exceeds conventional technologies by 60-80%, creating resistance particularly among small to medium enterprises. Additionally, uncertainty regarding membrane longevity in real-world industrial conditions introduces risk factors that impact economic calculations.
Infrastructure compatibility represents another economic consideration. Retrofitting existing carbon capture facilities with perfluorinated polymer membrane systems requires substantial modification, with adaptation costs estimated at 40-60% of new installation expenses. However, modular design approaches are emerging that could reduce these integration costs by 25-30%.
Economies of scale demonstrate clear thresholds for viability. Analysis indicates that perfluorinated polymer membrane systems become economically competitive at processing capacities above 100,000 tons of CO2 annually, suggesting initial adoption will likely concentrate in large industrial facilities such as power plants and major chemical manufacturing operations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!