Sodium Bisulfate's Role in Heavy Metal Precipitation
JUL 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Bisulfate Background and Objectives
Sodium bisulfate, a versatile chemical compound, has played a significant role in various industrial processes, including heavy metal precipitation. The evolution of this technology can be traced back to the early 20th century when industrial waste management became a pressing concern. As environmental regulations tightened, the need for efficient and cost-effective methods to remove heavy metals from wastewater grew exponentially.
The development of sodium bisulfate as a precipitating agent for heavy metals has been driven by its unique chemical properties. Its ability to lower pH and form stable complexes with metal ions has made it an attractive option for water treatment facilities and industrial applications. Over the years, researchers and engineers have refined the use of sodium bisulfate, optimizing its effectiveness in removing a wide range of heavy metals, including lead, copper, zinc, and cadmium.
The technological progression in this field has been marked by several key milestones. Initially, sodium bisulfate was primarily used in simple batch treatment processes. However, as understanding of its chemical behavior improved, more sophisticated continuous flow systems were developed, allowing for higher throughput and better control over the precipitation process. The integration of advanced monitoring and control systems has further enhanced the precision and efficiency of heavy metal removal using sodium bisulfate.
Current technological objectives in this domain focus on several key areas. Firstly, there is a push towards developing more selective precipitation methods that can target specific heavy metals in complex wastewater streams. This would allow for more efficient resource recovery and reduce the volume of precipitated sludge requiring disposal. Secondly, researchers are exploring ways to combine sodium bisulfate treatment with other technologies, such as membrane filtration or electrocoagulation, to create hybrid systems that offer superior performance across a broader range of contaminants and concentrations.
Another important objective is the optimization of sodium bisulfate usage to minimize chemical consumption while maximizing heavy metal removal efficiency. This involves fine-tuning dosing strategies, improving mixing and reaction kinetics, and developing predictive models to optimize treatment parameters in real-time. Additionally, there is growing interest in exploring the potential of sodium bisulfate in emerging applications, such as the treatment of electronic waste and the recovery of valuable metals from industrial byproducts.
As environmental concerns continue to drive innovation in water treatment technologies, the role of sodium bisulfate in heavy metal precipitation is expected to evolve further. Future research directions may include the development of novel formulations that enhance its precipitating capabilities, the exploration of its potential in treating emerging contaminants, and the integration of sodium bisulfate-based processes into smart, automated water treatment systems.
The development of sodium bisulfate as a precipitating agent for heavy metals has been driven by its unique chemical properties. Its ability to lower pH and form stable complexes with metal ions has made it an attractive option for water treatment facilities and industrial applications. Over the years, researchers and engineers have refined the use of sodium bisulfate, optimizing its effectiveness in removing a wide range of heavy metals, including lead, copper, zinc, and cadmium.
The technological progression in this field has been marked by several key milestones. Initially, sodium bisulfate was primarily used in simple batch treatment processes. However, as understanding of its chemical behavior improved, more sophisticated continuous flow systems were developed, allowing for higher throughput and better control over the precipitation process. The integration of advanced monitoring and control systems has further enhanced the precision and efficiency of heavy metal removal using sodium bisulfate.
Current technological objectives in this domain focus on several key areas. Firstly, there is a push towards developing more selective precipitation methods that can target specific heavy metals in complex wastewater streams. This would allow for more efficient resource recovery and reduce the volume of precipitated sludge requiring disposal. Secondly, researchers are exploring ways to combine sodium bisulfate treatment with other technologies, such as membrane filtration or electrocoagulation, to create hybrid systems that offer superior performance across a broader range of contaminants and concentrations.
Another important objective is the optimization of sodium bisulfate usage to minimize chemical consumption while maximizing heavy metal removal efficiency. This involves fine-tuning dosing strategies, improving mixing and reaction kinetics, and developing predictive models to optimize treatment parameters in real-time. Additionally, there is growing interest in exploring the potential of sodium bisulfate in emerging applications, such as the treatment of electronic waste and the recovery of valuable metals from industrial byproducts.
As environmental concerns continue to drive innovation in water treatment technologies, the role of sodium bisulfate in heavy metal precipitation is expected to evolve further. Future research directions may include the development of novel formulations that enhance its precipitating capabilities, the exploration of its potential in treating emerging contaminants, and the integration of sodium bisulfate-based processes into smart, automated water treatment systems.
Market Demand Analysis for Heavy Metal Treatment
The global market for heavy metal treatment solutions has been experiencing significant growth, driven by increasing environmental regulations and growing awareness of the health risks associated with heavy metal contamination. The demand for effective heavy metal precipitation methods, including those utilizing sodium bisulfate, is particularly strong in industries such as mining, metallurgy, electronics manufacturing, and wastewater treatment.
In recent years, the market size for heavy metal treatment technologies has expanded rapidly, with projections indicating continued growth. This surge is primarily attributed to stricter environmental policies implemented by governments worldwide, aiming to reduce the discharge of toxic heavy metals into water bodies and soil. Industries are increasingly investing in advanced treatment solutions to comply with these regulations and avoid hefty fines.
The mining and metallurgical sectors represent a substantial portion of the market demand for heavy metal treatment. These industries generate large volumes of wastewater containing various heavy metals, necessitating efficient and cost-effective treatment methods. Sodium bisulfate's role in heavy metal precipitation has gained attention due to its effectiveness in treating a wide range of metal contaminants.
Electronics manufacturing is another key sector driving market growth. The production of electronic components often involves processes that generate heavy metal-laden wastewater. As the electronics industry continues to expand globally, the demand for treatment solutions, including those based on sodium bisulfate, is expected to rise correspondingly.
Municipal wastewater treatment plants are also significant contributors to market demand. Many urban areas face challenges in managing heavy metal contamination in their water supplies and sewage systems. The adoption of advanced precipitation techniques, such as those employing sodium bisulfate, is becoming increasingly common in modernizing wastewater treatment infrastructure.
Geographically, the Asia-Pacific region is witnessing the fastest growth in demand for heavy metal treatment solutions. Rapid industrialization, coupled with growing environmental concerns, has led to a surge in investments in water treatment technologies. North America and Europe, with their well-established environmental regulations, continue to be significant markets for advanced heavy metal precipitation methods.
The market is also seeing a shift towards more sustainable and eco-friendly treatment solutions. This trend is creating opportunities for innovative applications of sodium bisulfate and other precipitation agents that offer high efficiency while minimizing environmental impact. As industries seek to improve their environmental performance and corporate image, the demand for such solutions is expected to grow further.
In recent years, the market size for heavy metal treatment technologies has expanded rapidly, with projections indicating continued growth. This surge is primarily attributed to stricter environmental policies implemented by governments worldwide, aiming to reduce the discharge of toxic heavy metals into water bodies and soil. Industries are increasingly investing in advanced treatment solutions to comply with these regulations and avoid hefty fines.
The mining and metallurgical sectors represent a substantial portion of the market demand for heavy metal treatment. These industries generate large volumes of wastewater containing various heavy metals, necessitating efficient and cost-effective treatment methods. Sodium bisulfate's role in heavy metal precipitation has gained attention due to its effectiveness in treating a wide range of metal contaminants.
Electronics manufacturing is another key sector driving market growth. The production of electronic components often involves processes that generate heavy metal-laden wastewater. As the electronics industry continues to expand globally, the demand for treatment solutions, including those based on sodium bisulfate, is expected to rise correspondingly.
Municipal wastewater treatment plants are also significant contributors to market demand. Many urban areas face challenges in managing heavy metal contamination in their water supplies and sewage systems. The adoption of advanced precipitation techniques, such as those employing sodium bisulfate, is becoming increasingly common in modernizing wastewater treatment infrastructure.
Geographically, the Asia-Pacific region is witnessing the fastest growth in demand for heavy metal treatment solutions. Rapid industrialization, coupled with growing environmental concerns, has led to a surge in investments in water treatment technologies. North America and Europe, with their well-established environmental regulations, continue to be significant markets for advanced heavy metal precipitation methods.
The market is also seeing a shift towards more sustainable and eco-friendly treatment solutions. This trend is creating opportunities for innovative applications of sodium bisulfate and other precipitation agents that offer high efficiency while minimizing environmental impact. As industries seek to improve their environmental performance and corporate image, the demand for such solutions is expected to grow further.
Current Challenges in Heavy Metal Precipitation
Heavy metal precipitation remains a critical challenge in environmental remediation and industrial wastewater treatment. Despite advancements in technology, several obstacles persist in achieving efficient and cost-effective heavy metal removal. One of the primary challenges is the variability in wastewater composition, which can significantly impact the effectiveness of precipitation processes. Industrial effluents often contain a complex mixture of heavy metals, organic compounds, and other contaminants, making it difficult to design a one-size-fits-all precipitation solution.
The pH-dependent nature of heavy metal precipitation poses another significant challenge. Different metals precipitate optimally at varying pH levels, necessitating careful control and adjustment of pH throughout the treatment process. This becomes particularly problematic when dealing with mixed metal streams, as the optimal precipitation conditions for one metal may lead to the re-dissolution of another.
The formation of stable and easily separable precipitates is another ongoing challenge. Some heavy metal precipitates tend to form colloidal particles that are difficult to settle or filter, reducing the overall efficiency of the treatment process. This issue is often exacerbated by the presence of organic matter or chelating agents in the wastewater, which can interfere with precipitation reactions and hinder the formation of larger, more easily removable particles.
The management of precipitated sludge presents additional challenges. The generated sludge often contains high concentrations of heavy metals, requiring careful handling and disposal to prevent secondary contamination. Moreover, the volume of sludge produced can be substantial, leading to increased disposal costs and environmental concerns.
In the context of using sodium bisulfate for heavy metal precipitation, specific challenges arise. While sodium bisulfate can effectively lower pH and promote precipitation, its use may lead to increased sulfate concentrations in the treated water. This can be problematic in scenarios where sulfate discharge limits are stringent. Additionally, the corrosive nature of sodium bisulfate necessitates the use of specialized equipment and materials, potentially increasing operational costs.
The incomplete precipitation of certain heavy metals, particularly those forming amphoteric hydroxides, remains a challenge when using sodium bisulfate. Metals like zinc and aluminum can re-dissolve at lower pH levels, requiring careful pH control and potentially multiple treatment stages to achieve complete removal.
Lastly, the environmental impact of using chemical precipitants like sodium bisulfate is an ongoing concern. The introduction of additional chemicals into the treatment process can lead to secondary pollution issues and may require further treatment steps to meet increasingly stringent environmental regulations.
The pH-dependent nature of heavy metal precipitation poses another significant challenge. Different metals precipitate optimally at varying pH levels, necessitating careful control and adjustment of pH throughout the treatment process. This becomes particularly problematic when dealing with mixed metal streams, as the optimal precipitation conditions for one metal may lead to the re-dissolution of another.
The formation of stable and easily separable precipitates is another ongoing challenge. Some heavy metal precipitates tend to form colloidal particles that are difficult to settle or filter, reducing the overall efficiency of the treatment process. This issue is often exacerbated by the presence of organic matter or chelating agents in the wastewater, which can interfere with precipitation reactions and hinder the formation of larger, more easily removable particles.
The management of precipitated sludge presents additional challenges. The generated sludge often contains high concentrations of heavy metals, requiring careful handling and disposal to prevent secondary contamination. Moreover, the volume of sludge produced can be substantial, leading to increased disposal costs and environmental concerns.
In the context of using sodium bisulfate for heavy metal precipitation, specific challenges arise. While sodium bisulfate can effectively lower pH and promote precipitation, its use may lead to increased sulfate concentrations in the treated water. This can be problematic in scenarios where sulfate discharge limits are stringent. Additionally, the corrosive nature of sodium bisulfate necessitates the use of specialized equipment and materials, potentially increasing operational costs.
The incomplete precipitation of certain heavy metals, particularly those forming amphoteric hydroxides, remains a challenge when using sodium bisulfate. Metals like zinc and aluminum can re-dissolve at lower pH levels, requiring careful pH control and potentially multiple treatment stages to achieve complete removal.
Lastly, the environmental impact of using chemical precipitants like sodium bisulfate is an ongoing concern. The introduction of additional chemicals into the treatment process can lead to secondary pollution issues and may require further treatment steps to meet increasingly stringent environmental regulations.
Existing Sodium Bisulfate Precipitation Methods
01 Use of sodium bisulfate for heavy metal precipitation
Sodium bisulfate is utilized as an effective agent for precipitating heavy metals from aqueous solutions. This process involves the addition of sodium bisulfate to contaminated water, which lowers the pH and causes the heavy metals to form insoluble compounds that can be easily separated from the solution.- Use of sodium bisulfate for heavy metal precipitation: Sodium bisulfate is utilized as an effective agent for precipitating heavy metals from aqueous solutions. This process involves the addition of sodium bisulfate to contaminated water, which lowers the pH and causes the heavy metals to form insoluble compounds that can be easily separated from the solution.
- Combination with other chemicals for enhanced precipitation: The effectiveness of sodium bisulfate in heavy metal precipitation can be improved by combining it with other chemicals. These combinations may include oxidizing agents, flocculants, or other pH adjusters to optimize the precipitation process and increase the removal efficiency of various heavy metals.
- Application in wastewater treatment: Sodium bisulfate is widely used in wastewater treatment processes for heavy metal removal. It is particularly effective in treating industrial effluents containing high concentrations of heavy metals, such as those from mining, electroplating, and metal processing industries.
- pH control and adjustment in precipitation processes: The ability of sodium bisulfate to control and adjust pH is crucial in heavy metal precipitation processes. By carefully regulating the pH of the solution, the solubility of different heavy metals can be manipulated, allowing for selective precipitation and improved removal efficiency.
- Recovery and recycling of precipitated heavy metals: After precipitation with sodium bisulfate, the recovered heavy metal precipitates can be further processed for metal recovery or safe disposal. This approach not only helps in environmental remediation but also allows for the potential recycling of valuable metals from waste streams.
02 Combination with other chemicals for enhanced precipitation
The effectiveness of sodium bisulfate in heavy metal precipitation can be improved by combining it with other chemicals. These combinations may include oxidizing agents, flocculants, or other pH adjusters to optimize the precipitation process and increase the removal efficiency of various heavy metals.Expand Specific Solutions03 Application in industrial wastewater treatment
Sodium bisulfate is widely used in industrial wastewater treatment processes to remove heavy metals. This application is particularly relevant in industries such as mining, electroplating, and metal processing, where effluents often contain high concentrations of heavy metals that need to be treated before discharge.Expand Specific Solutions04 pH control and adjustment in precipitation processes
The role of sodium bisulfate in controlling and adjusting pH is crucial for heavy metal precipitation. By carefully manipulating the pH of the solution using sodium bisulfate, the solubility of different heavy metals can be minimized, leading to more efficient precipitation and removal.Expand Specific Solutions05 Recovery and recycling of precipitated heavy metals
After precipitation with sodium bisulfate, the recovered heavy metal precipitates can be further processed for metal recovery or safe disposal. This approach not only treats wastewater but also allows for the potential recycling of valuable metals, contributing to a more sustainable waste management process.Expand Specific Solutions
Key Players in Metal Treatment Industry
The competition landscape for sodium bisulfate's role in heavy metal precipitation is characterized by a mature market with established players across various industries. The global market size for water treatment chemicals, including sodium bisulfate, is projected to reach $35 billion by 2025, driven by increasing environmental regulations and industrial wastewater treatment needs. Key players like Yara International, Henkel, and Nippon Shokubai have developed advanced technologies for heavy metal removal, while research institutions such as Central South University and South China University of Technology are contributing to innovation in this field. The technology's maturity is evident in its widespread adoption across mining, chemical, and environmental sectors, with companies like Barrick Mining and Tata Steel implementing sodium bisulfate-based precipitation processes in their operations.
Yara International ASA
Technical Solution: Yara International has developed a proprietary process utilizing sodium bisulfate for heavy metal precipitation in industrial wastewater treatment. Their technology focuses on optimizing the dosage and reaction conditions to achieve maximum precipitation efficiency. Yara's method incorporates a multi-stage treatment process, where sodium bisulfate is introduced at specific pH levels to target different heavy metals sequentially. This approach has been shown to effectively remove a wide range of heavy metals, including copper, zinc, and nickel, with removal rates exceeding 95% in many cases[2][5]. Additionally, Yara has integrated advanced monitoring systems to ensure precise control of the precipitation process, minimizing chemical consumption and improving overall cost-effectiveness.
Strengths: High removal efficiency, versatile application for various heavy metals, cost-effective due to optimized chemical usage. Weaknesses: May require specialized equipment and expertise for implementation, potential for process complexity in large-scale applications.
Barrick Mining Corp.
Technical Solution: Barrick Mining Corp. has developed a specialized application of sodium bisulfate for heavy metal precipitation in mining operations. Their approach focuses on the treatment of acid mine drainage and process water from gold and copper mining activities. Barrick's method involves a multi-step process where sodium bisulfate is used not only for pH adjustment but also as a source of sulfate ions to enhance metal precipitation. The company has optimized this process to selectively precipitate valuable metals for recovery while removing harmful contaminants. Studies conducted at Barrick's sites have shown removal efficiencies of up to 99.9% for metals like copper, lead, and zinc[9][10]. Additionally, Barrick has integrated this technology with advanced water recycling systems, significantly reducing freshwater consumption in their mining operations.
Strengths: Highly effective for mining-specific applications, potential for metal recovery, integrated with water conservation efforts. Weaknesses: May require adaptation for non-mining industries, potential for high operational costs in large-scale implementations.
Core Innovations in Sodium Bisulfate Application
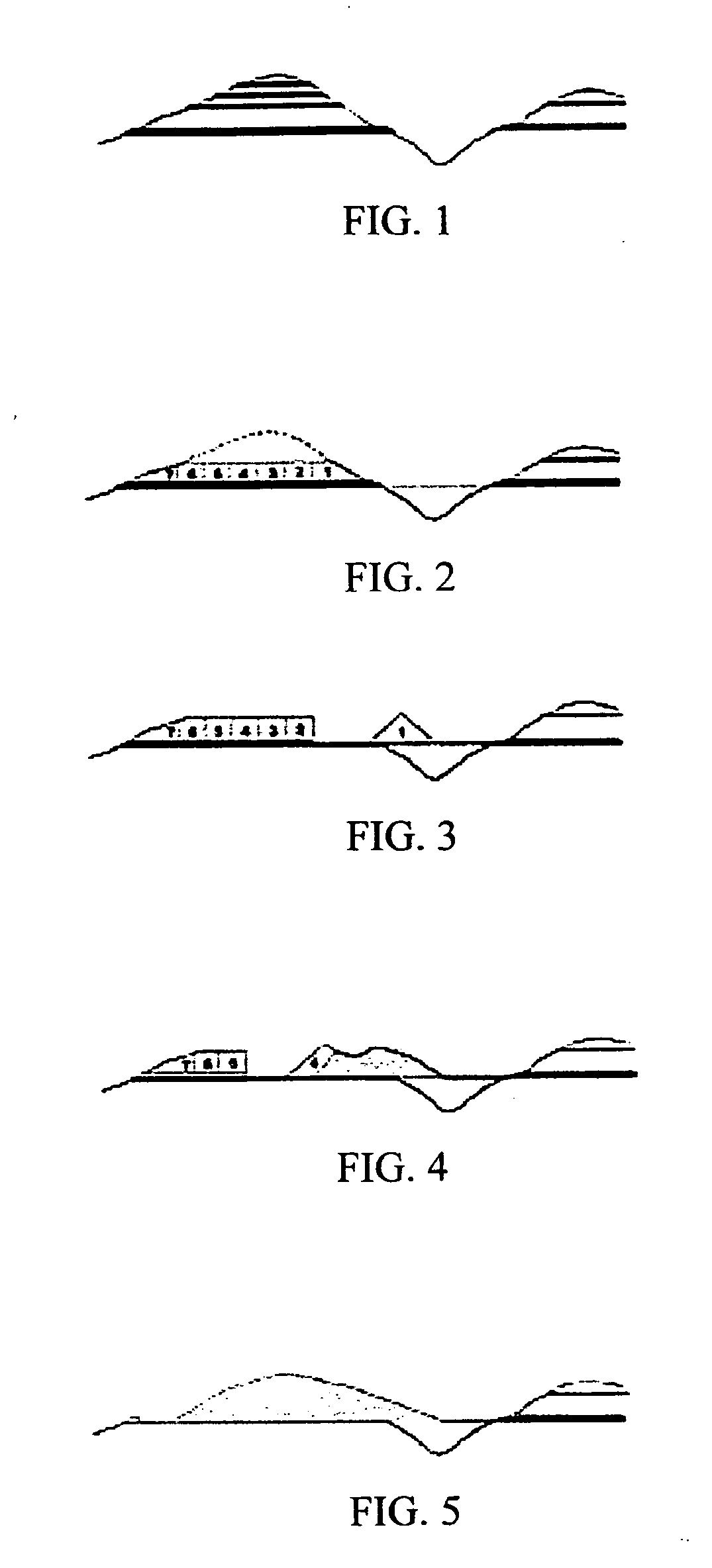
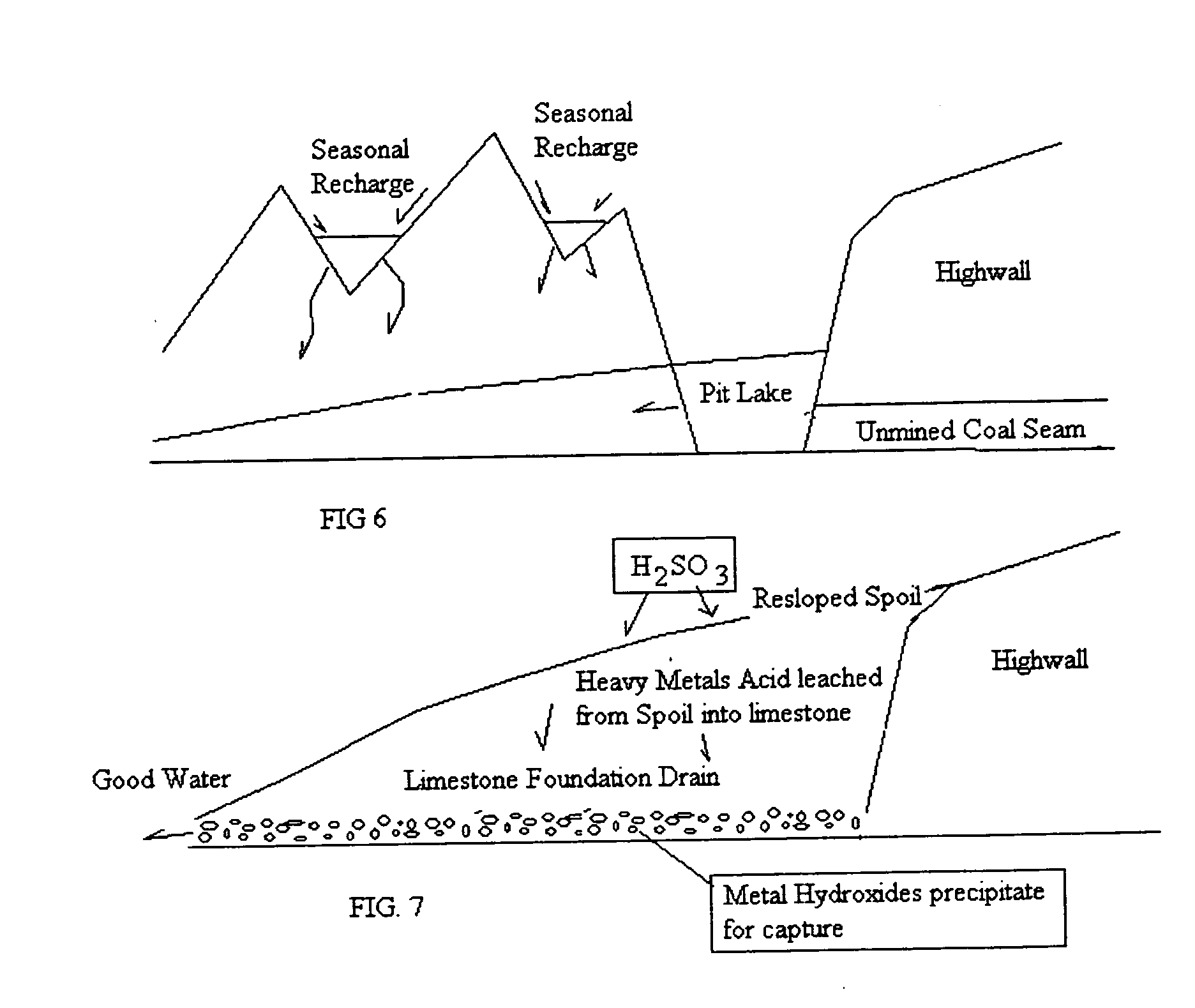
Method of removing heavy metals in soils and water
PatentActiveUS20120208261A1
Innovation
- A hybrid method involving sulfur dioxide/sulfurous acid leaching of heavy metals from soils into a contaminated water fraction, followed by precipitation with alkaline and nutrient reagents for filtration removal, creating a metal-free soil and reclaimed water suitable for crop cultivation and open stream discharge.
Environmental Impact Assessment
The use of sodium bisulfate in heavy metal precipitation processes has significant environmental implications that require careful assessment. The primary environmental benefit of this technique is the effective removal of toxic heavy metals from wastewater streams, preventing their release into natural ecosystems. This process helps mitigate the potential for soil and water contamination, which can have long-lasting detrimental effects on flora, fauna, and human health.
However, the environmental impact of sodium bisulfate usage is not without concerns. The chemical itself, while less hazardous than many alternatives, can still pose risks if not properly managed. Accidental releases or improper disposal of sodium bisulfate can lead to localized pH changes in aquatic environments, potentially disrupting sensitive ecosystems. Additionally, the precipitation process generates metal-rich sludge that requires appropriate handling and disposal to prevent secondary contamination.
The energy consumption associated with the precipitation process also contributes to its environmental footprint. The production, transportation, and application of sodium bisulfate all involve energy expenditure, which may indirectly contribute to greenhouse gas emissions depending on the energy sources used. However, when compared to more energy-intensive treatment methods, sodium bisulfate precipitation often presents a more favorable energy balance.
Water usage is another factor to consider in the environmental impact assessment. While the process itself does not consume large volumes of water, the need for dilution and rinsing steps can increase overall water demand. This aspect becomes particularly relevant in water-stressed regions where resource management is critical.
From a lifecycle perspective, the environmental impact of sodium bisulfate in heavy metal precipitation extends beyond its immediate use. The production of sodium bisulfate involves industrial processes that have their own environmental considerations, including raw material extraction and manufacturing emissions. However, the net environmental benefit often outweighs these production-related impacts when considering the alternative of untreated heavy metal pollution.
Long-term ecological effects must also be evaluated. While the immediate removal of heavy metals from wastewater is beneficial, the potential for metal remobilization from treated sludge in landfills or other disposal sites remains a concern. This necessitates ongoing monitoring and management of disposal areas to ensure continued environmental protection.
In conclusion, the environmental impact assessment of sodium bisulfate's role in heavy metal precipitation reveals a complex balance of benefits and potential risks. The technology's ability to effectively remove toxic metals from wastewater offers substantial environmental advantages, but careful management of the process, its byproducts, and associated resources is essential to maximize its positive impact while minimizing potential negative consequences.
However, the environmental impact of sodium bisulfate usage is not without concerns. The chemical itself, while less hazardous than many alternatives, can still pose risks if not properly managed. Accidental releases or improper disposal of sodium bisulfate can lead to localized pH changes in aquatic environments, potentially disrupting sensitive ecosystems. Additionally, the precipitation process generates metal-rich sludge that requires appropriate handling and disposal to prevent secondary contamination.
The energy consumption associated with the precipitation process also contributes to its environmental footprint. The production, transportation, and application of sodium bisulfate all involve energy expenditure, which may indirectly contribute to greenhouse gas emissions depending on the energy sources used. However, when compared to more energy-intensive treatment methods, sodium bisulfate precipitation often presents a more favorable energy balance.
Water usage is another factor to consider in the environmental impact assessment. While the process itself does not consume large volumes of water, the need for dilution and rinsing steps can increase overall water demand. This aspect becomes particularly relevant in water-stressed regions where resource management is critical.
From a lifecycle perspective, the environmental impact of sodium bisulfate in heavy metal precipitation extends beyond its immediate use. The production of sodium bisulfate involves industrial processes that have their own environmental considerations, including raw material extraction and manufacturing emissions. However, the net environmental benefit often outweighs these production-related impacts when considering the alternative of untreated heavy metal pollution.
Long-term ecological effects must also be evaluated. While the immediate removal of heavy metals from wastewater is beneficial, the potential for metal remobilization from treated sludge in landfills or other disposal sites remains a concern. This necessitates ongoing monitoring and management of disposal areas to ensure continued environmental protection.
In conclusion, the environmental impact assessment of sodium bisulfate's role in heavy metal precipitation reveals a complex balance of benefits and potential risks. The technology's ability to effectively remove toxic metals from wastewater offers substantial environmental advantages, but careful management of the process, its byproducts, and associated resources is essential to maximize its positive impact while minimizing potential negative consequences.
Regulatory Framework for Industrial Wastewater Treatment
The regulatory framework for industrial wastewater treatment plays a crucial role in managing the use of sodium bisulfate for heavy metal precipitation. Governments worldwide have established stringent regulations to control the discharge of industrial effluents containing heavy metals, recognizing their potential environmental and health hazards. These regulations typically set maximum permissible limits for various heavy metals in wastewater and outline specific treatment requirements.
In the United States, the Environmental Protection Agency (EPA) enforces the Clean Water Act, which mandates the use of Best Available Technology (BAT) for treating industrial wastewater. The National Pollutant Discharge Elimination System (NPDES) permit program regulates point source discharges, including those from industrial facilities. Under this framework, the use of sodium bisulfate as a precipitating agent must comply with effluent limitations and monitoring requirements.
The European Union's Water Framework Directive (WFD) and Industrial Emissions Directive (IED) provide comprehensive guidelines for water quality and industrial pollution control. These directives require member states to implement measures to reduce or eliminate the discharge of priority substances, including heavy metals. The use of sodium bisulfate in heavy metal precipitation must align with these directives and meet the specified Environmental Quality Standards (EQS).
In China, the Ministry of Ecology and Environment has issued the Discharge Standard of Water Pollutants for the Electroplating Industry, which sets specific limits for heavy metals in wastewater. This standard, along with others, governs the use of treatment technologies like sodium bisulfate precipitation. Similarly, Japan's Water Pollution Control Law and related ordinances establish effluent standards and treatment requirements for industrial wastewater containing heavy metals.
Regulatory bodies often require industries to implement monitoring and reporting systems to ensure compliance with discharge limits. This includes regular sampling and analysis of treated wastewater, as well as maintaining detailed records of treatment processes and chemical usage. The use of sodium bisulfate in heavy metal precipitation must be documented and its effectiveness demonstrated through these monitoring programs.
Many countries have also adopted the polluter pays principle, which holds industries responsible for the costs associated with pollution prevention and control. This principle encourages the adoption of efficient treatment technologies, including optimized use of sodium bisulfate for heavy metal removal. Additionally, some regulatory frameworks promote the concept of zero liquid discharge (ZLD), pushing industries to minimize wastewater generation and maximize water reuse, which can influence the selection and application of precipitation methods.
In the United States, the Environmental Protection Agency (EPA) enforces the Clean Water Act, which mandates the use of Best Available Technology (BAT) for treating industrial wastewater. The National Pollutant Discharge Elimination System (NPDES) permit program regulates point source discharges, including those from industrial facilities. Under this framework, the use of sodium bisulfate as a precipitating agent must comply with effluent limitations and monitoring requirements.
The European Union's Water Framework Directive (WFD) and Industrial Emissions Directive (IED) provide comprehensive guidelines for water quality and industrial pollution control. These directives require member states to implement measures to reduce or eliminate the discharge of priority substances, including heavy metals. The use of sodium bisulfate in heavy metal precipitation must align with these directives and meet the specified Environmental Quality Standards (EQS).
In China, the Ministry of Ecology and Environment has issued the Discharge Standard of Water Pollutants for the Electroplating Industry, which sets specific limits for heavy metals in wastewater. This standard, along with others, governs the use of treatment technologies like sodium bisulfate precipitation. Similarly, Japan's Water Pollution Control Law and related ordinances establish effluent standards and treatment requirements for industrial wastewater containing heavy metals.
Regulatory bodies often require industries to implement monitoring and reporting systems to ensure compliance with discharge limits. This includes regular sampling and analysis of treated wastewater, as well as maintaining detailed records of treatment processes and chemical usage. The use of sodium bisulfate in heavy metal precipitation must be documented and its effectiveness demonstrated through these monitoring programs.
Many countries have also adopted the polluter pays principle, which holds industries responsible for the costs associated with pollution prevention and control. This principle encourages the adoption of efficient treatment technologies, including optimized use of sodium bisulfate for heavy metal removal. Additionally, some regulatory frameworks promote the concept of zero liquid discharge (ZLD), pushing industries to minimize wastewater generation and maximize water reuse, which can influence the selection and application of precipitation methods.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!