Solvent Recovery Techniques for Glacial Acetic Acid
AUG 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glacial Acetic Acid Recovery Background and Objectives
Glacial acetic acid, a vital chemical compound in various industries, has been a subject of significant interest in the realm of solvent recovery techniques. The evolution of these techniques has been driven by the increasing demand for sustainable and cost-effective production processes. Over the years, the focus has shifted from simple distillation methods to more sophisticated approaches that aim to maximize recovery efficiency while minimizing energy consumption and environmental impact.
The primary objective of glacial acetic acid recovery is to reclaim and purify the compound from waste streams or process byproducts, thereby reducing raw material costs and minimizing waste generation. This aligns with the broader industry goals of circular economy and resource conservation. As environmental regulations become more stringent, the importance of efficient recovery techniques has grown exponentially, pushing researchers and industry professionals to innovate and refine existing methods.
The development of recovery techniques for glacial acetic acid has been influenced by advancements in separation technologies, process intensification, and the integration of smart control systems. Early recovery methods often relied on energy-intensive thermal separation processes, which, while effective, were not always economically viable or environmentally friendly. The technological trajectory has since moved towards more efficient membrane-based separations, reactive distillation, and hybrid processes that combine multiple separation principles.
One of the key drivers in the evolution of these techniques has been the need to handle increasingly complex mixtures and achieve higher purity levels. As industrial processes become more intricate, the recovery of glacial acetic acid from multi-component systems has presented new challenges, spurring research into novel separation materials and process configurations. The goal is not only to recover the acid but to do so with minimal loss and maximum purity, ensuring its suitability for reuse or resale.
The current technological landscape for glacial acetic acid recovery is characterized by a blend of traditional and cutting-edge approaches. While conventional distillation and extraction methods still play a role, emerging technologies such as pervaporation, adsorption using specialized materials, and electrochemical separation are gaining traction. These newer techniques promise enhanced selectivity, reduced energy requirements, and improved overall process economics.
Looking ahead, the objectives for future developments in glacial acetic acid recovery techniques are multifaceted. They include further improving energy efficiency, reducing the environmental footprint of recovery processes, enhancing the ability to handle diverse feed compositions, and developing more robust and scalable technologies suitable for industrial implementation. Additionally, there is a growing emphasis on integrating recovery systems with existing production processes to create closed-loop systems that maximize resource utilization and minimize waste generation.
The primary objective of glacial acetic acid recovery is to reclaim and purify the compound from waste streams or process byproducts, thereby reducing raw material costs and minimizing waste generation. This aligns with the broader industry goals of circular economy and resource conservation. As environmental regulations become more stringent, the importance of efficient recovery techniques has grown exponentially, pushing researchers and industry professionals to innovate and refine existing methods.
The development of recovery techniques for glacial acetic acid has been influenced by advancements in separation technologies, process intensification, and the integration of smart control systems. Early recovery methods often relied on energy-intensive thermal separation processes, which, while effective, were not always economically viable or environmentally friendly. The technological trajectory has since moved towards more efficient membrane-based separations, reactive distillation, and hybrid processes that combine multiple separation principles.
One of the key drivers in the evolution of these techniques has been the need to handle increasingly complex mixtures and achieve higher purity levels. As industrial processes become more intricate, the recovery of glacial acetic acid from multi-component systems has presented new challenges, spurring research into novel separation materials and process configurations. The goal is not only to recover the acid but to do so with minimal loss and maximum purity, ensuring its suitability for reuse or resale.
The current technological landscape for glacial acetic acid recovery is characterized by a blend of traditional and cutting-edge approaches. While conventional distillation and extraction methods still play a role, emerging technologies such as pervaporation, adsorption using specialized materials, and electrochemical separation are gaining traction. These newer techniques promise enhanced selectivity, reduced energy requirements, and improved overall process economics.
Looking ahead, the objectives for future developments in glacial acetic acid recovery techniques are multifaceted. They include further improving energy efficiency, reducing the environmental footprint of recovery processes, enhancing the ability to handle diverse feed compositions, and developing more robust and scalable technologies suitable for industrial implementation. Additionally, there is a growing emphasis on integrating recovery systems with existing production processes to create closed-loop systems that maximize resource utilization and minimize waste generation.
Industrial Demand for Solvent Recovery
The industrial demand for solvent recovery techniques in the context of glacial acetic acid has been steadily increasing due to several key factors. Firstly, the growing emphasis on environmental sustainability and regulatory compliance has pushed industries to adopt more efficient and eco-friendly processes. Solvent recovery, particularly for glacial acetic acid, aligns with these goals by reducing waste and minimizing the environmental impact of industrial operations.
The chemical industry, being a major consumer of glacial acetic acid, has been at the forefront of driving demand for solvent recovery techniques. This sector utilizes acetic acid in various processes, including the production of vinyl acetate monomer, purified terephthalic acid, and acetic anhydride. As production scales continue to expand, the economic incentives for recovering and reusing solvents have become more pronounced.
Pharmaceutical manufacturing represents another significant sector contributing to the demand for glacial acetic acid solvent recovery. The industry's stringent quality requirements and the high cost of pharmaceutical-grade solvents make recovery processes particularly attractive. By implementing efficient recovery techniques, pharmaceutical companies can substantially reduce their raw material costs while maintaining product purity.
The textile industry, which uses glacial acetic acid in dyeing processes and as a pH regulator, has also shown increased interest in solvent recovery. As textile production volumes grow, especially in developing economies, the need for cost-effective and environmentally responsible solvent management has become more pressing.
Furthermore, the food and beverage industry, where glacial acetic acid is used in the production of various additives and preservatives, has been exploring solvent recovery options to optimize their production costs and reduce waste streams. This trend is particularly evident in large-scale operations where the volume of solvent used justifies the investment in recovery systems.
The electronics industry, particularly in the manufacturing of printed circuit boards and semiconductors, has also contributed to the demand for glacial acetic acid solvent recovery. As the industry continues to expand and face pressure to reduce its environmental footprint, efficient solvent management has become a priority.
Economically, the volatility in acetic acid prices has been a significant driver for industries to invest in recovery techniques. By reducing dependence on fresh solvent purchases, companies can better manage their operational costs and mitigate the impact of market fluctuations. This economic incentive has led to increased research and development efforts in improving solvent recovery technologies, focusing on enhancing efficiency, reducing energy consumption, and minimizing solvent losses during the recovery process.
The chemical industry, being a major consumer of glacial acetic acid, has been at the forefront of driving demand for solvent recovery techniques. This sector utilizes acetic acid in various processes, including the production of vinyl acetate monomer, purified terephthalic acid, and acetic anhydride. As production scales continue to expand, the economic incentives for recovering and reusing solvents have become more pronounced.
Pharmaceutical manufacturing represents another significant sector contributing to the demand for glacial acetic acid solvent recovery. The industry's stringent quality requirements and the high cost of pharmaceutical-grade solvents make recovery processes particularly attractive. By implementing efficient recovery techniques, pharmaceutical companies can substantially reduce their raw material costs while maintaining product purity.
The textile industry, which uses glacial acetic acid in dyeing processes and as a pH regulator, has also shown increased interest in solvent recovery. As textile production volumes grow, especially in developing economies, the need for cost-effective and environmentally responsible solvent management has become more pressing.
Furthermore, the food and beverage industry, where glacial acetic acid is used in the production of various additives and preservatives, has been exploring solvent recovery options to optimize their production costs and reduce waste streams. This trend is particularly evident in large-scale operations where the volume of solvent used justifies the investment in recovery systems.
The electronics industry, particularly in the manufacturing of printed circuit boards and semiconductors, has also contributed to the demand for glacial acetic acid solvent recovery. As the industry continues to expand and face pressure to reduce its environmental footprint, efficient solvent management has become a priority.
Economically, the volatility in acetic acid prices has been a significant driver for industries to invest in recovery techniques. By reducing dependence on fresh solvent purchases, companies can better manage their operational costs and mitigate the impact of market fluctuations. This economic incentive has led to increased research and development efforts in improving solvent recovery technologies, focusing on enhancing efficiency, reducing energy consumption, and minimizing solvent losses during the recovery process.
Current Challenges in Acetic Acid Recovery
The recovery of glacial acetic acid presents several significant challenges in industrial processes. One of the primary issues is the formation of azeotropes with water, which makes conventional distillation techniques inefficient and energy-intensive. The azeotropic mixture of acetic acid and water at 84.2 wt% acid concentration requires specialized separation methods to achieve high purity levels.
Another major challenge is the corrosive nature of acetic acid, particularly at elevated temperatures. This necessitates the use of specialized materials for equipment construction, such as stainless steel or glass-lined vessels, which can significantly increase capital costs. The corrosivity also leads to maintenance issues and potential safety hazards in industrial settings.
Energy consumption remains a critical concern in acetic acid recovery processes. Traditional distillation methods require substantial thermal energy input, contributing to high operational costs and environmental impact. The need for multiple distillation stages to achieve desired purity levels further exacerbates this issue.
The presence of impurities in the acetic acid stream poses additional separation challenges. Organic contaminants, such as acetaldehyde or ethyl acetate, often have similar boiling points to acetic acid, making their removal difficult through conventional means. These impurities can affect the quality of the recovered acid and limit its potential applications.
Membrane-based separation techniques, while promising, face limitations in terms of flux rates and membrane stability when exposed to concentrated acetic acid solutions. The development of robust, high-performance membranes capable of withstanding harsh chemical environments remains an ongoing challenge in the field.
Environmental concerns also play a significant role in acetic acid recovery. The potential release of volatile organic compounds (VOCs) during the recovery process necessitates stringent emission control measures. Additionally, the disposal of byproducts and waste streams from the recovery process requires careful management to comply with environmental regulations.
Scaling up laboratory-proven recovery techniques to industrial levels presents its own set of challenges. Issues such as heat transfer efficiency, fluid dynamics, and process control become more complex at larger scales, requiring significant engineering efforts to maintain process effectiveness and economic viability.
Another major challenge is the corrosive nature of acetic acid, particularly at elevated temperatures. This necessitates the use of specialized materials for equipment construction, such as stainless steel or glass-lined vessels, which can significantly increase capital costs. The corrosivity also leads to maintenance issues and potential safety hazards in industrial settings.
Energy consumption remains a critical concern in acetic acid recovery processes. Traditional distillation methods require substantial thermal energy input, contributing to high operational costs and environmental impact. The need for multiple distillation stages to achieve desired purity levels further exacerbates this issue.
The presence of impurities in the acetic acid stream poses additional separation challenges. Organic contaminants, such as acetaldehyde or ethyl acetate, often have similar boiling points to acetic acid, making their removal difficult through conventional means. These impurities can affect the quality of the recovered acid and limit its potential applications.
Membrane-based separation techniques, while promising, face limitations in terms of flux rates and membrane stability when exposed to concentrated acetic acid solutions. The development of robust, high-performance membranes capable of withstanding harsh chemical environments remains an ongoing challenge in the field.
Environmental concerns also play a significant role in acetic acid recovery. The potential release of volatile organic compounds (VOCs) during the recovery process necessitates stringent emission control measures. Additionally, the disposal of byproducts and waste streams from the recovery process requires careful management to comply with environmental regulations.
Scaling up laboratory-proven recovery techniques to industrial levels presents its own set of challenges. Issues such as heat transfer efficiency, fluid dynamics, and process control become more complex at larger scales, requiring significant engineering efforts to maintain process effectiveness and economic viability.
Existing Acetic Acid Recovery Methods
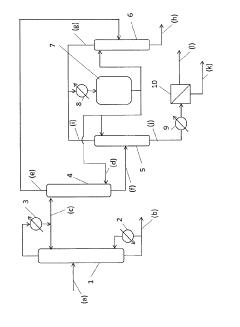
01 Distillation and rectification processes
Glacial acetic acid can be recovered through distillation and rectification processes. These methods involve separating the acetic acid from other components based on their different boiling points. The process may include multiple distillation columns and heat exchangers to achieve high purity recovery.- Distillation and rectification processes: Glacial acetic acid can be recovered through distillation and rectification processes. These methods involve separating the acetic acid from other components based on their different boiling points. The process may include multiple distillation columns and heat exchangers to achieve high purity recovery.
- Membrane separation techniques: Membrane separation techniques, such as pervaporation or nanofiltration, can be used to recover glacial acetic acid. These methods utilize selective membranes to separate acetic acid from other components in the mixture, offering advantages in energy efficiency and operational flexibility.
- Extraction and liquid-liquid separation: Glacial acetic acid can be recovered using extraction and liquid-liquid separation methods. These techniques involve using suitable solvents to selectively extract acetic acid from the mixture, followed by separation of the solvent and acid phases. The process may include multiple extraction stages to improve recovery efficiency.
- Crystallization and freezing techniques: Crystallization and freezing techniques can be employed for glacial acetic acid recovery. These methods exploit the differences in freezing points between acetic acid and other components in the mixture. The process may involve controlled cooling and separation of solid acetic acid crystals from the liquid phase.
- Adsorption and desorption processes: Adsorption and desorption processes can be used to recover glacial acetic acid. These methods utilize adsorbent materials with high selectivity for acetic acid. The acid is first adsorbed onto the material and then desorbed using appropriate conditions, such as temperature or pressure changes, to recover the purified acetic acid.
02 Membrane separation technology
Membrane separation techniques, such as pervaporation or nanofiltration, can be used to recover glacial acetic acid. These methods utilize selective membranes to separate acetic acid from other components in the mixture, offering advantages in energy efficiency and operational flexibility.Expand Specific Solutions03 Extraction and liquid-liquid separation
Glacial acetic acid can be recovered using extraction methods, including liquid-liquid separation. This involves using a solvent to selectively extract acetic acid from the mixture, followed by subsequent separation steps to isolate the pure acid.Expand Specific Solutions04 Crystallization and freezing techniques
Recovery of glacial acetic acid can be achieved through crystallization or freezing processes. These methods exploit the differences in freezing points between acetic acid and other components in the mixture, allowing for separation and purification.Expand Specific Solutions05 Adsorption and desorption processes
Adsorption-based methods can be used to recover glacial acetic acid. These techniques involve using adsorbent materials to selectively capture acetic acid from the mixture, followed by desorption processes to recover the purified acid.Expand Specific Solutions
Key Players in Solvent Recovery Industry
The solvent recovery techniques for glacial acetic acid market is in a growth phase, driven by increasing environmental regulations and cost-saving initiatives in chemical industries. The market size is expanding, with a projected CAGR of 5-7% over the next five years. Technologically, the field is moderately mature, with established players like Daicel Corp., LG Chem Ltd., and China Petroleum & Chemical Corp. leading innovations. These companies are investing in advanced separation technologies and process optimization to improve recovery efficiency and reduce energy consumption. Emerging players like Zea2 LLC are exploring bio-based alternatives, indicating potential disruption in the future.
Daicel Corp.
Technical Solution: Daicel Corp. has pioneered a novel solvent recovery technique for glacial acetic acid using a combination of reactive distillation and pervaporation. The process begins with a reactive distillation column where acetic acid is selectively esterified with a low-boiling alcohol, facilitating easier separation from water and other impurities. The ester is then hydrolyzed back to acetic acid in a second column. Finally, a pervaporation membrane system is used to remove any remaining water, achieving glacial grade purity (>99.85%)[2]. This method significantly reduces energy consumption compared to conventional azeotropic distillation, with reported energy savings of up to 30%[4].
Strengths: Lower energy consumption, high purity output, reduced equipment footprint. Weaknesses: Requires careful control of reaction conditions, potential for membrane fouling over time.
Hitachi Ltd.
Technical Solution: Hitachi Ltd. has developed an innovative solvent recovery system for glacial acetic acid utilizing advanced adsorption technology coupled with thermal swing regeneration. The process employs specially designed molecular sieves that selectively adsorb acetic acid from waste streams. Once saturated, the adsorbent is regenerated using a low-temperature thermal swing process, which desorbs the acetic acid at high purity. This cyclic operation allows for continuous recovery. The system incorporates smart sensors and AI-driven process control to optimize adsorption-desorption cycles, maximizing efficiency and minimizing energy use[5]. Hitachi reports recovery rates of up to 98% with purity levels exceeding 99.7%[6].
Strengths: High recovery efficiency, low energy consumption, adaptable to varying feed compositions. Weaknesses: Periodic replacement of adsorbent material required, potential for fouling in presence of certain impurities.
Innovative Techniques for Glacial Acetic Acid Recovery
Method of recovery of acetic acid
PatentInactiveUS4353784A
Innovation
- A method using a tertiary amine with a boiling point higher than acetic acid, combined with an oxygen-containing organic solvent like 3,3,5-trimethylcyclohexanone, for extraction followed by dehydration and distillation in a reduced pressure column to achieve efficient separation and recovery of water-free acetic acid.
Method for recovering acetic acid from acetic acid-containing aqueous solution
PatentInactiveJP2019131526A
Innovation
- A method involving distillation, extraction with an acetic acid ester as an extractant, followed by distillation of the extract and raffinate, and finally membrane separation to concentrate and recover acetic acid and the extractant.
Environmental Impact of Acetic Acid Recovery
The environmental impact of acetic acid recovery is a critical consideration in the implementation of solvent recovery techniques for glacial acetic acid. The process of recovering acetic acid can have both positive and negative effects on the environment, necessitating a comprehensive assessment of its overall ecological footprint.
One of the primary environmental benefits of acetic acid recovery is the reduction of waste and emissions. By implementing effective recovery techniques, industries can significantly decrease the amount of acetic acid released into the environment. This not only conserves resources but also minimizes the potential for soil and water contamination. The recovered acetic acid can be reused in various industrial processes, reducing the need for fresh production and thereby lowering the overall environmental impact associated with its manufacture.
However, the recovery process itself may have environmental implications. Energy consumption is a key factor to consider, as many recovery techniques require substantial thermal or electrical input. The energy source used for these processes plays a crucial role in determining the net environmental impact. Facilities utilizing renewable energy sources for recovery operations can significantly reduce their carbon footprint compared to those relying on fossil fuels.
Water usage is another important environmental aspect of acetic acid recovery. Some recovery methods, particularly those involving distillation or extraction, may require substantial amounts of water for cooling or as a process medium. Efficient water management systems and closed-loop cooling processes can help mitigate this impact, but the potential strain on local water resources must be carefully evaluated.
The handling and storage of recovered acetic acid also present environmental challenges. Proper containment systems are essential to prevent leaks or spills that could harm local ecosystems. Additionally, the transportation of recovered acetic acid, whether for reuse on-site or distribution to other facilities, must be conducted with stringent safety measures to minimize the risk of environmental contamination during transit.
Air quality is another environmental concern in acetic acid recovery. Volatile organic compound (VOC) emissions may occur during the recovery process, potentially contributing to air pollution and smog formation. Advanced air pollution control technologies, such as scrubbers or thermal oxidizers, are often necessary to mitigate these emissions and ensure compliance with environmental regulations.
The disposal of byproducts and waste materials generated during the recovery process also requires careful consideration. These may include spent catalysts, contaminated adsorbents, or residual sludges, which must be handled and disposed of in an environmentally responsible manner. Proper waste management practices, including recycling and safe disposal methods, are crucial to minimizing the environmental footprint of the recovery operation.
In conclusion, while acetic acid recovery techniques offer significant environmental benefits through resource conservation and waste reduction, their implementation must be carefully designed to address potential negative impacts. A holistic approach considering energy efficiency, water management, emission control, and waste handling is essential to ensure that the recovery process contributes positively to overall environmental sustainability.
One of the primary environmental benefits of acetic acid recovery is the reduction of waste and emissions. By implementing effective recovery techniques, industries can significantly decrease the amount of acetic acid released into the environment. This not only conserves resources but also minimizes the potential for soil and water contamination. The recovered acetic acid can be reused in various industrial processes, reducing the need for fresh production and thereby lowering the overall environmental impact associated with its manufacture.
However, the recovery process itself may have environmental implications. Energy consumption is a key factor to consider, as many recovery techniques require substantial thermal or electrical input. The energy source used for these processes plays a crucial role in determining the net environmental impact. Facilities utilizing renewable energy sources for recovery operations can significantly reduce their carbon footprint compared to those relying on fossil fuels.
Water usage is another important environmental aspect of acetic acid recovery. Some recovery methods, particularly those involving distillation or extraction, may require substantial amounts of water for cooling or as a process medium. Efficient water management systems and closed-loop cooling processes can help mitigate this impact, but the potential strain on local water resources must be carefully evaluated.
The handling and storage of recovered acetic acid also present environmental challenges. Proper containment systems are essential to prevent leaks or spills that could harm local ecosystems. Additionally, the transportation of recovered acetic acid, whether for reuse on-site or distribution to other facilities, must be conducted with stringent safety measures to minimize the risk of environmental contamination during transit.
Air quality is another environmental concern in acetic acid recovery. Volatile organic compound (VOC) emissions may occur during the recovery process, potentially contributing to air pollution and smog formation. Advanced air pollution control technologies, such as scrubbers or thermal oxidizers, are often necessary to mitigate these emissions and ensure compliance with environmental regulations.
The disposal of byproducts and waste materials generated during the recovery process also requires careful consideration. These may include spent catalysts, contaminated adsorbents, or residual sludges, which must be handled and disposed of in an environmentally responsible manner. Proper waste management practices, including recycling and safe disposal methods, are crucial to minimizing the environmental footprint of the recovery operation.
In conclusion, while acetic acid recovery techniques offer significant environmental benefits through resource conservation and waste reduction, their implementation must be carefully designed to address potential negative impacts. A holistic approach considering energy efficiency, water management, emission control, and waste handling is essential to ensure that the recovery process contributes positively to overall environmental sustainability.
Economic Feasibility of Recovery Techniques
The economic feasibility of solvent recovery techniques for glacial acetic acid is a critical consideration for industries utilizing this compound. The recovery process aims to reduce raw material costs, minimize waste, and improve overall operational efficiency. Several factors influence the economic viability of these techniques, including the initial investment, operational costs, recovery efficiency, and market conditions.
One of the primary recovery methods, distillation, offers high purity but comes with significant energy costs. The economic analysis must consider the energy consumption against the value of recovered acetic acid. In many cases, the energy costs can be offset by the savings in raw material purchases and waste disposal fees. However, the feasibility heavily depends on the scale of operation and the local energy prices.
Membrane-based recovery techniques, such as pervaporation and nanofiltration, present a potentially more energy-efficient alternative. While the initial investment in membrane technology can be substantial, the lower operational costs and reduced energy consumption can lead to favorable long-term economics. The economic viability of these methods improves with advancements in membrane materials and increased durability, reducing replacement frequency and associated costs.
Adsorption-based recovery using activated carbon or specialized resins offers another economically attractive option, particularly for smaller-scale operations. The lower capital investment and operational simplicity make it suitable for facilities with limited resources. However, the economic feasibility is sensitive to the cost and lifespan of the adsorbent materials, as well as the efficiency of the regeneration process.
The market price of glacial acetic acid plays a crucial role in determining the economic feasibility of recovery techniques. Fluctuations in acetic acid prices directly impact the return on investment for recovery systems. In periods of high acetic acid prices, even more expensive recovery methods may become economically viable. Conversely, during low-price periods, only the most efficient and cost-effective recovery techniques remain economically justifiable.
Environmental regulations and waste disposal costs significantly influence the economic equation. Stringent environmental policies and high disposal fees for acetic acid-containing waste streams can make recovery techniques more attractive, even if the direct economic benefits are marginal. In such scenarios, the avoided costs of regulatory compliance and waste management contribute substantially to the overall economic feasibility.
The scale of operation is a critical factor in the economic assessment. Larger facilities can benefit from economies of scale, making more sophisticated recovery techniques economically viable. For smaller operations, simpler and less capital-intensive methods may be more suitable, despite potentially lower recovery efficiencies.
In conclusion, the economic feasibility of solvent recovery techniques for glacial acetic acid varies depending on multiple interrelated factors. A comprehensive economic analysis must consider not only the direct costs and benefits but also the broader operational context, regulatory environment, and market conditions to determine the most suitable recovery strategy for a given facility.
One of the primary recovery methods, distillation, offers high purity but comes with significant energy costs. The economic analysis must consider the energy consumption against the value of recovered acetic acid. In many cases, the energy costs can be offset by the savings in raw material purchases and waste disposal fees. However, the feasibility heavily depends on the scale of operation and the local energy prices.
Membrane-based recovery techniques, such as pervaporation and nanofiltration, present a potentially more energy-efficient alternative. While the initial investment in membrane technology can be substantial, the lower operational costs and reduced energy consumption can lead to favorable long-term economics. The economic viability of these methods improves with advancements in membrane materials and increased durability, reducing replacement frequency and associated costs.
Adsorption-based recovery using activated carbon or specialized resins offers another economically attractive option, particularly for smaller-scale operations. The lower capital investment and operational simplicity make it suitable for facilities with limited resources. However, the economic feasibility is sensitive to the cost and lifespan of the adsorbent materials, as well as the efficiency of the regeneration process.
The market price of glacial acetic acid plays a crucial role in determining the economic feasibility of recovery techniques. Fluctuations in acetic acid prices directly impact the return on investment for recovery systems. In periods of high acetic acid prices, even more expensive recovery methods may become economically viable. Conversely, during low-price periods, only the most efficient and cost-effective recovery techniques remain economically justifiable.
Environmental regulations and waste disposal costs significantly influence the economic equation. Stringent environmental policies and high disposal fees for acetic acid-containing waste streams can make recovery techniques more attractive, even if the direct economic benefits are marginal. In such scenarios, the avoided costs of regulatory compliance and waste management contribute substantially to the overall economic feasibility.
The scale of operation is a critical factor in the economic assessment. Larger facilities can benefit from economies of scale, making more sophisticated recovery techniques economically viable. For smaller operations, simpler and less capital-intensive methods may be more suitable, despite potentially lower recovery efficiencies.
In conclusion, the economic feasibility of solvent recovery techniques for glacial acetic acid varies depending on multiple interrelated factors. A comprehensive economic analysis must consider not only the direct costs and benefits but also the broader operational context, regulatory environment, and market conditions to determine the most suitable recovery strategy for a given facility.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!