Study of Electrode Kinetics in CO2 Capture Membrane Functionality
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Capture Membrane Technology Background and Objectives
Carbon dioxide capture and sequestration (CCS) has emerged as a critical technology in the global effort to mitigate climate change. The evolution of CO2 capture membrane technology represents one of the most promising approaches within this domain, offering potential advantages in energy efficiency, operational flexibility, and environmental impact compared to conventional absorption-based capture methods. The development trajectory of membrane-based CO2 capture spans several decades, beginning with rudimentary polymer membranes in the 1980s and progressing through significant advancements in material science and engineering.
The fundamental principle underlying membrane-based CO2 separation involves selective permeation of gas molecules through specialized membrane structures. Early research focused primarily on polymeric membranes, which offered modest selectivity but suffered from performance limitations under industrial conditions. The field has since expanded to include inorganic membranes, mixed matrix membranes (MMMs), facilitated transport membranes, and most recently, electrochemically-mediated separation membranes that leverage electrode kinetics to enhance capture efficiency.
Electrode kinetics in CO2 capture membranes represents a frontier area where electrochemical principles are being applied to address the persistent challenges of selectivity, permeability, and stability. This approach utilizes electrical potential differences to modulate the transport behavior of CO2 across membrane interfaces, potentially overcoming the traditional permeability-selectivity trade-off that has limited membrane performance.
The technical objectives of current research in electrode kinetics for CO2 capture membranes are multifaceted. Primary goals include enhancing CO2/N2 selectivity beyond 100 while maintaining high permeability (>1000 Barrer), achieving operational stability under industrial flue gas conditions for at least 5,000 hours, and reducing the energy penalty of capture to below 1 GJ/ton CO2. Additionally, researchers aim to develop membranes capable of functioning effectively across a broad temperature range (25-120°C) and in the presence of contaminants such as SOx, NOx, and water vapor.
The evolution of this technology is being driven by both environmental imperatives and economic considerations. With global CO2 emissions continuing to rise despite climate agreements, there is urgent need for scalable, cost-effective capture technologies. Simultaneously, the potential market for effective CO2 capture solutions spans multiple industries, including power generation, cement production, steel manufacturing, and natural gas processing, representing a combined addressable market estimated to exceed $50 billion by 2030.
The fundamental principle underlying membrane-based CO2 separation involves selective permeation of gas molecules through specialized membrane structures. Early research focused primarily on polymeric membranes, which offered modest selectivity but suffered from performance limitations under industrial conditions. The field has since expanded to include inorganic membranes, mixed matrix membranes (MMMs), facilitated transport membranes, and most recently, electrochemically-mediated separation membranes that leverage electrode kinetics to enhance capture efficiency.
Electrode kinetics in CO2 capture membranes represents a frontier area where electrochemical principles are being applied to address the persistent challenges of selectivity, permeability, and stability. This approach utilizes electrical potential differences to modulate the transport behavior of CO2 across membrane interfaces, potentially overcoming the traditional permeability-selectivity trade-off that has limited membrane performance.
The technical objectives of current research in electrode kinetics for CO2 capture membranes are multifaceted. Primary goals include enhancing CO2/N2 selectivity beyond 100 while maintaining high permeability (>1000 Barrer), achieving operational stability under industrial flue gas conditions for at least 5,000 hours, and reducing the energy penalty of capture to below 1 GJ/ton CO2. Additionally, researchers aim to develop membranes capable of functioning effectively across a broad temperature range (25-120°C) and in the presence of contaminants such as SOx, NOx, and water vapor.
The evolution of this technology is being driven by both environmental imperatives and economic considerations. With global CO2 emissions continuing to rise despite climate agreements, there is urgent need for scalable, cost-effective capture technologies. Simultaneously, the potential market for effective CO2 capture solutions spans multiple industries, including power generation, cement production, steel manufacturing, and natural gas processing, representing a combined addressable market estimated to exceed $50 billion by 2030.
Market Analysis for CO2 Capture Membrane Solutions
The global CO2 capture membrane market is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. Current market valuations indicate the global carbon capture and storage (CCS) market reached approximately 3.5 billion USD in 2022, with membrane technologies representing a rapidly expanding segment projected to grow at a CAGR of 19.2% through 2030.
Electrode kinetics-enhanced membranes are emerging as a particularly promising segment within this market. These advanced membranes leverage electrochemical principles to actively facilitate CO2 separation, offering potential efficiency improvements of 30-40% compared to conventional passive membrane systems. This technological advancement addresses a critical market need for more energy-efficient capture solutions.
Geographically, North America currently dominates the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is demonstrating the fastest growth rate, particularly in China and India where industrial decarbonization initiatives are accelerating rapidly. These regions are implementing increasingly stringent carbon emission regulations, creating substantial market pull for advanced membrane technologies.
By application sector, power generation represents the largest market segment (45%), followed by industrial processes (30%) and natural gas processing (15%). The cement and steel industries are showing particularly strong demand growth, with projected adoption rates increasing by 25% annually as these hard-to-abate sectors seek viable decarbonization pathways.
Customer requirements are evolving toward solutions that offer lower energy penalties, reduced operational costs, and smaller physical footprints. Market research indicates that end-users are willing to pay premium prices for membrane systems that can demonstrate CO2 capture costs below 40 USD per ton, representing a key price threshold for widespread commercial adoption.
The market is further segmented by membrane material type, with polymeric membranes currently holding 60% market share due to their cost advantages. However, advanced composite membranes incorporating electrode kinetics functionality are gaining traction, with projected market penetration increasing from 5% to 20% over the next five years.
Competitive dynamics are intensifying as traditional membrane manufacturers face new entrants from the electrochemical and materials science sectors. This convergence is creating a more diverse supplier ecosystem and accelerating innovation cycles. Strategic partnerships between membrane technology developers and large industrial end-users are becoming increasingly common, creating new commercialization pathways and market access strategies.
Electrode kinetics-enhanced membranes are emerging as a particularly promising segment within this market. These advanced membranes leverage electrochemical principles to actively facilitate CO2 separation, offering potential efficiency improvements of 30-40% compared to conventional passive membrane systems. This technological advancement addresses a critical market need for more energy-efficient capture solutions.
Geographically, North America currently dominates the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is demonstrating the fastest growth rate, particularly in China and India where industrial decarbonization initiatives are accelerating rapidly. These regions are implementing increasingly stringent carbon emission regulations, creating substantial market pull for advanced membrane technologies.
By application sector, power generation represents the largest market segment (45%), followed by industrial processes (30%) and natural gas processing (15%). The cement and steel industries are showing particularly strong demand growth, with projected adoption rates increasing by 25% annually as these hard-to-abate sectors seek viable decarbonization pathways.
Customer requirements are evolving toward solutions that offer lower energy penalties, reduced operational costs, and smaller physical footprints. Market research indicates that end-users are willing to pay premium prices for membrane systems that can demonstrate CO2 capture costs below 40 USD per ton, representing a key price threshold for widespread commercial adoption.
The market is further segmented by membrane material type, with polymeric membranes currently holding 60% market share due to their cost advantages. However, advanced composite membranes incorporating electrode kinetics functionality are gaining traction, with projected market penetration increasing from 5% to 20% over the next five years.
Competitive dynamics are intensifying as traditional membrane manufacturers face new entrants from the electrochemical and materials science sectors. This convergence is creating a more diverse supplier ecosystem and accelerating innovation cycles. Strategic partnerships between membrane technology developers and large industrial end-users are becoming increasingly common, creating new commercialization pathways and market access strategies.
Current Electrode Kinetics Challenges in CO2 Capture
The current landscape of electrode kinetics in CO2 capture membrane systems presents several significant challenges that impede optimal performance and widespread implementation. One primary obstacle is the slow reaction kinetics at the electrode-electrolyte interface, which substantially limits the rate of CO2 capture and conversion. This sluggishness results in lower throughput and efficiency, making large-scale applications economically challenging.
Electrode degradation represents another critical issue, as materials often suffer from performance decline over operational cycles. This degradation manifests through surface fouling, catalyst poisoning, and structural changes that progressively reduce active sites available for CO2 interaction. The resulting decrease in electrode lifespan significantly impacts the economic viability of membrane-based capture systems.
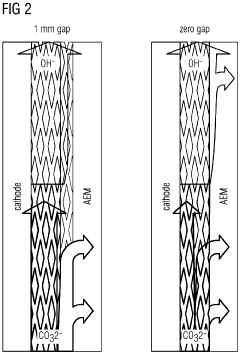
Mass transport limitations further complicate electrode functionality, particularly in the boundary layer where CO2 molecules must efficiently reach reaction sites. Current electrode designs struggle to maintain optimal diffusion pathways, leading to concentration polarization that severely restricts reaction rates even when sufficient electrical potential is applied.
Selectivity challenges persist across various electrode materials and configurations. Many systems exhibit poor discrimination between CO2 and other gases like O2 and N2, resulting in reduced capture efficiency and purity. This selectivity issue becomes particularly problematic in real-world applications where flue gas compositions vary considerably.
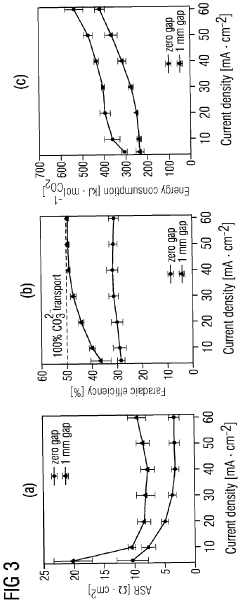
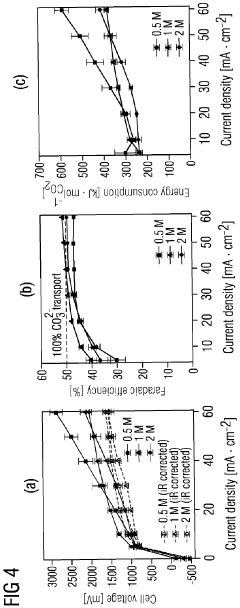
Energy efficiency remains a significant hurdle, with high overpotentials required to drive CO2 reduction reactions. These excessive energy requirements undermine the sustainability benefits of carbon capture technologies. Current electrode materials often demand substantial electrical input that exceeds practical implementation thresholds for industrial deployment.
Stability under varying operational conditions presents ongoing difficulties, as electrode performance can fluctuate dramatically with changes in temperature, pressure, and gas composition. This inconsistency creates reliability concerns for continuous industrial operations where conditions rarely remain constant.
Integration challenges between electrode components and membrane structures further complicate system design. The interface between these elements often suffers from compatibility issues, leading to increased resistance, reduced ion transport, and compromised overall system performance.
Recent research has identified promising directions for addressing these challenges, including novel nanostructured catalysts, advanced electrode architectures, and innovative membrane-electrode assembly techniques. However, significant gaps remain between laboratory demonstrations and commercially viable solutions that can operate reliably at industrial scales.
Electrode degradation represents another critical issue, as materials often suffer from performance decline over operational cycles. This degradation manifests through surface fouling, catalyst poisoning, and structural changes that progressively reduce active sites available for CO2 interaction. The resulting decrease in electrode lifespan significantly impacts the economic viability of membrane-based capture systems.
Mass transport limitations further complicate electrode functionality, particularly in the boundary layer where CO2 molecules must efficiently reach reaction sites. Current electrode designs struggle to maintain optimal diffusion pathways, leading to concentration polarization that severely restricts reaction rates even when sufficient electrical potential is applied.
Selectivity challenges persist across various electrode materials and configurations. Many systems exhibit poor discrimination between CO2 and other gases like O2 and N2, resulting in reduced capture efficiency and purity. This selectivity issue becomes particularly problematic in real-world applications where flue gas compositions vary considerably.
Energy efficiency remains a significant hurdle, with high overpotentials required to drive CO2 reduction reactions. These excessive energy requirements undermine the sustainability benefits of carbon capture technologies. Current electrode materials often demand substantial electrical input that exceeds practical implementation thresholds for industrial deployment.
Stability under varying operational conditions presents ongoing difficulties, as electrode performance can fluctuate dramatically with changes in temperature, pressure, and gas composition. This inconsistency creates reliability concerns for continuous industrial operations where conditions rarely remain constant.
Integration challenges between electrode components and membrane structures further complicate system design. The interface between these elements often suffers from compatibility issues, leading to increased resistance, reduced ion transport, and compromised overall system performance.
Recent research has identified promising directions for addressing these challenges, including novel nanostructured catalysts, advanced electrode architectures, and innovative membrane-electrode assembly techniques. However, significant gaps remain between laboratory demonstrations and commercially viable solutions that can operate reliably at industrial scales.
State-of-the-Art Electrode Kinetics Solutions
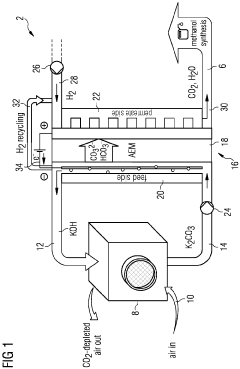
01 Membrane-electrode assembly for CO2 capture
Membrane-electrode assemblies (MEAs) are used for electrochemical CO2 capture systems. These assemblies typically consist of a membrane sandwiched between two electrodes, where CO2 is captured at one electrode and released at the other. The kinetics of these systems depend on the electrode materials, membrane properties, and operating conditions. Advanced MEA designs can significantly improve CO2 capture efficiency and selectivity while reducing energy requirements.- Membrane electrode assembly for CO2 capture: Membrane electrode assemblies (MEAs) are designed specifically for carbon dioxide capture applications. These assemblies integrate selective membranes with electrodes to facilitate the separation and capture of CO2 from gas mixtures. The membrane materials are engineered to have high CO2 permeability and selectivity, while the electrode components are optimized for electrochemical reactions involving carbon dioxide. This integration enhances the overall efficiency of the CO2 capture process by combining membrane separation with electrochemical mechanisms.
- Electrode kinetics optimization for CO2 reduction: Optimizing electrode kinetics is crucial for efficient CO2 reduction reactions. This involves designing electrodes with specific catalytic properties that lower activation energy barriers and increase reaction rates. Various materials and structures are employed to enhance electron transfer processes and improve the selectivity toward desired carbon dioxide reduction products. The electrode surface chemistry and morphology are engineered to maximize active sites and facilitate mass transport, resulting in improved conversion efficiency and reduced energy requirements for CO2 capture and utilization processes.
- Integrated membrane-catalyst systems: Integrated membrane-catalyst systems combine selective membrane technology with catalytic materials to enhance CO2 capture performance. These systems feature catalytic layers directly incorporated into or onto membrane structures, creating synergistic effects that improve both separation and reaction processes. The integration allows for simultaneous CO2 separation and conversion, reducing mass transfer limitations and improving overall system efficiency. Advanced fabrication techniques ensure optimal interface between membrane and catalyst components, leading to enhanced stability and longer operational lifetimes.
- Electrochemical CO2 capture mechanisms: Electrochemical mechanisms for CO2 capture utilize electrical potential to drive carbon dioxide separation and concentration processes. These systems employ various electrochemical reactions that selectively interact with CO2 molecules, facilitating their transport across membranes or binding them to specific sites. The mechanisms may involve redox reactions, electrochemically-mediated sorbent regeneration, or direct electrochemical pumping of carbon dioxide. By controlling electrode potentials and current densities, these systems can achieve high capture efficiencies while minimizing energy consumption compared to traditional thermal separation methods.
- Novel materials for CO2 capture electrodes: Advanced materials are being developed specifically for CO2 capture electrodes to improve performance and durability. These materials include modified carbon structures, metal-organic frameworks, conductive polymers, and novel metal alloys with enhanced catalytic properties. The materials are designed to provide high surface area, improved conductivity, and selective interaction with carbon dioxide molecules. Nanostructured materials and composite formulations offer advantages in terms of mass transport, reaction kinetics, and resistance to degradation under operating conditions, leading to more efficient and longer-lasting CO2 capture systems.
02 Electrode materials affecting CO2 capture kinetics
The choice of electrode materials significantly impacts the kinetics of CO2 capture processes. Catalytic materials such as metal oxides, noble metals, and carbon-based composites can enhance the reaction rates at the electrode-electrolyte interface. Nanostructured electrodes with high surface area improve mass transfer and reaction kinetics. The development of specialized electrode materials with optimized porosity and conductivity has led to substantial improvements in CO2 capture performance and energy efficiency.Expand Specific Solutions03 Membrane technology for selective CO2 transport
Advanced membrane technologies play a crucial role in CO2 capture systems by providing selective transport pathways for CO2 molecules or ions. Ion-exchange membranes, facilitated transport membranes, and composite membranes with functionalized surfaces can enhance CO2 separation efficiency. The kinetics of CO2 transport through these membranes depend on factors such as membrane thickness, porosity, hydration level, and chemical composition. Optimizing these parameters can significantly improve the overall performance of CO2 capture systems.Expand Specific Solutions04 Electrochemical reaction mechanisms in CO2 capture
Understanding the electrochemical reaction mechanisms is essential for improving CO2 capture kinetics. The process typically involves the reduction of CO2 at the cathode and complementary reactions at the anode. Factors affecting reaction kinetics include electrode potential, electrolyte composition, temperature, and pressure. Advanced characterization techniques such as impedance spectroscopy and in-situ spectroscopy help elucidate these mechanisms, enabling the design of more efficient CO2 capture systems with enhanced reaction rates and selectivity.Expand Specific Solutions05 System integration and operational parameters
The integration of membrane-electrode assemblies into complete CO2 capture systems requires careful consideration of operational parameters that affect capture kinetics. These include temperature control, pressure management, flow rates, and electrical parameters such as current density and applied voltage. Optimizing these parameters can significantly enhance the kinetics of CO2 capture processes. Advanced control systems and process intensification techniques help maintain optimal operating conditions, resulting in improved energy efficiency and capture performance in practical applications.Expand Specific Solutions
Leading Research Institutions and Industrial Players
The electrode kinetics in CO2 capture membrane functionality market is currently in a growth phase, characterized by increasing research intensity and technological advancements. The global carbon capture market, estimated at $7-10 billion, is expected to expand significantly as climate regulations tighten. While the technology remains in early commercial maturity, key players are driving innovation across different approaches. Research institutions like Paul Scherrer Institut and universities (Michigan, Shanghai Jiao Tong) focus on fundamental science, while energy giants including TotalEnergies, Siemens Energy, Saudi Aramco, and CNPC invest in scalable applications. Specialized firms like Dioxycle are developing targeted electrolyser solutions. The competitive landscape shows collaboration between academic institutions and industry partners to overcome efficiency and cost barriers in membrane electrode technology.
Paul Scherrer Institut PSI
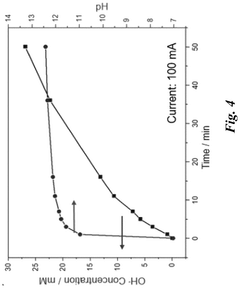
Technical Solution: Paul Scherrer Institut (PSI) has developed advanced electrochemical membrane systems for CO2 capture utilizing ionic liquid-based composite membranes. Their technology focuses on enhancing electrode kinetics through novel catalyst designs that reduce activation energy barriers at the electrode-electrolyte interface. PSI employs in-situ X-ray photoelectron spectroscopy and impedance spectroscopy to characterize electrode surfaces during CO2 reduction reactions, allowing real-time monitoring of reaction intermediates. Their membrane functionality incorporates specialized ionic conductors with tailored pore structures that facilitate selective CO2 transport while maintaining high ionic conductivity. The institute has demonstrated membrane systems achieving over 90% CO2 capture efficiency with significantly reduced energy penalties compared to conventional amine scrubbing technologies.
Strengths: Superior characterization capabilities through advanced analytical techniques; strong fundamental understanding of interfacial phenomena; excellent integration of materials science with electrochemical engineering. Weaknesses: Potential scaling challenges from laboratory to industrial implementation; higher manufacturing costs compared to conventional technologies; possible durability issues under real-world operating conditions.
Siemens AG
Technical Solution: Siemens AG has pioneered electrochemical membrane technology for CO2 capture through their proprietary Electrochemical Carbon Capture (ECC) system. Their approach focuses on optimizing electrode kinetics using specially designed mixed metal oxide catalysts that enhance the electron transfer rates during CO2 separation processes. The company has developed composite membrane electrode assemblies with hierarchical pore structures that maximize the three-phase boundary regions where gas, electrolyte, and electrode meet. Siemens' technology employs advanced impedance analysis to characterize and optimize the electrode-electrolyte interfaces, resulting in reduced overpotentials and improved energy efficiency. Their membrane systems incorporate temperature-responsive polymers that adapt to varying operating conditions, maintaining optimal ionic conductivity while preventing membrane fouling. The technology has been demonstrated at pilot scale, achieving CO2 capture rates exceeding 85% with energy consumption approximately 30% lower than conventional amine-based systems.
Strengths: Strong industrial engineering capabilities for system integration and scale-up; robust manufacturing infrastructure; extensive experience in process automation and control systems for optimal operation. Weaknesses: Higher initial capital investment compared to conventional technologies; technology still requires further optimization for certain industrial applications; potential challenges with long-term membrane stability under industrial conditions.
Key Patents and Research in CO2 Capture Membranes
Electrochemical capture of carbon dioxide from air with electricity storage
PatentPendingUS20250121325A1
Innovation
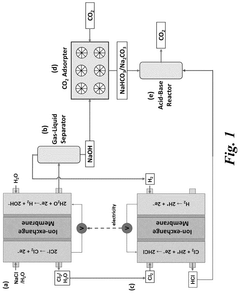
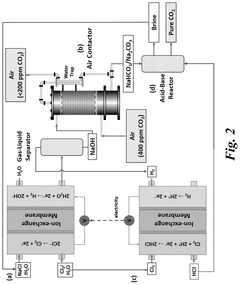
- The system employs an electrochemical approach with a series of membrane reactors, gas-liquid separators, CO2 absorption reactors, and acid-base reactors to capture CO2 from the atmosphere using ion-exchange membranes and electrochemically produced acid and base, allowing for efficient capture and regeneration under ambient conditions.
A system for electrochemically releasing carbon dioxide being captured in an aqueous solution to a hydrogen gas stream
PatentInactiveEP4268934A1
Innovation
- A system using an anion-exchange membrane electrochemical cell with a cathode and anode compartment, where an aqueous alkaline solution captures CO2, which is then electrochemically released as a hydrogen gas stream, regenerating the solution, with a hydrogen depolarized anode and recycling of hydrogen to reduce energy requirements and enhance CO2 transport efficiency.
Environmental Impact and Sustainability Assessment
The environmental implications of CO2 capture membrane technologies extend far beyond their immediate technical performance. When evaluating electrode kinetics in these systems, we must consider the complete lifecycle environmental footprint, from raw material extraction to end-of-life disposal. Current membrane-based carbon capture systems demonstrate significant potential for reducing greenhouse gas emissions, with estimates suggesting potential capture efficiencies of 85-95% when operating at optimal electrode kinetic parameters.
The sustainability profile of these membranes varies considerably depending on material composition and manufacturing processes. Membranes utilizing rare earth elements or precious metals as electrode catalysts present resource scarcity concerns, despite their superior kinetic performance. Research indicates that platinum-based electrodes, while offering excellent reaction rates, contribute to approximately 40% of the total environmental impact of some membrane systems due to mining-related ecosystem disruption and energy-intensive refining processes.
Energy consumption represents another critical sustainability factor. The electrode kinetics directly influence the energy penalty associated with CO2 separation, which typically ranges from 0.5-2.5 GJ/ton CO2 captured. Advanced electrode designs with optimized kinetic properties have demonstrated potential to reduce this energy requirement by 15-30%, significantly improving the technology's overall carbon balance. This improvement is essential for ensuring that the carbon captured exceeds the carbon emitted during the capture process.
Water usage presents an additional environmental consideration, particularly in membrane systems requiring aqueous electrolytes. Studies show that electrode materials affecting reaction kinetics can influence water consumption rates by modifying operational parameters such as temperature and pressure requirements. Membranes operating at lower temperatures due to enhanced electrode kinetics can reduce cooling water requirements by up to 40%.
Chemical waste generation from membrane degradation and electrode material leaching must also be evaluated. Long-term environmental monitoring has identified potential concerns regarding metal ion release from certain electrode materials, with potential aquatic toxicity implications. Membranes designed with kinetically stable electrodes have demonstrated up to three times longer operational lifespans, substantially reducing waste generation rates and replacement frequency.
From a circular economy perspective, the recyclability of electrode materials significantly impacts sustainability. Research indicates that approximately 60-85% of precious metals from spent electrodes can be recovered through appropriate recycling processes, though recovery rates for composite materials remain considerably lower. Designing electrodes with both optimal kinetic properties and end-of-life recoverability represents a frontier challenge in sustainable membrane development.
The sustainability profile of these membranes varies considerably depending on material composition and manufacturing processes. Membranes utilizing rare earth elements or precious metals as electrode catalysts present resource scarcity concerns, despite their superior kinetic performance. Research indicates that platinum-based electrodes, while offering excellent reaction rates, contribute to approximately 40% of the total environmental impact of some membrane systems due to mining-related ecosystem disruption and energy-intensive refining processes.
Energy consumption represents another critical sustainability factor. The electrode kinetics directly influence the energy penalty associated with CO2 separation, which typically ranges from 0.5-2.5 GJ/ton CO2 captured. Advanced electrode designs with optimized kinetic properties have demonstrated potential to reduce this energy requirement by 15-30%, significantly improving the technology's overall carbon balance. This improvement is essential for ensuring that the carbon captured exceeds the carbon emitted during the capture process.
Water usage presents an additional environmental consideration, particularly in membrane systems requiring aqueous electrolytes. Studies show that electrode materials affecting reaction kinetics can influence water consumption rates by modifying operational parameters such as temperature and pressure requirements. Membranes operating at lower temperatures due to enhanced electrode kinetics can reduce cooling water requirements by up to 40%.
Chemical waste generation from membrane degradation and electrode material leaching must also be evaluated. Long-term environmental monitoring has identified potential concerns regarding metal ion release from certain electrode materials, with potential aquatic toxicity implications. Membranes designed with kinetically stable electrodes have demonstrated up to three times longer operational lifespans, substantially reducing waste generation rates and replacement frequency.
From a circular economy perspective, the recyclability of electrode materials significantly impacts sustainability. Research indicates that approximately 60-85% of precious metals from spent electrodes can be recovered through appropriate recycling processes, though recovery rates for composite materials remain considerably lower. Designing electrodes with both optimal kinetic properties and end-of-life recoverability represents a frontier challenge in sustainable membrane development.
Techno-Economic Analysis of Membrane Implementation
The implementation of CO2 capture membranes represents a significant capital investment that must be justified through comprehensive techno-economic analysis. Current cost estimates for membrane-based carbon capture systems range from $40-80 per ton of CO2 captured, depending on the membrane material, system configuration, and operational parameters. These costs are competitive with traditional amine scrubbing technologies, which typically range from $50-100 per ton.
Membrane implementation economics are heavily influenced by several key factors. Material costs constitute approximately 30-40% of total capital expenditure, with high-performance polymers and advanced composite materials commanding premium prices. Manufacturing scalability presents another critical economic consideration, as current production methods for specialized CO2-selective membranes often involve complex processes that limit economies of scale.
Operational expenses must also be carefully evaluated, including energy requirements for maintaining pressure differentials across membranes, periodic replacement costs due to membrane fouling or degradation, and maintenance of supporting infrastructure. Energy consumption typically ranges from 0.5-1.2 GJ per ton of CO2 captured, significantly lower than the 3.5-4.2 GJ required by conventional amine systems.
Lifecycle cost analysis reveals that membrane systems generally achieve payback periods of 3-7 years in industrial applications, depending on carbon pricing mechanisms and regulatory frameworks. Sensitivity analysis indicates that membrane performance improvements—particularly enhancements in CO2 permeability and selectivity resulting from optimized electrode kinetics—can dramatically improve economic viability. A 20% improvement in these parameters can reduce capture costs by approximately 15-25%.
Market adoption scenarios suggest that membrane technologies will achieve cost parity with conventional systems by 2025-2027, with potential for significant cost advantages thereafter as manufacturing scales and materials advance. Integration costs with existing industrial infrastructure must be factored into implementation strategies, typically adding 15-30% to initial capital expenditures but offering operational synergies that reduce long-term costs.
Future economic projections indicate that continued research into electrode kinetics could reduce membrane implementation costs to below $30 per ton of CO2 by 2030, representing a disruptive shift in carbon capture economics. This trajectory depends heavily on successful translation of laboratory advances in electrode-membrane interfaces to commercial-scale manufacturing processes.
Membrane implementation economics are heavily influenced by several key factors. Material costs constitute approximately 30-40% of total capital expenditure, with high-performance polymers and advanced composite materials commanding premium prices. Manufacturing scalability presents another critical economic consideration, as current production methods for specialized CO2-selective membranes often involve complex processes that limit economies of scale.
Operational expenses must also be carefully evaluated, including energy requirements for maintaining pressure differentials across membranes, periodic replacement costs due to membrane fouling or degradation, and maintenance of supporting infrastructure. Energy consumption typically ranges from 0.5-1.2 GJ per ton of CO2 captured, significantly lower than the 3.5-4.2 GJ required by conventional amine systems.
Lifecycle cost analysis reveals that membrane systems generally achieve payback periods of 3-7 years in industrial applications, depending on carbon pricing mechanisms and regulatory frameworks. Sensitivity analysis indicates that membrane performance improvements—particularly enhancements in CO2 permeability and selectivity resulting from optimized electrode kinetics—can dramatically improve economic viability. A 20% improvement in these parameters can reduce capture costs by approximately 15-25%.
Market adoption scenarios suggest that membrane technologies will achieve cost parity with conventional systems by 2025-2027, with potential for significant cost advantages thereafter as manufacturing scales and materials advance. Integration costs with existing industrial infrastructure must be factored into implementation strategies, typically adding 15-30% to initial capital expenditures but offering operational synergies that reduce long-term costs.
Future economic projections indicate that continued research into electrode kinetics could reduce membrane implementation costs to below $30 per ton of CO2 by 2030, representing a disruptive shift in carbon capture economics. This trajectory depends heavily on successful translation of laboratory advances in electrode-membrane interfaces to commercial-scale manufacturing processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!