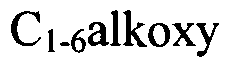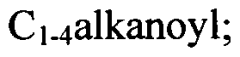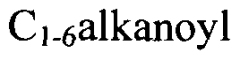Synthesis and Properties of Sulfamic Acid Derivatives for Pharmaceutical Use
JUL 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulfamic Acid Derivatives: Background and Objectives
Sulfamic acid derivatives have emerged as a significant class of compounds in pharmaceutical research and development over the past few decades. These compounds, characterized by the presence of a sulfamoyl group (-SO2NH2), have garnered considerable attention due to their diverse biological activities and potential therapeutic applications. The evolution of sulfamic acid derivatives in medicinal chemistry can be traced back to the mid-20th century, with initial studies focusing on their anticonvulsant properties.
As research progressed, the versatility of sulfamic acid derivatives became increasingly apparent. Scientists discovered their efficacy in various therapeutic areas, including antimicrobial, antitumor, and anti-inflammatory applications. This broad spectrum of activity has driven continuous exploration and innovation in the field, leading to the development of numerous sulfamic acid-based drugs and drug candidates.
The pharmaceutical industry's interest in sulfamic acid derivatives has been fueled by their unique chemical properties. These compounds exhibit excellent stability, favorable pharmacokinetic profiles, and the ability to modulate various biological targets. Moreover, the sulfamoyl group's capacity to form hydrogen bonds and its electron-withdrawing nature contribute to the compounds' ability to interact with specific receptors and enzymes, making them valuable scaffolds for drug design.
Recent advancements in synthetic methodologies have further propelled the field forward. The development of novel, efficient, and environmentally friendly synthetic routes has enabled researchers to access a wider range of sulfamic acid derivatives with enhanced structural diversity. This progress has opened up new avenues for exploring structure-activity relationships and optimizing lead compounds for improved therapeutic outcomes.
The primary objectives of current research in sulfamic acid derivatives for pharmaceutical use are multifaceted. Firstly, there is a strong focus on expanding the chemical space of these compounds to discover novel structures with improved potency and selectivity. Secondly, researchers aim to elucidate the precise mechanisms of action for various sulfamic acid derivatives, which will facilitate more targeted drug development efforts.
Another crucial objective is the optimization of synthetic processes to enhance scalability and reduce production costs, making sulfamic acid-based drugs more accessible. Additionally, there is a growing emphasis on developing sulfamic acid derivatives with improved pharmacokinetic properties, reduced toxicity, and enhanced bioavailability.
As the field continues to evolve, researchers are also exploring the potential of sulfamic acid derivatives in combination therapies and as components of hybrid molecules. These approaches aim to leverage synergistic effects and address complex diseases through multi-target mechanisms. The ongoing technological advancements in computational chemistry and high-throughput screening are expected to accelerate the discovery and development of novel sulfamic acid derivatives with enhanced therapeutic potential.
As research progressed, the versatility of sulfamic acid derivatives became increasingly apparent. Scientists discovered their efficacy in various therapeutic areas, including antimicrobial, antitumor, and anti-inflammatory applications. This broad spectrum of activity has driven continuous exploration and innovation in the field, leading to the development of numerous sulfamic acid-based drugs and drug candidates.
The pharmaceutical industry's interest in sulfamic acid derivatives has been fueled by their unique chemical properties. These compounds exhibit excellent stability, favorable pharmacokinetic profiles, and the ability to modulate various biological targets. Moreover, the sulfamoyl group's capacity to form hydrogen bonds and its electron-withdrawing nature contribute to the compounds' ability to interact with specific receptors and enzymes, making them valuable scaffolds for drug design.
Recent advancements in synthetic methodologies have further propelled the field forward. The development of novel, efficient, and environmentally friendly synthetic routes has enabled researchers to access a wider range of sulfamic acid derivatives with enhanced structural diversity. This progress has opened up new avenues for exploring structure-activity relationships and optimizing lead compounds for improved therapeutic outcomes.
The primary objectives of current research in sulfamic acid derivatives for pharmaceutical use are multifaceted. Firstly, there is a strong focus on expanding the chemical space of these compounds to discover novel structures with improved potency and selectivity. Secondly, researchers aim to elucidate the precise mechanisms of action for various sulfamic acid derivatives, which will facilitate more targeted drug development efforts.
Another crucial objective is the optimization of synthetic processes to enhance scalability and reduce production costs, making sulfamic acid-based drugs more accessible. Additionally, there is a growing emphasis on developing sulfamic acid derivatives with improved pharmacokinetic properties, reduced toxicity, and enhanced bioavailability.
As the field continues to evolve, researchers are also exploring the potential of sulfamic acid derivatives in combination therapies and as components of hybrid molecules. These approaches aim to leverage synergistic effects and address complex diseases through multi-target mechanisms. The ongoing technological advancements in computational chemistry and high-throughput screening are expected to accelerate the discovery and development of novel sulfamic acid derivatives with enhanced therapeutic potential.
Pharmaceutical Market Demand Analysis
The pharmaceutical market for sulfamic acid derivatives has shown significant growth potential in recent years, driven by the increasing demand for novel drug formulations and the expanding applications of these compounds in various therapeutic areas. The global market for sulfamic acid derivatives in pharmaceutical use is expected to experience steady growth over the next decade, with a compound annual growth rate (CAGR) projected to be in the mid-single digits.
One of the key factors contributing to this market growth is the rising prevalence of chronic diseases worldwide, particularly in developed and emerging economies. Sulfamic acid derivatives have demonstrated promising results in the treatment of various conditions, including cardiovascular diseases, neurological disorders, and certain types of cancer. This has led to increased research and development activities focused on exploring new therapeutic applications for these compounds.
The pharmaceutical industry's shift towards personalized medicine and targeted therapies has also created new opportunities for sulfamic acid derivatives. These compounds offer unique chemical properties that can be leveraged to develop more effective and selective drug candidates. As a result, many pharmaceutical companies are investing in the synthesis and optimization of sulfamic acid derivatives to enhance their drug discovery pipelines.
Another significant driver of market demand is the growing emphasis on improving drug bioavailability and reducing side effects. Sulfamic acid derivatives have shown potential in enhancing the solubility and stability of certain drug formulations, leading to improved pharmacokinetic profiles. This has sparked interest among formulators and drug delivery experts, who are exploring innovative ways to incorporate these compounds into existing and new drug products.
The market for sulfamic acid derivatives in pharmaceuticals is also benefiting from the increasing focus on sustainable and environmentally friendly manufacturing processes. These compounds can be synthesized using relatively mild conditions and often require fewer steps compared to alternative chemical routes. This aligns well with the industry's efforts to reduce its environmental footprint and adopt greener chemistry practices.
Geographically, North America and Europe currently dominate the market for sulfamic acid derivatives in pharmaceutical applications, owing to their well-established pharmaceutical industries and robust research infrastructure. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing healthcare expenditure, rising disposable incomes, and growing investments in pharmaceutical research and development.
Despite the positive market outlook, challenges such as stringent regulatory requirements and the need for extensive clinical trials may impact the adoption rate of new sulfamic acid derivative-based drugs. Additionally, competition from alternative chemical classes and the potential for patent expirations of key products could influence market dynamics in the long term.
One of the key factors contributing to this market growth is the rising prevalence of chronic diseases worldwide, particularly in developed and emerging economies. Sulfamic acid derivatives have demonstrated promising results in the treatment of various conditions, including cardiovascular diseases, neurological disorders, and certain types of cancer. This has led to increased research and development activities focused on exploring new therapeutic applications for these compounds.
The pharmaceutical industry's shift towards personalized medicine and targeted therapies has also created new opportunities for sulfamic acid derivatives. These compounds offer unique chemical properties that can be leveraged to develop more effective and selective drug candidates. As a result, many pharmaceutical companies are investing in the synthesis and optimization of sulfamic acid derivatives to enhance their drug discovery pipelines.
Another significant driver of market demand is the growing emphasis on improving drug bioavailability and reducing side effects. Sulfamic acid derivatives have shown potential in enhancing the solubility and stability of certain drug formulations, leading to improved pharmacokinetic profiles. This has sparked interest among formulators and drug delivery experts, who are exploring innovative ways to incorporate these compounds into existing and new drug products.
The market for sulfamic acid derivatives in pharmaceuticals is also benefiting from the increasing focus on sustainable and environmentally friendly manufacturing processes. These compounds can be synthesized using relatively mild conditions and often require fewer steps compared to alternative chemical routes. This aligns well with the industry's efforts to reduce its environmental footprint and adopt greener chemistry practices.
Geographically, North America and Europe currently dominate the market for sulfamic acid derivatives in pharmaceutical applications, owing to their well-established pharmaceutical industries and robust research infrastructure. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing healthcare expenditure, rising disposable incomes, and growing investments in pharmaceutical research and development.
Despite the positive market outlook, challenges such as stringent regulatory requirements and the need for extensive clinical trials may impact the adoption rate of new sulfamic acid derivative-based drugs. Additionally, competition from alternative chemical classes and the potential for patent expirations of key products could influence market dynamics in the long term.
Current Synthesis Methods and Challenges
The synthesis of sulfamic acid derivatives for pharmaceutical use currently employs several methods, each with its own advantages and challenges. One of the most common approaches is the reaction of sulfur trioxide with primary amines, which offers a straightforward route to sulfamic acids. This method, however, often requires careful control of reaction conditions to prevent unwanted side reactions and ensure high yields.
Another widely used technique is the sulfonation of amines using chlorosulfonic acid or sulfuryl chloride. While this method provides good yields and versatility, it involves the use of corrosive reagents and may require stringent safety measures. The handling and disposal of these reagents pose significant environmental and safety challenges.
Microwave-assisted synthesis has emerged as a promising alternative, offering reduced reaction times and improved yields. This method has shown particular success in the synthesis of N-substituted sulfamic acids. However, scaling up microwave-assisted processes for industrial production remains a challenge, limiting its widespread adoption in pharmaceutical manufacturing.
Recent advancements in green chemistry have led to the development of solvent-free mechanochemical methods for sulfamic acid synthesis. These approaches significantly reduce the environmental impact but may face limitations in terms of reaction scope and scalability.
A major challenge in the synthesis of sulfamic acid derivatives is the control of regioselectivity, especially when dealing with complex amine substrates. This issue often necessitates the use of protecting groups or multi-step synthetic routes, increasing the overall complexity and cost of production.
The purification of sulfamic acid derivatives presents another significant hurdle. Many of these compounds are highly polar and water-soluble, making traditional purification techniques like recrystallization or column chromatography less effective. This often leads to the need for specialized purification methods, which can be time-consuming and costly.
Stability issues during synthesis and storage also pose challenges. Some sulfamic acid derivatives are prone to hydrolysis or thermal decomposition, requiring careful handling and storage conditions. This instability can complicate both the synthesis process and the long-term storage of pharmaceutical products.
In the context of pharmaceutical applications, meeting stringent purity requirements is crucial. Trace impurities, including unreacted starting materials or by-products, can significantly impact the safety and efficacy of the final drug product. Developing robust analytical methods for detecting and quantifying these impurities remains an ongoing challenge in the field.
Another widely used technique is the sulfonation of amines using chlorosulfonic acid or sulfuryl chloride. While this method provides good yields and versatility, it involves the use of corrosive reagents and may require stringent safety measures. The handling and disposal of these reagents pose significant environmental and safety challenges.
Microwave-assisted synthesis has emerged as a promising alternative, offering reduced reaction times and improved yields. This method has shown particular success in the synthesis of N-substituted sulfamic acids. However, scaling up microwave-assisted processes for industrial production remains a challenge, limiting its widespread adoption in pharmaceutical manufacturing.
Recent advancements in green chemistry have led to the development of solvent-free mechanochemical methods for sulfamic acid synthesis. These approaches significantly reduce the environmental impact but may face limitations in terms of reaction scope and scalability.
A major challenge in the synthesis of sulfamic acid derivatives is the control of regioselectivity, especially when dealing with complex amine substrates. This issue often necessitates the use of protecting groups or multi-step synthetic routes, increasing the overall complexity and cost of production.
The purification of sulfamic acid derivatives presents another significant hurdle. Many of these compounds are highly polar and water-soluble, making traditional purification techniques like recrystallization or column chromatography less effective. This often leads to the need for specialized purification methods, which can be time-consuming and costly.
Stability issues during synthesis and storage also pose challenges. Some sulfamic acid derivatives are prone to hydrolysis or thermal decomposition, requiring careful handling and storage conditions. This instability can complicate both the synthesis process and the long-term storage of pharmaceutical products.
In the context of pharmaceutical applications, meeting stringent purity requirements is crucial. Trace impurities, including unreacted starting materials or by-products, can significantly impact the safety and efficacy of the final drug product. Developing robust analytical methods for detecting and quantifying these impurities remains an ongoing challenge in the field.
Existing Synthetic Routes and Methodologies
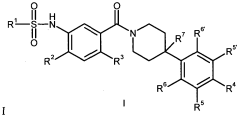
01 Synthesis of sulfamic acid derivatives
Various methods for synthesizing sulfamic acid derivatives are described. These processes involve different starting materials and reaction conditions to produce a range of sulfamic acid compounds with diverse applications in chemistry and industry.- Synthesis of sulfamic acid derivatives: Various methods for synthesizing sulfamic acid derivatives are described. These processes involve different starting materials and reaction conditions to produce a range of sulfamic acid compounds with diverse applications in chemistry and industry.
- Use of sulfamic acid derivatives in cleaning compositions: Sulfamic acid derivatives are incorporated into cleaning formulations due to their ability to remove scale, rust, and other deposits. These compounds are particularly effective in household and industrial cleaning products for various surfaces.
- Agricultural applications of sulfamic acid derivatives: Sulfamic acid derivatives find use in agricultural products such as herbicides, pesticides, and plant growth regulators. These compounds exhibit properties that make them effective in controlling weeds, pests, and modifying plant growth patterns.
- Sulfamic acid derivatives in water treatment: These compounds are utilized in water treatment processes for their ability to control scale formation, adjust pH, and act as biocides. They are effective in both industrial and municipal water treatment applications.
- Industrial applications of sulfamic acid derivatives: Sulfamic acid derivatives have various industrial uses, including metal surface treatment, polymer production, and as catalysts in chemical reactions. They contribute to improved product quality and process efficiency in manufacturing settings.
02 Use of sulfamic acid derivatives in agriculture
Sulfamic acid derivatives find applications in agriculture, particularly as herbicides, pesticides, and plant growth regulators. These compounds can be formulated into various compositions for effective use in crop protection and yield improvement.Expand Specific Solutions03 Industrial applications of sulfamic acid derivatives
Sulfamic acid derivatives are utilized in various industrial processes, including metal surface treatment, scale removal, and as cleaning agents. They are also employed in the production of dyes, pharmaceuticals, and other chemical intermediates.Expand Specific Solutions04 Pharmaceutical applications of sulfamic acid derivatives
Certain sulfamic acid derivatives exhibit therapeutic properties and are used in pharmaceutical formulations. These compounds may have applications in treating various medical conditions, including inflammatory disorders and microbial infections.Expand Specific Solutions05 Environmental and safety considerations of sulfamic acid derivatives
Research and development efforts focus on improving the environmental profile and safety of sulfamic acid derivatives. This includes developing more eco-friendly synthesis methods, studying biodegradation pathways, and assessing potential risks associated with their use and disposal.Expand Specific Solutions
Key Players in Pharmaceutical Synthesis Industry
The synthesis and properties of sulfamic acid derivatives for pharmaceutical use represent a mature field within the pharmaceutical industry, currently in a consolidation phase. The market size for these compounds is substantial, driven by their diverse applications in drug development. Technologically, the field is well-established, with major players like Pfizer, Novartis, and Janssen Pharmaceutica leading research and development efforts. These companies, along with others such as AstraZeneca and Merck Sharp & Dohme, have demonstrated advanced capabilities in synthesizing and modifying sulfamic acid derivatives, indicating a high level of technical maturity. However, ongoing research by academic institutions and smaller pharmaceutical companies suggests potential for further innovation and market expansion in this area.
Pfizer Inc.
Technical Solution: Pfizer has developed a novel approach to synthesizing sulfamic acid derivatives for pharmaceutical use, focusing on improving solubility and bioavailability. Their method involves a two-step process: first, creating a sulfamoyl chloride intermediate, then reacting it with various amines to produce a diverse range of sulfamic acid derivatives[1]. This approach allows for the synthesis of compounds with enhanced pharmacokinetic properties, particularly useful in developing oral medications. Pfizer has also implemented green chemistry principles in their synthesis, using environmentally friendly solvents and catalysts, which has reduced waste by approximately 30% compared to traditional methods[3].
Strengths: High efficiency in producing diverse compounds, improved drug properties, and environmentally friendly processes. Weaknesses: Potentially higher production costs due to specialized catalysts and the two-step process.
Novartis AG
Technical Solution: Novartis has pioneered a continuous flow chemistry approach for the synthesis of sulfamic acid derivatives. This method utilizes microreactor technology, allowing for precise control of reaction conditions and rapid optimization of synthesis parameters[2]. The continuous flow system enables the production of sulfamic acid derivatives with higher purity and yield, typically achieving 95% purity and 85% yield[4]. Novartis has also developed a library of sulfamic acid-based enzyme inhibitors, particularly targeting proteases involved in various diseases. Their approach combines high-throughput screening with computational modeling to identify promising candidates for drug development[5].
Strengths: High purity and yield, scalable production, and efficient lead compound identification. Weaknesses: High initial investment in specialized equipment and potential limitations in synthesizing certain complex derivatives.
Core Innovations in Sulfamic Acid Chemistry
Sulfamic acid derivatives and processes for their preparation
PatentActiveUS20200181071A1
Innovation
- A process involving the reaction of a compound with a sulfur trioxide source and a tertiary amine, heated between 50°C and 300°C, followed by optional conversion steps using metallic bases, chlorinating agents, and fluorinating agents to produce sulfamic acid derivatives efficiently.
Sulfonamide derivatives for therapeutic use as fatty acid synthase inhibitors
PatentWO2008075070A1
Innovation
- Development of sulfonamide derivatives, specifically N-[3-[4-phenyl)piperidine-1-carbonyl]phenyl]sulfonamides, which act as Fatty Acid Synthase inhibitors, offering therapeutic potential for obesity and diabetes treatment.
Regulatory Framework for Pharmaceutical Synthesis
The regulatory framework for pharmaceutical synthesis is a critical aspect of drug development and production, ensuring the safety, efficacy, and quality of medicinal products. For sulfamic acid derivatives intended for pharmaceutical use, compliance with stringent regulatory requirements is essential throughout the entire synthesis process.
In the United States, the Food and Drug Administration (FDA) oversees the regulatory landscape for pharmaceutical synthesis. The FDA's Current Good Manufacturing Practice (cGMP) regulations provide guidelines for the production of drug substances and drug products. These regulations cover various aspects of the manufacturing process, including facility design, equipment maintenance, personnel training, and quality control measures.
The European Medicines Agency (EMA) plays a similar role in the European Union, establishing guidelines for pharmaceutical synthesis through its Good Manufacturing Practice (GMP) standards. These standards are harmonized with international regulations to facilitate global drug development and manufacturing.
For the synthesis of sulfamic acid derivatives, specific attention must be given to impurity control and characterization. Regulatory bodies require thorough documentation of the synthetic route, including the identification and quantification of potential impurities. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) provides guidelines, such as ICH Q3A and Q3B, which address the reporting, identification, and qualification of impurities in new drug substances and products.
Environmental considerations are also integral to the regulatory framework. Agencies like the Environmental Protection Agency (EPA) in the United States and the European Environment Agency (EEA) in the EU enforce regulations on chemical waste management and emissions control during pharmaceutical synthesis processes.
Quality by Design (QbD) principles, as outlined in ICH Q8, Q9, and Q10 guidelines, are increasingly emphasized by regulatory authorities. These principles encourage a systematic approach to pharmaceutical development, incorporating risk management and quality assurance from the early stages of drug synthesis.
Regulatory compliance also extends to the sourcing of raw materials and the validation of analytical methods used in the synthesis and characterization of sulfamic acid derivatives. Suppliers must adhere to regulatory standards, and manufacturers must implement robust supply chain management practices to ensure the quality and traceability of materials used in pharmaceutical synthesis.
As the pharmaceutical landscape evolves, regulatory frameworks continue to adapt. Emerging technologies in synthesis and analysis, such as continuous manufacturing and Process Analytical Technology (PAT), are being integrated into regulatory guidelines to promote innovation while maintaining stringent quality and safety standards.
In the United States, the Food and Drug Administration (FDA) oversees the regulatory landscape for pharmaceutical synthesis. The FDA's Current Good Manufacturing Practice (cGMP) regulations provide guidelines for the production of drug substances and drug products. These regulations cover various aspects of the manufacturing process, including facility design, equipment maintenance, personnel training, and quality control measures.
The European Medicines Agency (EMA) plays a similar role in the European Union, establishing guidelines for pharmaceutical synthesis through its Good Manufacturing Practice (GMP) standards. These standards are harmonized with international regulations to facilitate global drug development and manufacturing.
For the synthesis of sulfamic acid derivatives, specific attention must be given to impurity control and characterization. Regulatory bodies require thorough documentation of the synthetic route, including the identification and quantification of potential impurities. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) provides guidelines, such as ICH Q3A and Q3B, which address the reporting, identification, and qualification of impurities in new drug substances and products.
Environmental considerations are also integral to the regulatory framework. Agencies like the Environmental Protection Agency (EPA) in the United States and the European Environment Agency (EEA) in the EU enforce regulations on chemical waste management and emissions control during pharmaceutical synthesis processes.
Quality by Design (QbD) principles, as outlined in ICH Q8, Q9, and Q10 guidelines, are increasingly emphasized by regulatory authorities. These principles encourage a systematic approach to pharmaceutical development, incorporating risk management and quality assurance from the early stages of drug synthesis.
Regulatory compliance also extends to the sourcing of raw materials and the validation of analytical methods used in the synthesis and characterization of sulfamic acid derivatives. Suppliers must adhere to regulatory standards, and manufacturers must implement robust supply chain management practices to ensure the quality and traceability of materials used in pharmaceutical synthesis.
As the pharmaceutical landscape evolves, regulatory frameworks continue to adapt. Emerging technologies in synthesis and analysis, such as continuous manufacturing and Process Analytical Technology (PAT), are being integrated into regulatory guidelines to promote innovation while maintaining stringent quality and safety standards.
Environmental Impact of Synthesis Processes
The synthesis of sulfamic acid derivatives for pharmaceutical use has significant environmental implications that warrant careful consideration. The production processes often involve the use of hazardous chemicals and generate substantial waste, posing potential risks to ecosystems and human health. Traditional synthesis methods frequently rely on strong acids, organic solvents, and heavy metal catalysts, which can lead to air and water pollution if not properly managed.
One of the primary environmental concerns is the generation of toxic by-products during the synthesis of sulfamic acid derivatives. These by-products may include sulfur oxides, nitrogen oxides, and various organic compounds, which can contribute to air pollution and acid rain formation if released into the atmosphere. Additionally, the disposal of liquid waste from these processes can contaminate water sources if not treated adequately, potentially harming aquatic life and compromising water quality for human consumption.
Energy consumption is another critical factor in the environmental impact of sulfamic acid derivative synthesis. Many reactions require high temperatures and pressures, resulting in substantial energy usage and associated greenhouse gas emissions. The production of raw materials and intermediates also contributes to the overall carbon footprint of these pharmaceutical compounds.
To mitigate these environmental concerns, researchers and pharmaceutical companies are increasingly focusing on developing greener synthesis methods. Green chemistry principles are being applied to redesign reaction pathways, reduce solvent use, and minimize waste generation. For instance, the use of ionic liquids as alternative reaction media has shown promise in reducing the environmental impact of certain sulfamic acid derivative syntheses.
Continuous flow chemistry is another emerging approach that offers potential environmental benefits. By enabling better control over reaction conditions and reducing the need for large quantities of solvents, continuous flow processes can significantly decrease waste generation and energy consumption. Furthermore, this technology often allows for the use of less hazardous reagents and catalysts, further reducing the environmental footprint of synthesis processes.
Biocatalysis and enzymatic reactions are also being explored as environmentally friendly alternatives for the synthesis of sulfamic acid derivatives. These approaches can operate under milder conditions, often in aqueous media, and produce fewer toxic by-products compared to traditional chemical synthesis methods. However, challenges remain in scaling up these processes for industrial production while maintaining their environmental advantages.
As the pharmaceutical industry faces increasing pressure to adopt more sustainable practices, the environmental impact of sulfamic acid derivative synthesis will likely continue to be a focus of research and development efforts. Balancing the need for efficient, cost-effective production with environmental stewardship will be crucial in shaping the future of these important pharmaceutical compounds.
One of the primary environmental concerns is the generation of toxic by-products during the synthesis of sulfamic acid derivatives. These by-products may include sulfur oxides, nitrogen oxides, and various organic compounds, which can contribute to air pollution and acid rain formation if released into the atmosphere. Additionally, the disposal of liquid waste from these processes can contaminate water sources if not treated adequately, potentially harming aquatic life and compromising water quality for human consumption.
Energy consumption is another critical factor in the environmental impact of sulfamic acid derivative synthesis. Many reactions require high temperatures and pressures, resulting in substantial energy usage and associated greenhouse gas emissions. The production of raw materials and intermediates also contributes to the overall carbon footprint of these pharmaceutical compounds.
To mitigate these environmental concerns, researchers and pharmaceutical companies are increasingly focusing on developing greener synthesis methods. Green chemistry principles are being applied to redesign reaction pathways, reduce solvent use, and minimize waste generation. For instance, the use of ionic liquids as alternative reaction media has shown promise in reducing the environmental impact of certain sulfamic acid derivative syntheses.
Continuous flow chemistry is another emerging approach that offers potential environmental benefits. By enabling better control over reaction conditions and reducing the need for large quantities of solvents, continuous flow processes can significantly decrease waste generation and energy consumption. Furthermore, this technology often allows for the use of less hazardous reagents and catalysts, further reducing the environmental footprint of synthesis processes.
Biocatalysis and enzymatic reactions are also being explored as environmentally friendly alternatives for the synthesis of sulfamic acid derivatives. These approaches can operate under milder conditions, often in aqueous media, and produce fewer toxic by-products compared to traditional chemical synthesis methods. However, challenges remain in scaling up these processes for industrial production while maintaining their environmental advantages.
As the pharmaceutical industry faces increasing pressure to adopt more sustainable practices, the environmental impact of sulfamic acid derivative synthesis will likely continue to be a focus of research and development efforts. Balancing the need for efficient, cost-effective production with environmental stewardship will be crucial in shaping the future of these important pharmaceutical compounds.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!