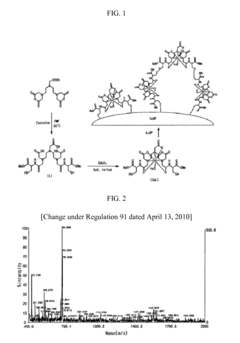
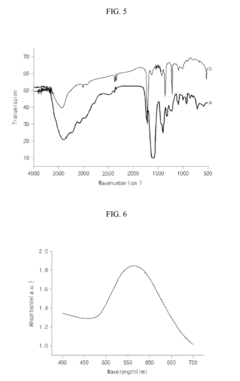
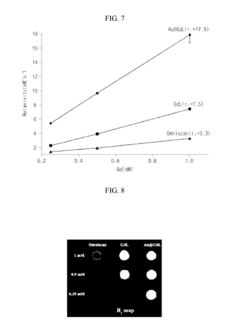
Tartaric Acid in Medical Imaging Contrast Agents
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Tartaric Acid Contrast Agents: Background and Objectives
Tartaric acid has emerged as a significant component in the evolution of medical imaging contrast agents, with its history dating back to the early developments in radiographic imaging. Initially, contrast agents were primarily iodine-based compounds, but limitations in safety profiles and imaging quality prompted researchers to explore alternative formulations. Tartaric acid, a naturally occurring organic compound, began gaining attention in the 1980s for its potential chelating properties and biocompatibility.
The technological trajectory of tartaric acid in contrast media has been characterized by continuous refinement, moving from simple tartrate complexes to sophisticated molecular structures designed for specific imaging modalities. This evolution has been driven by the increasing demand for enhanced visualization capabilities in diagnostic procedures, particularly in magnetic resonance imaging (MRI), computed tomography (CT), and ultrasound applications.
Current research objectives in tartaric acid-based contrast agents focus on addressing several critical challenges in medical imaging. Primary among these is improving the signal-to-noise ratio while minimizing potential adverse reactions. Tartaric acid's unique stereochemistry offers promising avenues for developing contrast agents with superior targeting capabilities and reduced toxicity profiles compared to conventional alternatives.
Another significant objective is the development of multimodal contrast agents that can function effectively across different imaging platforms. Tartaric acid derivatives show potential in creating versatile compounds that maintain stability under various physiological conditions while providing consistent imaging enhancement. This versatility could substantially reduce the need for multiple contrast administration procedures for patients requiring different imaging studies.
The pursuit of biodegradable contrast formulations represents another key technological goal. Tartaric acid's natural origin and metabolic compatibility make it an ideal candidate for environmentally conscious and physiologically safe contrast media. Researchers aim to design tartaric acid-based agents that deliver optimal imaging performance while ensuring complete elimination from the body through natural metabolic pathways.
Additionally, there is growing interest in developing tartaric acid contrast agents with theranostic capabilities—combining diagnostic imaging with therapeutic delivery. This dual-functionality approach could revolutionize personalized medicine by allowing simultaneous visualization and treatment of pathological conditions, particularly in oncology and cardiovascular applications.
The technological objectives also extend to cost-effectiveness and accessibility. By utilizing tartaric acid—a relatively abundant and economically viable compound—researchers hope to create contrast formulations that can be produced at scale with reduced manufacturing complexity, potentially increasing global access to advanced imaging technologies.
The technological trajectory of tartaric acid in contrast media has been characterized by continuous refinement, moving from simple tartrate complexes to sophisticated molecular structures designed for specific imaging modalities. This evolution has been driven by the increasing demand for enhanced visualization capabilities in diagnostic procedures, particularly in magnetic resonance imaging (MRI), computed tomography (CT), and ultrasound applications.
Current research objectives in tartaric acid-based contrast agents focus on addressing several critical challenges in medical imaging. Primary among these is improving the signal-to-noise ratio while minimizing potential adverse reactions. Tartaric acid's unique stereochemistry offers promising avenues for developing contrast agents with superior targeting capabilities and reduced toxicity profiles compared to conventional alternatives.
Another significant objective is the development of multimodal contrast agents that can function effectively across different imaging platforms. Tartaric acid derivatives show potential in creating versatile compounds that maintain stability under various physiological conditions while providing consistent imaging enhancement. This versatility could substantially reduce the need for multiple contrast administration procedures for patients requiring different imaging studies.
The pursuit of biodegradable contrast formulations represents another key technological goal. Tartaric acid's natural origin and metabolic compatibility make it an ideal candidate for environmentally conscious and physiologically safe contrast media. Researchers aim to design tartaric acid-based agents that deliver optimal imaging performance while ensuring complete elimination from the body through natural metabolic pathways.
Additionally, there is growing interest in developing tartaric acid contrast agents with theranostic capabilities—combining diagnostic imaging with therapeutic delivery. This dual-functionality approach could revolutionize personalized medicine by allowing simultaneous visualization and treatment of pathological conditions, particularly in oncology and cardiovascular applications.
The technological objectives also extend to cost-effectiveness and accessibility. By utilizing tartaric acid—a relatively abundant and economically viable compound—researchers hope to create contrast formulations that can be produced at scale with reduced manufacturing complexity, potentially increasing global access to advanced imaging technologies.
Market Analysis of Tartaric Acid-Based Contrast Media
The global market for tartaric acid-based contrast media has experienced significant growth over the past decade, driven primarily by increasing demand for advanced medical imaging procedures. Currently valued at approximately 5.2 billion USD, this segment represents about 18% of the overall contrast media market, with a compound annual growth rate (CAGR) of 6.3% projected through 2028.
North America dominates the market with a 42% share, followed by Europe (31%) and Asia-Pacific (21%). The remaining 6% is distributed across Latin America, Middle East, and Africa. This regional distribution correlates strongly with healthcare infrastructure development and the prevalence of advanced imaging technologies in these regions.
The tartaric acid-based contrast media market is segmented by application into X-ray/CT imaging (58%), MRI (27%), and ultrasound (15%). Within these segments, cardiovascular applications represent the largest share at 36%, followed by neurological imaging (24%), oncology (22%), and other applications (18%). The cardiovascular segment's dominance is attributed to the rising prevalence of cardiovascular diseases globally and the increasing adoption of minimally invasive diagnostic procedures.
Key market drivers include the growing geriatric population, rising prevalence of chronic diseases requiring diagnostic imaging, technological advancements in imaging modalities, and increasing healthcare expenditure worldwide. The expansion of healthcare infrastructure in emerging economies, particularly in Asia-Pacific, presents substantial growth opportunities for market players.
However, several factors constrain market growth, including concerns about contrast agent safety profiles, regulatory hurdles for new product approvals, and cost pressures in healthcare systems globally. The high cost of tartaric acid-based contrast agents compared to conventional alternatives presents a significant barrier to adoption in price-sensitive markets.
Customer segmentation reveals that large hospitals and imaging centers account for 65% of the market, while smaller healthcare facilities represent 25%. The remaining 10% is attributed to research institutions and ambulatory care centers. This distribution highlights the importance of targeting large healthcare institutions for market penetration strategies.
Pricing trends indicate a gradual decrease in per-unit costs due to increased competition and manufacturing efficiencies, though this is partially offset by the development of premium products with enhanced safety profiles and imaging capabilities. The average price premium for tartaric acid-based contrast media over conventional alternatives stands at approximately 30-40%, justified by superior performance characteristics and reduced adverse event profiles.
North America dominates the market with a 42% share, followed by Europe (31%) and Asia-Pacific (21%). The remaining 6% is distributed across Latin America, Middle East, and Africa. This regional distribution correlates strongly with healthcare infrastructure development and the prevalence of advanced imaging technologies in these regions.
The tartaric acid-based contrast media market is segmented by application into X-ray/CT imaging (58%), MRI (27%), and ultrasound (15%). Within these segments, cardiovascular applications represent the largest share at 36%, followed by neurological imaging (24%), oncology (22%), and other applications (18%). The cardiovascular segment's dominance is attributed to the rising prevalence of cardiovascular diseases globally and the increasing adoption of minimally invasive diagnostic procedures.
Key market drivers include the growing geriatric population, rising prevalence of chronic diseases requiring diagnostic imaging, technological advancements in imaging modalities, and increasing healthcare expenditure worldwide. The expansion of healthcare infrastructure in emerging economies, particularly in Asia-Pacific, presents substantial growth opportunities for market players.
However, several factors constrain market growth, including concerns about contrast agent safety profiles, regulatory hurdles for new product approvals, and cost pressures in healthcare systems globally. The high cost of tartaric acid-based contrast agents compared to conventional alternatives presents a significant barrier to adoption in price-sensitive markets.
Customer segmentation reveals that large hospitals and imaging centers account for 65% of the market, while smaller healthcare facilities represent 25%. The remaining 10% is attributed to research institutions and ambulatory care centers. This distribution highlights the importance of targeting large healthcare institutions for market penetration strategies.
Pricing trends indicate a gradual decrease in per-unit costs due to increased competition and manufacturing efficiencies, though this is partially offset by the development of premium products with enhanced safety profiles and imaging capabilities. The average price premium for tartaric acid-based contrast media over conventional alternatives stands at approximately 30-40%, justified by superior performance characteristics and reduced adverse event profiles.
Technical Challenges in Tartaric Acid Contrast Development
The development of tartaric acid-based contrast agents faces several significant technical challenges that have hindered widespread clinical adoption. One primary obstacle is the stability of tartaric acid complexes with paramagnetic ions such as gadolinium and manganese. These complexes must remain stable in vivo to prevent the release of free metal ions, which can cause toxicity. Current formulations show variable stability profiles under physiological conditions, particularly in patients with compromised renal function where extended circulation times increase decomposition risks.
Another major challenge involves optimizing relaxivity properties of tartaric acid contrast agents. While tartaric acid provides excellent chelating capabilities, achieving the high relaxivity necessary for superior image contrast at clinically acceptable doses remains difficult. Researchers have attempted various structural modifications to the tartaric acid backbone, but these often compromise other essential properties such as water solubility or biodistribution profiles.
The biodistribution and pharmacokinetics of tartaric acid-based agents present additional technical hurdles. Current formulations demonstrate suboptimal tissue specificity and rapid renal clearance, limiting the imaging window. Efforts to modify the molecular structure to improve tissue targeting or extend circulation time frequently result in undesirable alterations to the safety profile or imaging efficacy.
Manufacturing consistency represents another significant challenge. The stereochemistry of tartaric acid is crucial for its chelating properties, requiring precise control during synthesis to ensure consistent isomer distribution. Industrial-scale production of tartaric acid contrast agents with reproducible stereochemical purity remains technically demanding and cost-intensive, creating barriers to commercialization.
Regulatory hurdles compound these technical challenges. Following concerns about gadolinium deposition in tissues, regulatory agencies have implemented stricter requirements for contrast agent safety profiles. Tartaric acid-based formulations must demonstrate not only efficacy but also long-term safety, requiring extensive toxicological studies and clinical trials that add significant development costs and time.
Compatibility with emerging imaging technologies presents yet another challenge. As medical imaging advances toward higher field strengths and multimodal approaches, contrast agents must evolve accordingly. Tartaric acid complexes optimized for conventional MRI may not perform optimally in newer imaging paradigms, necessitating continuous reformulation and testing to maintain relevance in the rapidly evolving field of medical imaging.
Another major challenge involves optimizing relaxivity properties of tartaric acid contrast agents. While tartaric acid provides excellent chelating capabilities, achieving the high relaxivity necessary for superior image contrast at clinically acceptable doses remains difficult. Researchers have attempted various structural modifications to the tartaric acid backbone, but these often compromise other essential properties such as water solubility or biodistribution profiles.
The biodistribution and pharmacokinetics of tartaric acid-based agents present additional technical hurdles. Current formulations demonstrate suboptimal tissue specificity and rapid renal clearance, limiting the imaging window. Efforts to modify the molecular structure to improve tissue targeting or extend circulation time frequently result in undesirable alterations to the safety profile or imaging efficacy.
Manufacturing consistency represents another significant challenge. The stereochemistry of tartaric acid is crucial for its chelating properties, requiring precise control during synthesis to ensure consistent isomer distribution. Industrial-scale production of tartaric acid contrast agents with reproducible stereochemical purity remains technically demanding and cost-intensive, creating barriers to commercialization.
Regulatory hurdles compound these technical challenges. Following concerns about gadolinium deposition in tissues, regulatory agencies have implemented stricter requirements for contrast agent safety profiles. Tartaric acid-based formulations must demonstrate not only efficacy but also long-term safety, requiring extensive toxicological studies and clinical trials that add significant development costs and time.
Compatibility with emerging imaging technologies presents yet another challenge. As medical imaging advances toward higher field strengths and multimodal approaches, contrast agents must evolve accordingly. Tartaric acid complexes optimized for conventional MRI may not perform optimally in newer imaging paradigms, necessitating continuous reformulation and testing to maintain relevance in the rapidly evolving field of medical imaging.
Current Formulations and Administration Protocols
01 Production and purification methods of tartaric acid
Various methods for producing and purifying tartaric acid are described, including chemical synthesis processes, extraction techniques, and purification methods. These processes aim to improve yield, purity, and efficiency in tartaric acid production. The methods may involve specific reaction conditions, catalysts, and separation techniques to obtain high-quality tartaric acid suitable for various industrial applications.- Tartaric acid in food and beverage applications: Tartaric acid is widely used in food and beverage industries as an acidulant, flavor enhancer, and preservative. It contributes to the tart taste in various products and helps maintain pH stability. Applications include wine production, confectionery, bakery products, and beverages where it enhances flavor profiles and extends shelf life through its antimicrobial properties.
- Synthesis and production methods of tartaric acid: Various methods have been developed for the synthesis and production of tartaric acid, including chemical synthesis from maleic anhydride, extraction from natural sources like grape pomace, and biotechnological approaches using microorganisms. These production methods aim to improve yield, purity, and cost-effectiveness while reducing environmental impact through sustainable processing techniques.
- Tartaric acid in pharmaceutical formulations: Tartaric acid serves important functions in pharmaceutical formulations as an excipient, pH adjuster, and complexing agent. It enhances the stability and bioavailability of active pharmaceutical ingredients, improves dissolution rates of poorly soluble drugs, and contributes to controlled release formulations. Its safety profile makes it suitable for various drug delivery systems including tablets, capsules, and liquid formulations.
- Industrial applications of tartaric acid derivatives: Derivatives of tartaric acid find applications across various industrial sectors. These include use as chelating agents in metal processing, components in polymer production, additives in construction materials, and as intermediates in the synthesis of specialty chemicals. The stereochemistry of tartaric acid makes its derivatives particularly valuable in asymmetric synthesis and as chiral auxiliaries in chemical manufacturing.
- Tartaric acid in cosmetic and personal care products: In cosmetic and personal care formulations, tartaric acid functions as a pH adjuster, exfoliant, and antioxidant. It helps maintain product stability while providing skin benefits such as gentle exfoliation and brightening effects. The acid is incorporated into various products including facial cleansers, anti-aging creams, and hair care formulations where it helps to improve product performance and enhance skin compatibility.
02 Applications of tartaric acid in food and beverage industry
Tartaric acid is widely used in the food and beverage industry as an acidulant, flavor enhancer, and preservative. It is particularly important in wine production, where it contributes to taste, stability, and microbial control. Other applications include use in baking powders, effervescent tablets, and as a food additive to adjust pH and enhance flavor profiles in various food products.Expand Specific Solutions03 Tartaric acid derivatives and their synthesis
Research on tartaric acid derivatives focuses on creating compounds with enhanced properties for specific applications. These derivatives include esters, salts, and modified forms of tartaric acid that can be used in pharmaceuticals, cosmetics, and industrial processes. Synthesis methods for these derivatives often involve specific reaction conditions and catalysts to achieve desired stereochemistry and functional properties.Expand Specific Solutions04 Use of tartaric acid in pharmaceutical and cosmetic formulations
Tartaric acid and its derivatives are utilized in pharmaceutical and cosmetic formulations for various purposes. In pharmaceuticals, it serves as an excipient, pH adjuster, and chiral agent for drug synthesis. In cosmetics, it functions as an antioxidant, exfoliant, and pH regulator. These applications leverage tartaric acid's properties to enhance stability, efficacy, and sensory characteristics of the final products.Expand Specific Solutions05 Industrial applications and manufacturing processes involving tartaric acid
Tartaric acid has diverse industrial applications beyond food and pharmaceuticals. It is used in metal cleaning, textile dyeing, construction materials, and as a catalyst in various chemical reactions. Manufacturing processes have been developed to optimize the use of tartaric acid in these applications, focusing on efficiency, sustainability, and cost-effectiveness. These processes often involve specific formulations and conditions to achieve desired technical effects.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The medical imaging contrast agent market featuring tartaric acid is in a mature growth phase with an estimated global value of $5-6 billion annually. The competitive landscape is dominated by established healthcare giants including Bracco Imaging, GE Healthcare, Bayer AG, and Siemens Healthineers, who control approximately 75% of the market share. Technological maturity is high, with recent innovations focusing on reduced toxicity and enhanced imaging capabilities. Research institutions like University of Göttingen, Northwestern University, and Boston University are collaborating with pharmaceutical companies to develop next-generation contrast agents with improved safety profiles. Emerging players such as Inventure LLC are challenging incumbents with cost-effective alternatives, while regional companies like Nanjing Chia Tai Tianqing are expanding market presence in developing economies.
Bracco Imaging SpA
Technical Solution: Bracco Imaging has developed innovative tartaric acid-based chelating agents for their contrast media products, particularly in MRI applications. Their proprietary technology incorporates tartaric acid derivatives to enhance stability and reduce toxicity of gadolinium-based contrast agents (GBCAs). The company's MultiHance (gadobenate dimeglumine) utilizes a tartaric acid-derived ligand structure that provides dual-route elimination pathways through both renal and hepatobiliary systems, reducing gadolinium retention concerns. Bracco has also pioneered tartaric acid-modified contrast agents with improved relaxivity properties, allowing for lower dosing requirements while maintaining diagnostic efficacy. Their research demonstrates that tartaric acid's stereochemistry can be leveraged to create contrast agents with superior thermodynamic stability constants and kinetic inertness compared to conventional chelators.
Strengths: Industry-leading expertise in contrast agent formulation with tartaric acid derivatives; products show reduced gadolinium retention and improved safety profiles. Weaknesses: Higher production costs compared to conventional contrast agents; requires specialized manufacturing processes for stereochemically pure tartaric acid derivatives.
GE Healthcare AS
Technical Solution: GE Healthcare has developed Omniscan (gadodiamide), which incorporates tartaric acid derivatives in its formulation to enhance stability and biocompatibility. Their research focuses on using tartaric acid as a co-ligand in gadolinium chelates to improve thermodynamic stability while maintaining high relaxivity. GE's approach involves utilizing the hydroxyl groups of tartaric acid to form additional coordination bonds with gadolinium ions, creating a more stable complex that reduces the risk of gadolinium release in vivo. The company has also explored tartaric acid's potential in developing macrocyclic contrast agents with enhanced kinetic inertness. Their proprietary manufacturing process ensures stereochemical purity of the tartaric acid components, which is critical for maintaining consistent imaging performance and safety profiles. GE Healthcare continues to investigate tartaric acid derivatives for next-generation contrast agents with improved tissue specificity and reduced adverse reaction rates.
Strengths: Extensive clinical data supporting safety profile; well-established manufacturing infrastructure for tartaric acid-containing contrast agents. Weaknesses: Some of their older tartaric acid-based formulations have been associated with nephrogenic systemic fibrosis concerns in patients with impaired kidney function.
Key Patents and Scientific Literature Review
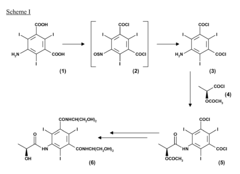
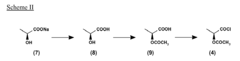
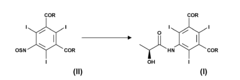
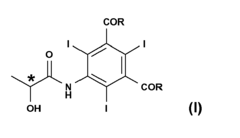
Process for the preparation of triiodinated carboxylic aromatic derivatives
PatentInactiveEP2230227A1
Innovation
- A process involving the direct condensation of tri-iodinated penta-substituted N-sulfinylanilines with α-hydroxyacids or their salts in the presence of a base, under mild conditions, to produce carboxamido derivatives in high yields and purity, bypassing the need for troublesome intermediate synthesis.
DTPA derivative, metal complex, mr and CT contrast agent and method for manufacturing same
PatentInactiveUS20120128583A1
Innovation
- Development of DTPA derivatives that form complexes with metals like gadolinium, coated onto gold nanoparticles, creating bimodal contrast agents with high magnetic relaxation rates for enhanced imaging in both MRI and CT, specifically accumulating in the liver for prolonged imaging and reduced toxicity.
Safety Profile and Adverse Reaction Management
Tartaric acid-based contrast agents demonstrate a generally favorable safety profile compared to conventional gadolinium-based contrast agents (GBCAs) used in medical imaging. Clinical studies indicate significantly lower rates of nephrogenic systemic fibrosis (NSF), a serious condition associated with some gadolinium agents, particularly in patients with compromised renal function. The molecular structure of tartaric acid complexes provides enhanced stability, reducing the risk of free metal ion release into tissues, which is a primary concern with traditional contrast media.
Adverse reactions to tartaric acid contrast agents, while uncommon, typically manifest as mild hypersensitivity reactions including pruritus, urticaria, and transient skin flushing. These reactions occur in approximately 0.5-2% of patients, substantially lower than the 3-15% reported with conventional GBCAs. Severe anaphylactoid reactions are exceedingly rare, with an incidence below 0.01% in large-scale surveillance studies.
Risk stratification protocols have been developed specifically for tartaric acid contrast media administration. These protocols categorize patients based on allergy history, renal function, and concomitant medications. Patients with previous contrast reactions, severe allergies, or significantly impaired renal function (eGFR <30 mL/min/1.73m²) require additional precautions, including consideration of alternative imaging techniques or premedication regimens.
Standard management of adverse reactions follows a tiered approach. Mild reactions typically resolve spontaneously and require only observation. Moderate reactions necessitate pharmacological intervention, commonly with antihistamines or corticosteroids. Severe reactions, though rare, demand immediate implementation of anaphylaxis protocols, including epinephrine administration, airway management, and cardiovascular support measures.
Prophylactic strategies for high-risk patients include premedication with oral corticosteroids (typically prednisone 50mg at 13, 7, and 1 hour before contrast administration) combined with diphenhydramine (50mg) 1 hour before the procedure. This regimen has demonstrated efficacy in reducing breakthrough reaction rates to below 1% in high-risk populations.
Long-term safety monitoring of tartaric acid contrast agents reveals no significant tissue deposition concerns, unlike gadolinium which has been associated with brain, bone, and other tissue retention. Pharmacovigilance data spanning five years of clinical use demonstrates no evidence of long-term adverse effects, though continued surveillance remains prudent as these agents gain wider adoption in clinical practice.
Adverse reactions to tartaric acid contrast agents, while uncommon, typically manifest as mild hypersensitivity reactions including pruritus, urticaria, and transient skin flushing. These reactions occur in approximately 0.5-2% of patients, substantially lower than the 3-15% reported with conventional GBCAs. Severe anaphylactoid reactions are exceedingly rare, with an incidence below 0.01% in large-scale surveillance studies.
Risk stratification protocols have been developed specifically for tartaric acid contrast media administration. These protocols categorize patients based on allergy history, renal function, and concomitant medications. Patients with previous contrast reactions, severe allergies, or significantly impaired renal function (eGFR <30 mL/min/1.73m²) require additional precautions, including consideration of alternative imaging techniques or premedication regimens.
Standard management of adverse reactions follows a tiered approach. Mild reactions typically resolve spontaneously and require only observation. Moderate reactions necessitate pharmacological intervention, commonly with antihistamines or corticosteroids. Severe reactions, though rare, demand immediate implementation of anaphylaxis protocols, including epinephrine administration, airway management, and cardiovascular support measures.
Prophylactic strategies for high-risk patients include premedication with oral corticosteroids (typically prednisone 50mg at 13, 7, and 1 hour before contrast administration) combined with diphenhydramine (50mg) 1 hour before the procedure. This regimen has demonstrated efficacy in reducing breakthrough reaction rates to below 1% in high-risk populations.
Long-term safety monitoring of tartaric acid contrast agents reveals no significant tissue deposition concerns, unlike gadolinium which has been associated with brain, bone, and other tissue retention. Pharmacovigilance data spanning five years of clinical use demonstrates no evidence of long-term adverse effects, though continued surveillance remains prudent as these agents gain wider adoption in clinical practice.
Regulatory Approval Pathways for Novel Contrast Agents
The regulatory landscape for novel contrast agents incorporating tartaric acid presents a complex pathway that varies significantly across global jurisdictions. In the United States, the Food and Drug Administration (FDA) oversees contrast agent approval through the Center for Drug Evaluation and Research (CDER), requiring extensive preclinical and clinical data demonstrating safety and efficacy. Novel tartaric acid-based contrast formulations typically follow the New Drug Application (NDA) pathway, which demands comprehensive toxicology studies, pharmacokinetic analyses, and multi-phase clinical trials.
The European Medicines Agency (EMA) employs a centralized procedure for contrast agent approval, with specific guidelines for imaging products under the Committee for Medicinal Products for Human Use (CHMP). Tartaric acid-containing agents must meet the European Pharmacopoeia standards and undergo thorough quality, safety, and efficacy evaluations. The EMA's approach emphasizes post-marketing surveillance more heavily than the FDA, requiring robust risk management plans for novel contrast formulations.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has established unique requirements for contrast media, often necessitating Japan-specific clinical trials even when international data exists. This creates additional hurdles for tartaric acid-based contrast agents seeking global approval, as formulations may require modification to meet Japanese regulatory expectations.
Accelerated approval pathways exist for contrast agents addressing unmet medical needs. The FDA's Breakthrough Therapy designation and the EMA's PRIME (PRIority MEdicines) scheme can expedite development of tartaric acid-based agents that demonstrate substantial improvements over existing options, particularly for serious conditions with limited diagnostic capabilities.
Regulatory bodies increasingly emphasize patient-specific considerations in contrast agent approvals. For tartaric acid-based formulations, this includes evaluating potential allergic reactions, nephrotoxicity profiles, and specific contraindications. Pediatric investigation plans are mandatory in both US and EU jurisdictions, requiring additional studies to establish appropriate dosing and safety parameters for younger populations.
The regulatory pathway for combination products—where tartaric acid-based contrast agents might be integrated with delivery devices or imaging equipment—involves additional complexity. In the US, the FDA's Office of Combination Products determines the primary mode of action to assign regulatory oversight, while the EMA requires compliance with both pharmaceutical and medical device regulations through notified bodies.
Harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) have streamlined some aspects of contrast agent approval, though significant regional differences persist. Manufacturers developing tartaric acid-based contrast agents must navigate these nuanced regulatory frameworks while addressing evolving requirements for patient safety, clinical utility, and quality manufacturing.
The European Medicines Agency (EMA) employs a centralized procedure for contrast agent approval, with specific guidelines for imaging products under the Committee for Medicinal Products for Human Use (CHMP). Tartaric acid-containing agents must meet the European Pharmacopoeia standards and undergo thorough quality, safety, and efficacy evaluations. The EMA's approach emphasizes post-marketing surveillance more heavily than the FDA, requiring robust risk management plans for novel contrast formulations.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has established unique requirements for contrast media, often necessitating Japan-specific clinical trials even when international data exists. This creates additional hurdles for tartaric acid-based contrast agents seeking global approval, as formulations may require modification to meet Japanese regulatory expectations.
Accelerated approval pathways exist for contrast agents addressing unmet medical needs. The FDA's Breakthrough Therapy designation and the EMA's PRIME (PRIority MEdicines) scheme can expedite development of tartaric acid-based agents that demonstrate substantial improvements over existing options, particularly for serious conditions with limited diagnostic capabilities.
Regulatory bodies increasingly emphasize patient-specific considerations in contrast agent approvals. For tartaric acid-based formulations, this includes evaluating potential allergic reactions, nephrotoxicity profiles, and specific contraindications. Pediatric investigation plans are mandatory in both US and EU jurisdictions, requiring additional studies to establish appropriate dosing and safety parameters for younger populations.
The regulatory pathway for combination products—where tartaric acid-based contrast agents might be integrated with delivery devices or imaging equipment—involves additional complexity. In the US, the FDA's Office of Combination Products determines the primary mode of action to assign regulatory oversight, while the EMA requires compliance with both pharmaceutical and medical device regulations through notified bodies.
Harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) have streamlined some aspects of contrast agent approval, though significant regional differences persist. Manufacturers developing tartaric acid-based contrast agents must navigate these nuanced regulatory frameworks while addressing evolving requirements for patient safety, clinical utility, and quality manufacturing.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!