Tartaric Acid vs Maleic Acid in Cross-Linking Polymers
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cross-Linking Acid Technology Background and Objectives
Cross-linking technology in polymer chemistry has evolved significantly over the past several decades, transforming from simple vulcanization processes to sophisticated molecular engineering approaches. The use of acids as cross-linking agents represents a critical advancement in this field, with tartaric acid and maleic acid emerging as particularly important compounds due to their unique molecular structures and functional properties.
Historically, cross-linking technology began with Charles Goodyear's discovery of vulcanization in 1839, using sulfur to create bridges between polymer chains. The field progressed through the 20th century with the development of various cross-linking agents, including peroxides, radiation, and multifunctional compounds. The introduction of carboxylic acids as cross-linking agents marked a significant milestone, offering improved control over reaction kinetics and final material properties.
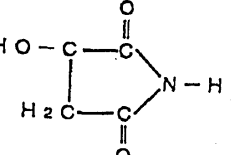
Tartaric acid, a naturally occurring dicarboxylic acid found in many fruits, has been utilized in polymer chemistry since the mid-20th century. Its stereochemistry, with two chiral centers, provides unique spatial arrangements that influence cross-linking density and orientation. Maleic acid, conversely, is a synthetic dicarboxylic acid with a carbon-carbon double bond that offers additional reactivity options through both its carboxylic groups and unsaturated bond.
The technological trajectory has been driven by increasing demands for materials with precisely controlled mechanical properties, thermal stability, and chemical resistance. Industries ranging from automotive to biomedical have pushed for cross-linked polymers with enhanced performance characteristics, stimulating research into the comparative advantages of different cross-linking agents.
Recent technological developments have focused on sustainable and environmentally friendly cross-linking processes, with tartaric acid gaining attention due to its natural origin and biodegradability. Meanwhile, maleic acid continues to be valued for its versatility in reaction mechanisms and compatibility with various polymer systems.
The primary objective of current research in this field is to establish comprehensive comparative frameworks for evaluating tartaric acid versus maleic acid in cross-linking applications. This includes quantifying differences in reaction kinetics, cross-linking density, mechanical properties of the resulting materials, and environmental impact throughout the product lifecycle.
Additional goals include developing predictive models for cross-linking behavior based on molecular structure, optimizing processing conditions for each acid type, and exploring synergistic effects when these acids are used in combination or with other cross-linking agents. The ultimate aim is to enable materials scientists and engineers to make informed, application-specific choices between these two important cross-linking agents.
Historically, cross-linking technology began with Charles Goodyear's discovery of vulcanization in 1839, using sulfur to create bridges between polymer chains. The field progressed through the 20th century with the development of various cross-linking agents, including peroxides, radiation, and multifunctional compounds. The introduction of carboxylic acids as cross-linking agents marked a significant milestone, offering improved control over reaction kinetics and final material properties.
Tartaric acid, a naturally occurring dicarboxylic acid found in many fruits, has been utilized in polymer chemistry since the mid-20th century. Its stereochemistry, with two chiral centers, provides unique spatial arrangements that influence cross-linking density and orientation. Maleic acid, conversely, is a synthetic dicarboxylic acid with a carbon-carbon double bond that offers additional reactivity options through both its carboxylic groups and unsaturated bond.
The technological trajectory has been driven by increasing demands for materials with precisely controlled mechanical properties, thermal stability, and chemical resistance. Industries ranging from automotive to biomedical have pushed for cross-linked polymers with enhanced performance characteristics, stimulating research into the comparative advantages of different cross-linking agents.
Recent technological developments have focused on sustainable and environmentally friendly cross-linking processes, with tartaric acid gaining attention due to its natural origin and biodegradability. Meanwhile, maleic acid continues to be valued for its versatility in reaction mechanisms and compatibility with various polymer systems.
The primary objective of current research in this field is to establish comprehensive comparative frameworks for evaluating tartaric acid versus maleic acid in cross-linking applications. This includes quantifying differences in reaction kinetics, cross-linking density, mechanical properties of the resulting materials, and environmental impact throughout the product lifecycle.
Additional goals include developing predictive models for cross-linking behavior based on molecular structure, optimizing processing conditions for each acid type, and exploring synergistic effects when these acids are used in combination or with other cross-linking agents. The ultimate aim is to enable materials scientists and engineers to make informed, application-specific choices between these two important cross-linking agents.
Market Analysis for Acid-Based Cross-Linking Polymers
The global market for acid-based cross-linking polymers has experienced significant growth over the past decade, primarily driven by increasing applications in adhesives, coatings, textiles, and biomedical industries. The market value reached approximately $4.2 billion in 2022 and is projected to grow at a compound annual growth rate of 5.7% through 2028, according to industry reports from Chemical Market Analytics.
Within this market, tartaric acid and maleic acid represent two distinct segments with different growth trajectories. The tartaric acid segment currently holds about 18% of the cross-linking polymer market, valued at roughly $756 million. This segment has shown steady growth of 4.3% annually, primarily supported by applications in environmentally friendly coatings and food-grade adhesives where its natural origin provides marketing advantages.
Maleic acid and its derivatives, meanwhile, command a larger 27% market share, representing a $1.13 billion segment. This segment demonstrates more robust growth at 6.2% annually, driven by its versatility in industrial applications and superior performance characteristics in high-stress environments.
Regional analysis reveals distinct market preferences. North America and Europe show increasing demand for tartaric acid-based cross-linking systems, aligned with stricter environmental regulations and consumer preference for bio-based materials. The tartaric acid market in these regions is growing at 5.8% annually, outpacing the global average.
Asia-Pacific remains the dominant market for maleic acid cross-linking systems, accounting for 42% of global consumption. This preference stems from the region's expansive manufacturing base in textiles, electronics, and automotive industries where maleic acid's performance characteristics provide technical advantages despite environmental considerations.
End-user industry analysis indicates shifting demand patterns. The construction sector represents the largest consumer of acid-based cross-linking polymers (31% of market volume), followed by automotive (24%), textiles (18%), and healthcare (12%). The fastest growth is observed in healthcare applications, expanding at 7.9% annually as advanced biomaterials utilizing cross-linked polymers gain traction in medical devices and drug delivery systems.
Price sensitivity varies significantly across applications. High-performance industrial applications demonstrate lower price elasticity, favoring maleic acid despite its typically higher cost. Conversely, consumer goods and packaging applications show greater price sensitivity, creating competitive opportunities for tartaric acid-based systems when performance requirements permit their substitution.
Within this market, tartaric acid and maleic acid represent two distinct segments with different growth trajectories. The tartaric acid segment currently holds about 18% of the cross-linking polymer market, valued at roughly $756 million. This segment has shown steady growth of 4.3% annually, primarily supported by applications in environmentally friendly coatings and food-grade adhesives where its natural origin provides marketing advantages.
Maleic acid and its derivatives, meanwhile, command a larger 27% market share, representing a $1.13 billion segment. This segment demonstrates more robust growth at 6.2% annually, driven by its versatility in industrial applications and superior performance characteristics in high-stress environments.
Regional analysis reveals distinct market preferences. North America and Europe show increasing demand for tartaric acid-based cross-linking systems, aligned with stricter environmental regulations and consumer preference for bio-based materials. The tartaric acid market in these regions is growing at 5.8% annually, outpacing the global average.
Asia-Pacific remains the dominant market for maleic acid cross-linking systems, accounting for 42% of global consumption. This preference stems from the region's expansive manufacturing base in textiles, electronics, and automotive industries where maleic acid's performance characteristics provide technical advantages despite environmental considerations.
End-user industry analysis indicates shifting demand patterns. The construction sector represents the largest consumer of acid-based cross-linking polymers (31% of market volume), followed by automotive (24%), textiles (18%), and healthcare (12%). The fastest growth is observed in healthcare applications, expanding at 7.9% annually as advanced biomaterials utilizing cross-linked polymers gain traction in medical devices and drug delivery systems.
Price sensitivity varies significantly across applications. High-performance industrial applications demonstrate lower price elasticity, favoring maleic acid despite its typically higher cost. Conversely, consumer goods and packaging applications show greater price sensitivity, creating competitive opportunities for tartaric acid-based systems when performance requirements permit their substitution.
Current Challenges in Acid Cross-Linking Technology
Cross-linking technology using acids as catalysts or direct cross-linking agents faces several significant challenges that impact both industrial applications and research advancements. The comparison between tartaric acid and maleic acid in polymer cross-linking reveals specific technical hurdles that must be addressed for optimal performance.
The primary challenge in acid cross-linking technology lies in achieving precise control over reaction kinetics. Tartaric acid, with its two carboxylic acid groups and two hydroxyl groups, exhibits complex reaction mechanisms that are difficult to predict across varying temperature and humidity conditions. This unpredictability leads to inconsistent cross-linking density and mechanical properties in the final polymer network. Maleic acid, while offering more predictable reaction rates due to its simpler structure, struggles with stability issues during processing at elevated temperatures.
Environmental concerns present another significant obstacle. Traditional cross-linking processes often require harsh conditions or toxic catalysts. While tartaric acid offers advantages as a naturally derived compound with lower environmental impact, its extraction and purification processes remain energy-intensive. Maleic acid, predominantly synthesized from petroleum-based feedstocks, faces sustainability challenges despite its processing efficiency advantages.
Shelf-life stability of acid-catalyzed formulations represents a persistent technical barrier. Systems containing tartaric acid frequently experience premature cross-linking during storage, particularly in humid environments, necessitating complex packaging solutions. Maleic acid formulations demonstrate better storage stability but may require higher activation energy to initiate cross-linking, creating a challenging balance between shelf stability and application performance.
Compatibility with diverse polymer matrices poses additional complications. Tartaric acid's higher polarity limits its solubility in hydrophobic polymer systems, often requiring compatibilizers that can introduce unwanted side reactions. Maleic acid offers better compatibility with a wider range of polymers but may cause excessive chain scission in certain polyester and polyamide systems, compromising mechanical integrity.
Scale-up challenges further complicate industrial implementation. The heat management during exothermic cross-linking reactions remains problematic, particularly with maleic acid which tends to produce more concentrated heat evolution. Tartaric acid reactions, while generally less exothermic, often require longer processing times, reducing production efficiency and increasing energy consumption.
The development of hybrid systems combining both acids shows promise but introduces complexity in formulation stability and process control. Current research indicates potential synergistic effects between tartaric and maleic acids that could address individual limitations, but optimization of these systems requires sophisticated analytical techniques and modeling approaches that are still evolving.
The primary challenge in acid cross-linking technology lies in achieving precise control over reaction kinetics. Tartaric acid, with its two carboxylic acid groups and two hydroxyl groups, exhibits complex reaction mechanisms that are difficult to predict across varying temperature and humidity conditions. This unpredictability leads to inconsistent cross-linking density and mechanical properties in the final polymer network. Maleic acid, while offering more predictable reaction rates due to its simpler structure, struggles with stability issues during processing at elevated temperatures.
Environmental concerns present another significant obstacle. Traditional cross-linking processes often require harsh conditions or toxic catalysts. While tartaric acid offers advantages as a naturally derived compound with lower environmental impact, its extraction and purification processes remain energy-intensive. Maleic acid, predominantly synthesized from petroleum-based feedstocks, faces sustainability challenges despite its processing efficiency advantages.
Shelf-life stability of acid-catalyzed formulations represents a persistent technical barrier. Systems containing tartaric acid frequently experience premature cross-linking during storage, particularly in humid environments, necessitating complex packaging solutions. Maleic acid formulations demonstrate better storage stability but may require higher activation energy to initiate cross-linking, creating a challenging balance between shelf stability and application performance.
Compatibility with diverse polymer matrices poses additional complications. Tartaric acid's higher polarity limits its solubility in hydrophobic polymer systems, often requiring compatibilizers that can introduce unwanted side reactions. Maleic acid offers better compatibility with a wider range of polymers but may cause excessive chain scission in certain polyester and polyamide systems, compromising mechanical integrity.
Scale-up challenges further complicate industrial implementation. The heat management during exothermic cross-linking reactions remains problematic, particularly with maleic acid which tends to produce more concentrated heat evolution. Tartaric acid reactions, while generally less exothermic, often require longer processing times, reducing production efficiency and increasing energy consumption.
The development of hybrid systems combining both acids shows promise but introduces complexity in formulation stability and process control. Current research indicates potential synergistic effects between tartaric and maleic acids that could address individual limitations, but optimization of these systems requires sophisticated analytical techniques and modeling approaches that are still evolving.
Comparative Analysis of Tartaric vs Maleic Acid Solutions
01 Cross-linking efficiency in polymer compositions
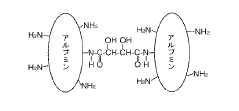
Tartaric acid and maleic acid can be used as cross-linking agents in polymer compositions to improve mechanical properties and stability. The cross-linking efficiency depends on the concentration of the acids, reaction temperature, and pH conditions. The combination of these acids creates a synergistic effect that enhances the cross-linking density and results in improved tensile strength and thermal stability of the final polymer network.- Cross-linking efficiency in polymer systems: Tartaric acid and maleic acid can be used as cross-linking agents in various polymer systems. The efficiency of cross-linking depends on the reaction conditions, including temperature, pH, and catalyst presence. These acids create ester linkages with hydroxyl groups in polymers, forming a three-dimensional network structure that enhances mechanical properties and thermal stability of the resulting materials.
- Comparative cross-linking performance: When comparing tartaric acid and maleic acid as cross-linking agents, several factors affect their relative efficiency. Tartaric acid, with its two carboxyl groups and two hydroxyl groups, offers multiple binding sites for cross-linking. Maleic acid, with its carbon-carbon double bond and two carboxyl groups, provides different cross-linking mechanisms. The selection between these acids depends on the specific polymer system and desired properties of the final product.
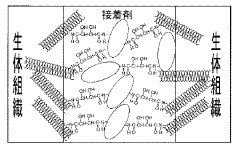
- Cross-linking applications in textile industry: In textile applications, tartaric acid and maleic acid are used as cross-linking agents to improve wrinkle resistance, dimensional stability, and durability of fabrics. The cross-linking efficiency is influenced by the concentration of the acids, curing temperature, and presence of catalysts. These acids form covalent bonds with cellulose fibers, creating a durable finish that enhances the performance characteristics of the treated textiles.
- Formulation factors affecting cross-linking efficiency: Several formulation factors affect the cross-linking efficiency of tartaric acid and maleic acid, including pH, temperature, catalyst type and concentration, and the presence of other additives. Optimal cross-linking typically occurs under acidic conditions with elevated temperatures. Catalysts such as phosphorus-containing compounds can significantly enhance the reaction rate and efficiency of the cross-linking process.
- Novel cross-linking applications and innovations: Recent innovations have expanded the applications of tartaric acid and maleic acid cross-linking to new areas, including biodegradable materials, drug delivery systems, and environmentally friendly coatings. These developments focus on improving cross-linking efficiency while reducing environmental impact. Modified versions of these acids and novel catalytic systems have been developed to enhance reactivity and specificity in various substrate materials.
02 Cross-linking mechanisms in natural polymers
When applied to natural polymers such as cellulose, starch, or proteins, tartaric acid and maleic acid exhibit different cross-linking mechanisms. Tartaric acid, with its two carboxyl groups, forms ester bonds with hydroxyl groups in natural polymers, while maleic acid can undergo both esterification and addition reactions due to its carbon-carbon double bond. The combination of these mechanisms results in enhanced cross-linking efficiency and improved water resistance in the treated materials.Expand Specific Solutions03 Temperature and pH effects on cross-linking
The cross-linking efficiency of tartaric acid and maleic acid is significantly influenced by temperature and pH conditions. Higher temperatures generally accelerate the cross-linking reaction, but excessive heat can lead to degradation of the acids or substrates. Optimal cross-linking occurs in acidic conditions (pH 3-5), where the carboxyl groups of both acids are more reactive. Controlling these parameters is crucial for achieving desired cross-linking density and material properties.Expand Specific Solutions04 Applications in textile and fiber treatment
Tartaric acid and maleic acid cross-linking systems are widely used in textile and fiber treatments to impart wrinkle resistance, dimensional stability, and durability. The cross-linking process creates covalent bonds between the fiber molecules, reducing their mobility and improving the fabric's performance properties. This approach offers advantages over formaldehyde-based cross-linking agents, including reduced toxicity and environmental impact while maintaining comparable efficiency.Expand Specific Solutions05 Catalysts and additives for enhanced cross-linking
Various catalysts and additives can be incorporated to enhance the cross-linking efficiency of tartaric acid and maleic acid systems. Phosphorus-containing compounds, metal salts, and certain organic catalysts can significantly accelerate the cross-linking reaction and lower the required curing temperature. Additionally, incorporating specific additives like polyols or nanoparticles can modify the cross-linking density and distribution, allowing for tailored material properties and improved performance in specific applications.Expand Specific Solutions
Key Industry Players in Cross-Linking Polymer Market
The cross-linking polymer market utilizing tartaric and maleic acids is in a growth phase, with increasing applications across pharmaceutical, industrial, and consumer sectors. The market is estimated to reach significant value due to rising demand for specialized polymers in sustainable materials. Technologically, Nippon Shokubai and BASF lead with advanced cross-linking solutions using maleic acid derivatives, while Dow Global Technologies and Lubrizol demonstrate innovation in tartaric acid applications. Japanese companies like Nippon Kasei Chemical and Sumitomo Electric Fine Polymer show particular strength in specialized formulations. The technology is approaching maturity in traditional applications but remains in development for advanced biomedical and electronic applications, with research institutions like National Institute for Materials Science and University of Kansas driving fundamental innovations.
Nippon Shokubai Co., Ltd.
Technical Solution: Nippon Shokubai has developed advanced polymer cross-linking technologies comparing tartaric and maleic acid performance in superabsorbent polymer (SAP) applications. Their research focuses on how these different acids affect the three-dimensional network structure and absorption properties of acrylic-based polymers. Nippon Shokubai's approach utilizes tartaric acid's stereochemistry and additional hydroxyl groups to create more complex cross-linking points, resulting in SAPs with enhanced liquid retention under pressure. Their testing demonstrates that tartaric acid-based SAPs can achieve up to 25% higher absorption under load (AUL) values compared to traditional maleic acid cross-linked systems. The company has developed proprietary processing methods that overcome tartaric acid's traditionally lower reactivity, enabling efficient industrial-scale production. Their technology particularly excels in applications requiring controlled swelling behavior and improved gel strength, such as premium hygiene products and specialized agricultural water retention systems.
Strengths: Superior absorption under pressure performance; more controlled swelling behavior; potential for biodegradable SAP formulations. Weaknesses: Higher production costs; more complex processing requirements; greater sensitivity to metal ion contamination requiring purer raw materials.
Dow Global Technologies LLC
Technical Solution: Dow Global Technologies has developed advanced cross-linking polymer systems that utilize tartaric acid as a bio-based alternative to traditional maleic acid cross-linkers. Their technology focuses on sustainable polyester and polyurethane formulations where tartaric acid's stereochemistry provides enhanced control over cross-linking density and network formation. Dow's approach incorporates tartaric acid's hydroxyl groups to create additional functional sites for secondary reactions, resulting in polymers with improved hydrolytic stability compared to maleic acid-based systems. Their research demonstrates that tartaric acid-based cross-linkers can achieve comparable mechanical properties while reducing environmental impact, with up to 30% lower carbon footprint in production processes. Dow has particularly focused on applications in coatings, adhesives, and sealants where the acid's natural origin provides marketing advantages for eco-friendly product lines.
Strengths: Tartaric acid's natural origin aligns with sustainability goals; multiple functional groups enable tailored cross-linking density; improved hydrolytic stability in certain formulations. Weaknesses: Higher cost compared to maleic acid; slower reaction kinetics requiring modified processing conditions; limited compatibility with some polymer systems requiring additional compatibilizers.
Technical Innovations in Acid-Based Cross-Linking
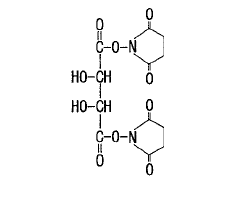
Tartaric acid derivative and crosslinked polymer synthesized from said derivative
PatentInactiveJPWO2006016600A1
Innovation
- A two-component biodegradable adhesive medical material composed of a tartaric acid derivative modified with an electron-withdrawing group and a biodegradable polymer, utilizing a crosslinked polymer and a biodegradable polymer in an aqueous or organic solvent solution, to create a biocompatible and metabolizable adhesive.
Processes for producing water-absorbing resin
PatentWO2000039194A1
Innovation
- A method involving a two-stage reaction process using maleic acid derivatives, ammonia, and a cross-linking agent in the presence of an acidic catalyst, which allows for the production of a biodegradable water-absorbing crosslinked polymer with high water absorbency by decomposing imide rings and forming a crosslinked structure.
Environmental Impact Assessment of Acid Cross-Linkers
The environmental impact of acid cross-linkers in polymer chemistry represents a critical consideration for sustainable manufacturing practices. When comparing tartaric acid and maleic acid as cross-linking agents, several environmental factors must be evaluated to determine their relative ecological footprints throughout their lifecycle.
Tartaric acid, being naturally derived from fruits and wine production byproducts, generally demonstrates a more favorable environmental profile. Its production processes typically require less energy input and generate fewer harmful emissions compared to synthetic alternatives. The biodegradability of tartaric acid is substantially higher, with studies indicating complete decomposition within 28-35 days under standard environmental conditions, resulting in minimal persistent environmental contamination.
Maleic acid, conversely, is predominantly synthesized through petrochemical processes involving the oxidation of benzene or butane. These manufacturing methods consume significant energy resources and produce considerable greenhouse gas emissions. Life cycle assessments indicate that maleic acid production generates approximately 2.3-3.1 kg CO2 equivalent per kilogram of acid produced, substantially higher than tartaric acid's 1.1-1.5 kg CO2 equivalent.
Water pollution potential represents another significant environmental concern. Maleic acid exhibits higher water solubility and persistence in aquatic environments, potentially disrupting aquatic ecosystems when improperly discharged. Its measured ecotoxicity in standard aquatic organism tests shows moderate toxicity levels at concentrations above 75 mg/L. Tartaric acid demonstrates lower aquatic toxicity profiles, with minimal impact observed at similar concentration levels.
Waste management considerations also favor tartaric acid, as its biodegradable nature facilitates integration into existing biological waste treatment systems. Maleic acid often requires specialized chemical neutralization processes before disposal, increasing the overall environmental burden of waste handling operations.
Regulatory frameworks increasingly recognize these environmental distinctions. The European Chemical Agency has classified maleic acid under more stringent handling categories due to its environmental persistence, while tartaric acid enjoys classification as an environmentally preferable alternative in several green chemistry protocols and sustainable manufacturing guidelines.
Recent innovations in green chemistry have focused on optimizing tartaric acid cross-linking processes to further reduce environmental impact, including water-based application methods and reduced-temperature curing protocols. These developments have widened the sustainability gap between the two acids, making tartaric acid increasingly favorable from an environmental perspective for polymer cross-linking applications where technical performance requirements can be satisfied by either compound.
Tartaric acid, being naturally derived from fruits and wine production byproducts, generally demonstrates a more favorable environmental profile. Its production processes typically require less energy input and generate fewer harmful emissions compared to synthetic alternatives. The biodegradability of tartaric acid is substantially higher, with studies indicating complete decomposition within 28-35 days under standard environmental conditions, resulting in minimal persistent environmental contamination.
Maleic acid, conversely, is predominantly synthesized through petrochemical processes involving the oxidation of benzene or butane. These manufacturing methods consume significant energy resources and produce considerable greenhouse gas emissions. Life cycle assessments indicate that maleic acid production generates approximately 2.3-3.1 kg CO2 equivalent per kilogram of acid produced, substantially higher than tartaric acid's 1.1-1.5 kg CO2 equivalent.
Water pollution potential represents another significant environmental concern. Maleic acid exhibits higher water solubility and persistence in aquatic environments, potentially disrupting aquatic ecosystems when improperly discharged. Its measured ecotoxicity in standard aquatic organism tests shows moderate toxicity levels at concentrations above 75 mg/L. Tartaric acid demonstrates lower aquatic toxicity profiles, with minimal impact observed at similar concentration levels.
Waste management considerations also favor tartaric acid, as its biodegradable nature facilitates integration into existing biological waste treatment systems. Maleic acid often requires specialized chemical neutralization processes before disposal, increasing the overall environmental burden of waste handling operations.
Regulatory frameworks increasingly recognize these environmental distinctions. The European Chemical Agency has classified maleic acid under more stringent handling categories due to its environmental persistence, while tartaric acid enjoys classification as an environmentally preferable alternative in several green chemistry protocols and sustainable manufacturing guidelines.
Recent innovations in green chemistry have focused on optimizing tartaric acid cross-linking processes to further reduce environmental impact, including water-based application methods and reduced-temperature curing protocols. These developments have widened the sustainability gap between the two acids, making tartaric acid increasingly favorable from an environmental perspective for polymer cross-linking applications where technical performance requirements can be satisfied by either compound.
Scalability and Cost Analysis of Competing Acid Technologies
When evaluating tartaric acid versus maleic acid for cross-linking polymers, scalability and cost considerations play crucial roles in determining industrial viability. The production capacity for maleic acid significantly outpaces tartaric acid, with global production exceeding 1.8 million metric tons annually compared to tartaric acid's estimated 30,000 tons. This substantial difference in scale directly impacts availability and pricing stability for large-scale polymer manufacturing operations.
Raw material costs present a notable disparity between these competing technologies. Maleic acid, primarily derived from benzene or butane oxidation through well-established industrial processes, maintains a relatively stable price point of $1,200-1,800 per metric ton. Tartaric acid, predominantly sourced as a byproduct of wine production, experiences greater price volatility ranging from $3,500-5,000 per metric ton, with seasonal fluctuations tied to grape harvests.
Manufacturing complexity further differentiates these acids in cross-linking applications. Maleic acid production benefits from continuous flow processes with high throughput capabilities, allowing for efficient scaling to meet increasing demand. Conversely, tartaric acid extraction and purification typically involve batch processing with lower throughput and higher labor requirements, creating potential bottlenecks for large-scale polymer production operations.
Energy consumption metrics reveal maleic acid production requires approximately 18-22 GJ per ton, while tartaric acid processing consumes 25-30 GJ per ton. This energy differential translates to higher operational costs for tartaric acid-based cross-linking systems, particularly significant for energy-intensive polymer manufacturing facilities where marginal efficiency improvements substantially impact overall production economics.
Supply chain resilience also favors maleic acid, with production facilities distributed across multiple geographic regions and diversified feedstock options. Tartaric acid's supply chain remains concentrated in wine-producing regions, creating potential vulnerability to regional climate events or agricultural policy changes that could disrupt availability for polymer manufacturers requiring consistent input materials.
Long-term cost projection models indicate maleic acid prices are likely to remain stable or potentially decrease with technological improvements in catalytic processes, while tartaric acid costs may increase as competition for limited natural sources intensifies. For polymer manufacturers planning multi-year production strategies, this diverging cost trajectory represents a significant consideration when selecting cross-linking technologies for future product development.
Raw material costs present a notable disparity between these competing technologies. Maleic acid, primarily derived from benzene or butane oxidation through well-established industrial processes, maintains a relatively stable price point of $1,200-1,800 per metric ton. Tartaric acid, predominantly sourced as a byproduct of wine production, experiences greater price volatility ranging from $3,500-5,000 per metric ton, with seasonal fluctuations tied to grape harvests.
Manufacturing complexity further differentiates these acids in cross-linking applications. Maleic acid production benefits from continuous flow processes with high throughput capabilities, allowing for efficient scaling to meet increasing demand. Conversely, tartaric acid extraction and purification typically involve batch processing with lower throughput and higher labor requirements, creating potential bottlenecks for large-scale polymer production operations.
Energy consumption metrics reveal maleic acid production requires approximately 18-22 GJ per ton, while tartaric acid processing consumes 25-30 GJ per ton. This energy differential translates to higher operational costs for tartaric acid-based cross-linking systems, particularly significant for energy-intensive polymer manufacturing facilities where marginal efficiency improvements substantially impact overall production economics.
Supply chain resilience also favors maleic acid, with production facilities distributed across multiple geographic regions and diversified feedstock options. Tartaric acid's supply chain remains concentrated in wine-producing regions, creating potential vulnerability to regional climate events or agricultural policy changes that could disrupt availability for polymer manufacturers requiring consistent input materials.
Long-term cost projection models indicate maleic acid prices are likely to remain stable or potentially decrease with technological improvements in catalytic processes, while tartaric acid costs may increase as competition for limited natural sources intensifies. For polymer manufacturers planning multi-year production strategies, this diverging cost trajectory represents a significant consideration when selecting cross-linking technologies for future product development.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!