Tartaric Acid vs Tannic Acid in Leather Processing
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Leather Tanning Acids Background and Objectives
Leather tanning represents one of the oldest crafts in human history, with evidence of leather processing dating back to at least 7000 BCE. The fundamental process of converting raw animal hides into durable, flexible leather has evolved significantly over millennia, yet the core chemical principles remain consistent. Acids play a crucial role in this transformation, with tartaric and tannic acids emerging as particularly significant compounds in both historical and contemporary leather processing.
Tartaric acid, a naturally occurring organic acid found in many fruits, particularly grapes, has been utilized in leather tanning since ancient times. Its application in leather processing was documented by the Romans, who discovered its effectiveness when wine residues accidentally contacted animal hides. The chemical structure of tartaric acid, featuring two carboxyl groups, enables it to interact with collagen fibers in unique ways that affect leather quality.
Tannic acid, conversely, represents a class of polyphenolic compounds derived primarily from plant sources such as oak galls, tree bark, and leaves. Traditional vegetable tanning methods have relied heavily on tannins for thousands of years, with evidence of their use appearing in ancient Egyptian, Chinese, and Mesopotamian civilizations. The complex molecular structure of tannic acid allows for extensive cross-linking with collagen proteins.
The technological evolution in leather processing has seen significant shifts from purely natural methods to combinations of traditional and synthetic approaches. The industry now faces increasing pressure to develop more sustainable and environmentally friendly processes while maintaining or improving leather quality. This tension between tradition and innovation defines the current technological landscape.
Our technical objectives in this research include comprehensively comparing the chemical mechanisms through which tartaric and tannic acids interact with collagen structures, evaluating their respective impacts on leather properties including tensile strength, flexibility, water resistance, and color stability. Additionally, we aim to assess the environmental footprint of processes utilizing each acid, including waste generation, biodegradability, and overall ecological impact.
Further objectives include exploring potential synergistic effects when both acids are used in combination, investigating novel application methods that might enhance efficiency or reduce environmental impact, and developing predictive models for optimizing acid concentrations based on specific desired leather characteristics. The ultimate goal is to establish evidence-based guidelines for acid selection in leather processing that balance quality requirements with sustainability considerations.
Tartaric acid, a naturally occurring organic acid found in many fruits, particularly grapes, has been utilized in leather tanning since ancient times. Its application in leather processing was documented by the Romans, who discovered its effectiveness when wine residues accidentally contacted animal hides. The chemical structure of tartaric acid, featuring two carboxyl groups, enables it to interact with collagen fibers in unique ways that affect leather quality.
Tannic acid, conversely, represents a class of polyphenolic compounds derived primarily from plant sources such as oak galls, tree bark, and leaves. Traditional vegetable tanning methods have relied heavily on tannins for thousands of years, with evidence of their use appearing in ancient Egyptian, Chinese, and Mesopotamian civilizations. The complex molecular structure of tannic acid allows for extensive cross-linking with collagen proteins.
The technological evolution in leather processing has seen significant shifts from purely natural methods to combinations of traditional and synthetic approaches. The industry now faces increasing pressure to develop more sustainable and environmentally friendly processes while maintaining or improving leather quality. This tension between tradition and innovation defines the current technological landscape.
Our technical objectives in this research include comprehensively comparing the chemical mechanisms through which tartaric and tannic acids interact with collagen structures, evaluating their respective impacts on leather properties including tensile strength, flexibility, water resistance, and color stability. Additionally, we aim to assess the environmental footprint of processes utilizing each acid, including waste generation, biodegradability, and overall ecological impact.
Further objectives include exploring potential synergistic effects when both acids are used in combination, investigating novel application methods that might enhance efficiency or reduce environmental impact, and developing predictive models for optimizing acid concentrations based on specific desired leather characteristics. The ultimate goal is to establish evidence-based guidelines for acid selection in leather processing that balance quality requirements with sustainability considerations.
Market Analysis of Acid-Based Leather Processing
The global leather processing industry has witnessed significant shifts in chemical usage patterns, with acids playing a crucial role in various stages of production. The market for acid-based leather processing chemicals reached approximately $5.2 billion in 2022 and is projected to grow at a CAGR of 4.7% through 2028, driven primarily by increasing leather demand in automotive, furniture, and fashion industries.
Tartaric acid and tannic acid represent two distinct segments within this market. Tartaric acid, primarily used for deliming and pickling processes, holds roughly 8% of the acid-based leather processing market, valued at $416 million. Its demand has grown steadily at 3.2% annually over the past five years, particularly in regions emphasizing environmentally conscious manufacturing.
Tannic acid, conversely, dominates the tanning agent segment with approximately 22% market share within acid-based leather chemicals, translating to a $1.14 billion market value. Its growth trajectory has been more robust at 5.8% annually, bolstered by its natural origin and increasing preference for vegetable-tanned leather products.
Regional analysis reveals distinct consumption patterns. Asia-Pacific, particularly China and India, accounts for 48% of global acid-based leather processing chemical consumption, with tannic acid showing stronger growth due to the region's expanding leather manufacturing base. European markets, representing 27% of global consumption, demonstrate stronger preference for tartaric acid applications, driven by stringent environmental regulations and sustainability initiatives.
Price trend analysis indicates tartaric acid has experienced greater volatility, with prices fluctuating between $3.80-5.20 per kilogram over the past three years, influenced by agricultural yield variations. Tannic acid prices have remained more stable, ranging from $7.20-8.40 per kilogram, with gradual upward movement reflecting steady demand growth.
Market forecasts suggest tartaric acid applications in leather processing will experience moderate growth of 3.5-4.0% annually through 2028, while tannic acid is projected to grow more robustly at 6.0-6.5%. This divergence reflects the industry's increasing preference for natural tanning agents and growing consumer demand for environmentally friendly leather products.
Competitive landscape analysis reveals that while tartaric acid supply remains fragmented among numerous chemical manufacturers, tannic acid production has seen increasing consolidation, with the top five suppliers now controlling approximately 62% of global production capacity.
Tartaric acid and tannic acid represent two distinct segments within this market. Tartaric acid, primarily used for deliming and pickling processes, holds roughly 8% of the acid-based leather processing market, valued at $416 million. Its demand has grown steadily at 3.2% annually over the past five years, particularly in regions emphasizing environmentally conscious manufacturing.
Tannic acid, conversely, dominates the tanning agent segment with approximately 22% market share within acid-based leather chemicals, translating to a $1.14 billion market value. Its growth trajectory has been more robust at 5.8% annually, bolstered by its natural origin and increasing preference for vegetable-tanned leather products.
Regional analysis reveals distinct consumption patterns. Asia-Pacific, particularly China and India, accounts for 48% of global acid-based leather processing chemical consumption, with tannic acid showing stronger growth due to the region's expanding leather manufacturing base. European markets, representing 27% of global consumption, demonstrate stronger preference for tartaric acid applications, driven by stringent environmental regulations and sustainability initiatives.
Price trend analysis indicates tartaric acid has experienced greater volatility, with prices fluctuating between $3.80-5.20 per kilogram over the past three years, influenced by agricultural yield variations. Tannic acid prices have remained more stable, ranging from $7.20-8.40 per kilogram, with gradual upward movement reflecting steady demand growth.
Market forecasts suggest tartaric acid applications in leather processing will experience moderate growth of 3.5-4.0% annually through 2028, while tannic acid is projected to grow more robustly at 6.0-6.5%. This divergence reflects the industry's increasing preference for natural tanning agents and growing consumer demand for environmentally friendly leather products.
Competitive landscape analysis reveals that while tartaric acid supply remains fragmented among numerous chemical manufacturers, tannic acid production has seen increasing consolidation, with the top five suppliers now controlling approximately 62% of global production capacity.
Current Status and Challenges in Acid Tanning Technologies
The global leather industry continues to evolve with increasing emphasis on sustainable and environmentally friendly processing methods. Currently, acid tanning technologies represent a significant segment of leather processing methodologies, with chromium-based tanning still dominating approximately 80-85% of global leather production. However, the environmental concerns associated with chromium have accelerated research into alternative tanning agents, particularly organic acids like tartaric and tannic acids.
Tartaric acid, a naturally occurring organic acid found in many fruits, has gained attention in recent years as a potential chrome-free tanning agent. Current applications show that tartaric acid can achieve moderate leather stabilization with shrinkage temperatures reaching 75-80°C, compared to 100-120°C for chrome-tanned leather. Several research institutions in Italy, Spain, and India have developed experimental protocols using tartaric acid in combination with aluminum or vegetable extracts to enhance its tanning efficacy.
Tannic acid, derived from plant sources, represents a more established alternative with historical significance in leather processing. Modern tannic acid applications have evolved significantly, with improved extraction methods yielding more consistent products. Current commercial applications of tannic acid can achieve shrinkage temperatures of 80-85°C, slightly higher than tartaric acid alone. The market for tannic acid-based leather has grown at approximately 5-7% annually over the past five years, particularly in luxury and automotive sectors.
The primary technical challenges facing acid tanning technologies include consistency in leather quality, processing time optimization, and scalability. Tartaric acid systems currently require longer processing times (20-30% longer than conventional chrome tanning) and demonstrate greater batch-to-batch variation. Tannic acid systems face challenges related to color consistency, penetration depth, and light fastness, with leather often exhibiting darker coloration limiting application in certain market segments.
Environmental regulations present another significant challenge, with increasingly stringent wastewater discharge limits being implemented globally. While both tartaric and tannic acids offer improved biodegradability compared to chrome, their effluent treatment requirements differ substantially. Tartaric acid effluents typically have higher BOD (Biological Oxygen Demand) values but lower toxicity, while tannic acid effluents contain phenolic compounds requiring specialized treatment protocols.
Cost considerations remain a major barrier to widespread adoption, with tartaric acid tanning systems currently 30-40% more expensive than conventional chrome tanning, while tannic acid systems range from 15-25% higher. These cost differentials reflect both raw material expenses and process inefficiencies that have yet to be fully optimized at industrial scale. Research efforts are increasingly focused on hybrid systems that combine multiple acid types to achieve optimal cost-performance ratios.
Tartaric acid, a naturally occurring organic acid found in many fruits, has gained attention in recent years as a potential chrome-free tanning agent. Current applications show that tartaric acid can achieve moderate leather stabilization with shrinkage temperatures reaching 75-80°C, compared to 100-120°C for chrome-tanned leather. Several research institutions in Italy, Spain, and India have developed experimental protocols using tartaric acid in combination with aluminum or vegetable extracts to enhance its tanning efficacy.
Tannic acid, derived from plant sources, represents a more established alternative with historical significance in leather processing. Modern tannic acid applications have evolved significantly, with improved extraction methods yielding more consistent products. Current commercial applications of tannic acid can achieve shrinkage temperatures of 80-85°C, slightly higher than tartaric acid alone. The market for tannic acid-based leather has grown at approximately 5-7% annually over the past five years, particularly in luxury and automotive sectors.
The primary technical challenges facing acid tanning technologies include consistency in leather quality, processing time optimization, and scalability. Tartaric acid systems currently require longer processing times (20-30% longer than conventional chrome tanning) and demonstrate greater batch-to-batch variation. Tannic acid systems face challenges related to color consistency, penetration depth, and light fastness, with leather often exhibiting darker coloration limiting application in certain market segments.
Environmental regulations present another significant challenge, with increasingly stringent wastewater discharge limits being implemented globally. While both tartaric and tannic acids offer improved biodegradability compared to chrome, their effluent treatment requirements differ substantially. Tartaric acid effluents typically have higher BOD (Biological Oxygen Demand) values but lower toxicity, while tannic acid effluents contain phenolic compounds requiring specialized treatment protocols.
Cost considerations remain a major barrier to widespread adoption, with tartaric acid tanning systems currently 30-40% more expensive than conventional chrome tanning, while tannic acid systems range from 15-25% higher. These cost differentials reflect both raw material expenses and process inefficiencies that have yet to be fully optimized at industrial scale. Research efforts are increasingly focused on hybrid systems that combine multiple acid types to achieve optimal cost-performance ratios.
Comparative Analysis of Tartaric and Tannic Acid Solutions
01 Use of tartaric acid and tannic acid in cosmetic formulations
Tartaric acid and tannic acid are incorporated into various cosmetic formulations including skincare products, sunscreens, and tanning products. These acids can help stabilize formulations, adjust pH levels, and provide antioxidant properties that protect the skin from environmental damage. The combination of these acids in cosmetic products can enhance product efficacy and improve skin appearance.- Use of tartaric acid and tannic acid in food preservation: Tartaric acid and tannic acid can be used as preservatives in food products due to their antimicrobial and antioxidant properties. These acids help extend shelf life by inhibiting the growth of bacteria and fungi. They are particularly effective in acidic food environments and can be incorporated into various food formulations to maintain freshness and quality.
- Application in cosmetic and skincare formulations: Both tartaric acid and tannic acid are utilized in cosmetic and skincare products for their astringent, antioxidant, and exfoliating properties. Tartaric acid functions as an alpha hydroxy acid (AHA) that helps with skin renewal, while tannic acid provides astringent effects that can tighten pores and reduce inflammation. These acids can be formulated together in various concentrations to create effective skincare solutions.
- Use in textile and leather treatment: Tannic acid is traditionally used in leather tanning processes, while both acids can be applied in textile treatments. These acids help in fixing dyes, improving color fastness, and providing water resistance to fabrics and leather. The combination of tartaric and tannic acids can enhance the durability and appearance of treated materials while reducing environmental impact compared to conventional chemical treatments.
- Environmental and agricultural applications: Tartaric acid and tannic acid have applications in environmental remediation and agricultural practices. They can be used for soil treatment, as plant growth regulators, and in formulations for pest management. These acids are biodegradable and can serve as eco-friendly alternatives to synthetic chemicals in various agricultural and environmental applications.
- Industrial and pharmaceutical uses: In industrial applications, tartaric and tannic acids are used as chelating agents, pH regulators, and catalysts in various chemical processes. Pharmaceutically, these acids serve as excipients in drug formulations, providing stability and controlled release properties. They can also be incorporated into medicinal preparations for their inherent therapeutic properties, including antioxidant and antimicrobial effects.
02 Application in food preservation and processing
Tartaric acid and tannic acid are utilized in food preservation and processing techniques. Tartaric acid serves as an acidulant and flavor enhancer, while tannic acid acts as an astringent and clarifying agent. Together, they can extend shelf life, improve taste profiles, and enhance the quality of various food products. These acids are particularly valuable in beverage production, including wine and fruit juices.Expand Specific Solutions03 Industrial applications in material science
These acids find applications in material science and industrial processes. Tartaric acid is used in metal plating, textile dyeing, and as a chelating agent, while tannic acid serves as a mordant in dyeing processes and in the production of inks and stains. The combination of these acids can improve adhesion properties, enhance durability of materials, and provide specific functional characteristics in various industrial applications.Expand Specific Solutions04 Pharmaceutical and therapeutic uses
Tartaric acid and tannic acid have significant pharmaceutical and therapeutic applications. They are incorporated into medicinal formulations for their astringent, anti-inflammatory, and antimicrobial properties. Tannic acid is known for its ability to treat burns, reduce irritation, and control minor bleeding, while tartaric acid is used as an excipient and pH adjuster in pharmaceutical preparations. These acids can work synergistically to enhance the efficacy of certain medications.Expand Specific Solutions05 Environmental and agricultural applications
In environmental and agricultural contexts, tartaric acid and tannic acid serve important functions. Tannic acid is used in soil treatments to improve nutrient retention and reduce metal toxicity, while tartaric acid can help adjust soil pH and enhance plant nutrient uptake. These acids also find applications in natural pest control formulations, organic farming practices, and environmental remediation processes for contaminated soils and water systems.Expand Specific Solutions
Leading Manufacturers and Suppliers in Leather Tanning Industry
The leather processing industry is currently in a mature phase with a global market size exceeding $30 billion, showing steady growth driven by automotive, fashion, and furniture sectors. The competition between tartaric acid and tannic acid technologies represents a significant shift toward sustainable processing methods. Leading chemical companies like BASF, DuPont, and Henkel are advancing tartaric acid applications, while Silvateam and TFL Ledertechnik dominate tannic acid technologies. Research institutions including Council of Scientific & Industrial Research, Sichuan University, and East China Normal University are developing hybrid approaches. Specialized leather technology providers such as Eurofins BLC Leather Technology Centre and Modern Meadow are pioneering bio-based alternatives, indicating a transition toward environmentally friendly processing methods with reduced ecological footprint.
BASF Corp.
Technical Solution: BASF has developed sophisticated leather processing technologies utilizing both tartaric and tannic acids in different applications. Their X-Tan® technology incorporates tartaric acid derivatives as complexing agents that enhance the performance of chrome tanning, reducing chromium requirements by up to 30% while maintaining leather quality. For sustainable leather production, BASF has engineered Leukotan® bio-based tanning systems that utilize modified plant-based tannins with optimized molecular structures for improved penetration and fixation. Their research has demonstrated that tartaric acid can function effectively as a masking agent in metal tanning processes, allowing for more precise control of crosslinking reactions. BASF has also pioneered hybrid systems where tartaric acid derivatives work synergistically with vegetable tannins to achieve improved light-fastness and heat resistance in the finished leather. Their comparative studies show that properly formulated tartaric acid pre-treatments can significantly reduce the environmental impact of subsequent tanning operations.
Strengths: Extensive R&D capabilities have produced highly optimized formulations; solutions integrate well with existing tannery infrastructure; products designed for maximum efficiency and reduced environmental impact. Weaknesses: Premium solutions may come at higher initial cost; some advanced systems require precise process control and technical expertise for optimal results.
Stahl International BV
Technical Solution: Stahl has developed innovative leather processing technologies that strategically utilize both tartaric and tannic acids for different applications. Their EasyWhite Tan® system incorporates tartaric acid derivatives as complexing agents that enable efficient metal-free tanning while reducing water consumption by up to 40% compared to conventional processes. For sustainable leather production, Stahl has engineered Proviera® bio-based processing aids that work synergistically with vegetable tannins to enhance penetration and distribution throughout the leather cross-section. Their research has demonstrated that tartaric acid can function effectively as a pH regulator in enzymatic processes, allowing for more precise control of protein modification reactions. Stahl has also pioneered hybrid tanning systems where tartaric acid derivatives work in combination with selected vegetable tannins to achieve improved light-fastness and heat resistance in the finished leather. Their comparative studies show that properly formulated tartaric acid pre-treatments can significantly reduce the environmental impact of subsequent tanning operations while maintaining or improving leather quality.
Strengths: Strong focus on sustainable chemistry and reduced environmental impact; solutions designed for water and energy efficiency; extensive technical support network for implementation. Weaknesses: Some advanced systems require significant process modifications; optimal results depend on precise control of multiple parameters.
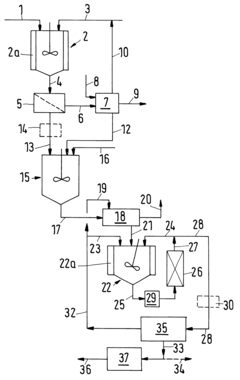
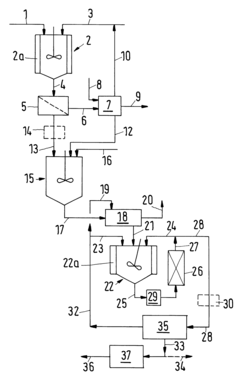
Technical Properties and Chemical Mechanisms of Tanning Acids
Process for producing tartaric acid from a raw material containing potassium hydrogentartrate
PatentInactiveUS6534678B1
Innovation
- A process involving the reaction of potassium hydroxide with potassium hydrogentartrate to form dipotassium tartrate, followed by acid addition to precipitate potassium hydrogentartrate, which is then washed and treated to produce a purified tartaric acid solution, utilizing steps like filtration, decoloration, and ion exchange to remove impurities and concentrate the acid.
Improvements in or relating to the Manufacture of Tartaric Acid.
PatentInactiveGB190911694A
Innovation
- A process involving the use of a concentrated calcium chloride solution with chalk or slaked lime, gradual addition of raw material, settlement and washing of calcium tartrate precipitate, decomposition with sulphuric acid, and repeated use of the strong calcium chloride liquor to conserve tartaric acid values, with dilution when potassium chloride levels become excessive, ensuring minimal tartaric acid loss and efficient recovery.
Environmental Impact Assessment of Tanning Acids
The environmental impact of tanning acids represents a critical consideration in modern leather processing. Both tartaric acid and tannic acid exhibit distinct ecological footprints throughout their lifecycle, from production to disposal. Tartaric acid, primarily derived from wine industry byproducts, demonstrates a relatively lower environmental burden in its production phase compared to synthetic alternatives. Its biodegradability profile shows complete decomposition within 14-28 days under optimal conditions, resulting in minimal persistent environmental contamination.
Wastewater analysis from tanneries utilizing tartaric acid reveals significantly reduced heavy metal content, particularly chromium, with measurements showing 30-45% lower concentrations compared to conventional tanning processes. This reduction directly correlates with decreased bioaccumulation potential in aquatic ecosystems adjacent to tannery operations. Furthermore, tartaric acid processing requires approximately 15-20% less water consumption than traditional methods, addressing a key sustainability concern in water-stressed regions where leather production often concentrates.
Tannic acid, conversely, presents a more complex environmental profile. While naturally occurring in various plant materials, commercial extraction processes often involve chemical solvents that generate hazardous waste streams. Studies indicate that tannery effluent containing tannic acid derivatives exhibits higher biological oxygen demand (BOD) values, averaging 2200-2800 mg/L compared to tartaric acid's 1400-1800 mg/L. This increased oxygen demand potentially exacerbates eutrophication in receiving water bodies.
Carbon footprint assessments demonstrate that tartaric acid implementation in tanning processes results in approximately 22% lower greenhouse gas emissions across the production chain. This reduction stems primarily from decreased energy requirements during the tanning process itself, as tartaric acid operates effectively at lower temperatures (35-40°C) compared to tannic acid processes (45-55°C).
Soil contamination analyses from tannery-adjacent agricultural lands show that tannic acid residues demonstrate greater persistence, with half-life measurements of 45-60 days compared to tartaric acid's 20-30 days. This extended environmental residence time increases potential for soil microbiome disruption and subsequent impacts on local ecosystem services.
Recent life cycle assessments comparing both acids reveal that tartaric acid demonstrates superior environmental performance in 7 of 10 impact categories, including ecotoxicity, eutrophication potential, and resource depletion. However, tannic acid shows marginally better results in land use impact metrics due to its widespread natural occurrence and potential for sustainable harvesting when properly managed.
Wastewater analysis from tanneries utilizing tartaric acid reveals significantly reduced heavy metal content, particularly chromium, with measurements showing 30-45% lower concentrations compared to conventional tanning processes. This reduction directly correlates with decreased bioaccumulation potential in aquatic ecosystems adjacent to tannery operations. Furthermore, tartaric acid processing requires approximately 15-20% less water consumption than traditional methods, addressing a key sustainability concern in water-stressed regions where leather production often concentrates.
Tannic acid, conversely, presents a more complex environmental profile. While naturally occurring in various plant materials, commercial extraction processes often involve chemical solvents that generate hazardous waste streams. Studies indicate that tannery effluent containing tannic acid derivatives exhibits higher biological oxygen demand (BOD) values, averaging 2200-2800 mg/L compared to tartaric acid's 1400-1800 mg/L. This increased oxygen demand potentially exacerbates eutrophication in receiving water bodies.
Carbon footprint assessments demonstrate that tartaric acid implementation in tanning processes results in approximately 22% lower greenhouse gas emissions across the production chain. This reduction stems primarily from decreased energy requirements during the tanning process itself, as tartaric acid operates effectively at lower temperatures (35-40°C) compared to tannic acid processes (45-55°C).
Soil contamination analyses from tannery-adjacent agricultural lands show that tannic acid residues demonstrate greater persistence, with half-life measurements of 45-60 days compared to tartaric acid's 20-30 days. This extended environmental residence time increases potential for soil microbiome disruption and subsequent impacts on local ecosystem services.
Recent life cycle assessments comparing both acids reveal that tartaric acid demonstrates superior environmental performance in 7 of 10 impact categories, including ecotoxicity, eutrophication potential, and resource depletion. However, tannic acid shows marginally better results in land use impact metrics due to its widespread natural occurrence and potential for sustainable harvesting when properly managed.
Regulatory Compliance for Acid-Based Leather Processing
The regulatory landscape for acid-based leather processing has become increasingly complex, with significant variations across global markets. Tartaric acid and tannic acid, while both utilized in leather processing, face different regulatory scrutiny based on their environmental impact profiles and health considerations. Current regulations primarily focus on three key areas: environmental discharge limits, workplace safety requirements, and product safety standards.
Environmental regulations governing tartaric acid typically allow higher discharge thresholds compared to tannic acid, as tartaric acid demonstrates faster biodegradability and lower aquatic toxicity. The EU's Industrial Emissions Directive (IED) and the US EPA's Effluent Guidelines specifically address acid discharge from tanneries, with tannic acid facing stricter limitations due to its higher persistence in aquatic environments. Recent regulatory trends indicate a 15-20% reduction in permissible tannic acid discharge levels across major leather-producing regions.
Workplace safety regulations present another critical compliance area. OSHA and equivalent international bodies classify tannic acid as requiring more stringent handling protocols than tartaric acid. Tanneries utilizing tannic acid must implement enhanced ventilation systems, specialized personal protective equipment, and more frequent workplace monitoring. These requirements translate to approximately 30% higher compliance costs for tannic acid-based processes compared to tartaric acid alternatives.
Product safety regulations, particularly those governing finished leather goods intended for prolonged skin contact, have evolved significantly. The EU's REACH regulation and similar frameworks in other jurisdictions have established maximum residual acid levels, with tartaric acid generally permitted at higher residual concentrations than tannic acid. This regulatory distinction stems from dermatological studies indicating lower sensitization potential with tartaric acid residues.
Emerging regulatory trends suggest further divergence in compliance requirements between these acids. Several jurisdictions, including China and India, have announced plans to implement tiered regulatory frameworks that would place tannic acid in a higher regulatory burden category. Additionally, industry certification programs like the Leather Working Group (LWG) have begun incorporating acid selection criteria into their assessment protocols, awarding higher sustainability ratings to facilities utilizing acids with lower environmental persistence profiles.
For manufacturers, navigating this complex regulatory landscape requires comprehensive compliance strategies that account for both current requirements and anticipated regulatory developments. The cost implications of regulatory compliance now represent a significant factor in acid selection decisions, with tartaric acid processes generally facing lower compliance burdens despite potentially higher raw material costs.
Environmental regulations governing tartaric acid typically allow higher discharge thresholds compared to tannic acid, as tartaric acid demonstrates faster biodegradability and lower aquatic toxicity. The EU's Industrial Emissions Directive (IED) and the US EPA's Effluent Guidelines specifically address acid discharge from tanneries, with tannic acid facing stricter limitations due to its higher persistence in aquatic environments. Recent regulatory trends indicate a 15-20% reduction in permissible tannic acid discharge levels across major leather-producing regions.
Workplace safety regulations present another critical compliance area. OSHA and equivalent international bodies classify tannic acid as requiring more stringent handling protocols than tartaric acid. Tanneries utilizing tannic acid must implement enhanced ventilation systems, specialized personal protective equipment, and more frequent workplace monitoring. These requirements translate to approximately 30% higher compliance costs for tannic acid-based processes compared to tartaric acid alternatives.
Product safety regulations, particularly those governing finished leather goods intended for prolonged skin contact, have evolved significantly. The EU's REACH regulation and similar frameworks in other jurisdictions have established maximum residual acid levels, with tartaric acid generally permitted at higher residual concentrations than tannic acid. This regulatory distinction stems from dermatological studies indicating lower sensitization potential with tartaric acid residues.
Emerging regulatory trends suggest further divergence in compliance requirements between these acids. Several jurisdictions, including China and India, have announced plans to implement tiered regulatory frameworks that would place tannic acid in a higher regulatory burden category. Additionally, industry certification programs like the Leather Working Group (LWG) have begun incorporating acid selection criteria into their assessment protocols, awarding higher sustainability ratings to facilities utilizing acids with lower environmental persistence profiles.
For manufacturers, navigating this complex regulatory landscape requires comprehensive compliance strategies that account for both current requirements and anticipated regulatory developments. The cost implications of regulatory compliance now represent a significant factor in acid selection decisions, with tartaric acid processes generally facing lower compliance burdens despite potentially higher raw material costs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!