The Applications of Fluoroantimonic Acid in Electrochemistry
JUN 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid in Electrochemistry: Background and Objectives
Fluoroantimonic acid, a superacid composed of a mixture of hydrogen fluoride (HF) and antimony pentafluoride (SbF5), has emerged as a subject of intense interest in the field of electrochemistry. This powerful acid, with its exceptional proton-donating ability, has opened new avenues for research and applications in various electrochemical processes.
The development of fluoroantimonic acid can be traced back to the mid-20th century when researchers began exploring superacids and their unique properties. Its extreme acidity, surpassing that of 100% sulfuric acid by many orders of magnitude, has made it a valuable tool in numerous chemical reactions and processes.
In the realm of electrochemistry, fluoroantimonic acid has garnered attention due to its potential to enhance electrode reactions, modify electrode surfaces, and facilitate novel electrocatalytic processes. The acid's ability to generate highly reactive species and stabilize unusual oxidation states of elements has led to its exploration in various electrochemical applications.
The evolution of fluoroantimonic acid in electrochemistry has been driven by the need for more efficient and selective electrochemical processes in industries such as energy storage, materials synthesis, and environmental remediation. As researchers continue to push the boundaries of electrochemical systems, the unique properties of fluoroantimonic acid offer promising avenues for innovation.
The primary objectives of investigating fluoroantimonic acid in electrochemistry include understanding its fundamental interactions with electrode materials, exploring its potential as an electrolyte or electrolyte additive, and developing novel electrochemical processes that leverage its extreme acidity. Researchers aim to harness its properties to overcome limitations in existing electrochemical systems and enable new reactions that were previously unfeasible.
Furthermore, the study of fluoroantimonic acid in electrochemistry seeks to elucidate the mechanisms by which it influences electron transfer processes, alters the kinetics of electrochemical reactions, and modifies the properties of electrode-electrolyte interfaces. These insights are crucial for designing more efficient and selective electrochemical systems for a wide range of applications.
As the field progresses, there is a growing interest in developing safer handling methods and more stable formulations of fluoroantimonic acid to facilitate its broader adoption in electrochemical research and industrial applications. The ultimate goal is to unlock the full potential of this superacid in advancing electrochemical technologies and contributing to the development of more sustainable and efficient processes across various sectors.
The development of fluoroantimonic acid can be traced back to the mid-20th century when researchers began exploring superacids and their unique properties. Its extreme acidity, surpassing that of 100% sulfuric acid by many orders of magnitude, has made it a valuable tool in numerous chemical reactions and processes.
In the realm of electrochemistry, fluoroantimonic acid has garnered attention due to its potential to enhance electrode reactions, modify electrode surfaces, and facilitate novel electrocatalytic processes. The acid's ability to generate highly reactive species and stabilize unusual oxidation states of elements has led to its exploration in various electrochemical applications.
The evolution of fluoroantimonic acid in electrochemistry has been driven by the need for more efficient and selective electrochemical processes in industries such as energy storage, materials synthesis, and environmental remediation. As researchers continue to push the boundaries of electrochemical systems, the unique properties of fluoroantimonic acid offer promising avenues for innovation.
The primary objectives of investigating fluoroantimonic acid in electrochemistry include understanding its fundamental interactions with electrode materials, exploring its potential as an electrolyte or electrolyte additive, and developing novel electrochemical processes that leverage its extreme acidity. Researchers aim to harness its properties to overcome limitations in existing electrochemical systems and enable new reactions that were previously unfeasible.
Furthermore, the study of fluoroantimonic acid in electrochemistry seeks to elucidate the mechanisms by which it influences electron transfer processes, alters the kinetics of electrochemical reactions, and modifies the properties of electrode-electrolyte interfaces. These insights are crucial for designing more efficient and selective electrochemical systems for a wide range of applications.
As the field progresses, there is a growing interest in developing safer handling methods and more stable formulations of fluoroantimonic acid to facilitate its broader adoption in electrochemical research and industrial applications. The ultimate goal is to unlock the full potential of this superacid in advancing electrochemical technologies and contributing to the development of more sustainable and efficient processes across various sectors.
Market Analysis for Electrochemical Applications
The market for electrochemical applications of fluoroantimonic acid is experiencing significant growth, driven by increasing demand in various industrial sectors. As the strongest known superacid, fluoroantimonic acid offers unique properties that make it valuable in electrochemical processes, particularly in the fields of energy storage, catalysis, and materials science.
In the energy storage sector, fluoroantimonic acid shows promise for enhancing the performance of advanced battery technologies. Its strong acidic properties can potentially improve electrode materials and electrolyte formulations, leading to higher energy density and faster charging capabilities. This aligns with the growing demand for more efficient energy storage solutions in electric vehicles and renewable energy systems.
The catalysis industry is another key market for fluoroantimonic acid's electrochemical applications. Its exceptional proton-donating ability makes it an attractive option for catalyzing various chemical reactions, particularly in the petrochemical and fine chemical industries. The acid's ability to activate certain compounds and facilitate challenging transformations could lead to more efficient and cost-effective production processes.
In materials science, fluoroantimonic acid's electrochemical properties are being explored for surface modification and etching applications. Its strong acidic nature allows for precise control over material surfaces, which is crucial in the semiconductor industry and in the development of advanced functional materials.
The market potential for fluoroantimonic acid in electrochemistry is further bolstered by ongoing research and development efforts. Academic institutions and industrial laboratories are actively investigating novel applications and seeking to overcome challenges associated with its handling and storage. This continuous innovation is likely to expand the acid's market reach and create new opportunities in emerging technologies.
However, the market growth is tempered by several factors. The extreme corrosiveness and reactivity of fluoroantimonic acid pose significant safety and handling challenges, necessitating specialized equipment and stringent safety protocols. This can increase operational costs and limit its adoption in certain applications. Additionally, environmental concerns and regulatory restrictions on the use of highly hazardous substances may impact market expansion in some regions.
Despite these challenges, the overall market trajectory for fluoroantimonic acid in electrochemical applications remains positive. The unique capabilities it offers in addressing complex chemical and materials challenges continue to drive interest across multiple industries. As research progresses and new applications emerge, the market is expected to diversify and expand, particularly in high-value, specialized applications where the acid's exceptional properties can provide significant advantages over conventional alternatives.
In the energy storage sector, fluoroantimonic acid shows promise for enhancing the performance of advanced battery technologies. Its strong acidic properties can potentially improve electrode materials and electrolyte formulations, leading to higher energy density and faster charging capabilities. This aligns with the growing demand for more efficient energy storage solutions in electric vehicles and renewable energy systems.
The catalysis industry is another key market for fluoroantimonic acid's electrochemical applications. Its exceptional proton-donating ability makes it an attractive option for catalyzing various chemical reactions, particularly in the petrochemical and fine chemical industries. The acid's ability to activate certain compounds and facilitate challenging transformations could lead to more efficient and cost-effective production processes.
In materials science, fluoroantimonic acid's electrochemical properties are being explored for surface modification and etching applications. Its strong acidic nature allows for precise control over material surfaces, which is crucial in the semiconductor industry and in the development of advanced functional materials.
The market potential for fluoroantimonic acid in electrochemistry is further bolstered by ongoing research and development efforts. Academic institutions and industrial laboratories are actively investigating novel applications and seeking to overcome challenges associated with its handling and storage. This continuous innovation is likely to expand the acid's market reach and create new opportunities in emerging technologies.
However, the market growth is tempered by several factors. The extreme corrosiveness and reactivity of fluoroantimonic acid pose significant safety and handling challenges, necessitating specialized equipment and stringent safety protocols. This can increase operational costs and limit its adoption in certain applications. Additionally, environmental concerns and regulatory restrictions on the use of highly hazardous substances may impact market expansion in some regions.
Despite these challenges, the overall market trajectory for fluoroantimonic acid in electrochemical applications remains positive. The unique capabilities it offers in addressing complex chemical and materials challenges continue to drive interest across multiple industries. As research progresses and new applications emerge, the market is expected to diversify and expand, particularly in high-value, specialized applications where the acid's exceptional properties can provide significant advantages over conventional alternatives.
Current State and Challenges in Fluoroantimonic Acid Usage
Fluoroantimonic acid, known as the world's strongest superacid, has garnered significant attention in the field of electrochemistry. Its current state of usage is characterized by both promising applications and substantial challenges. The acid's extreme acidity, with a Hammett acidity function estimated at -21.6, provides unique capabilities in various electrochemical processes.
In recent years, researchers have made notable progress in harnessing fluoroantimonic acid's potential for electrochemical applications. Its ability to protonate even extremely weak bases has led to its use in electrocatalysis, where it can activate inert substrates for electrochemical reactions. This property has shown particular promise in the development of novel electrocatalysts for fuel cells and water splitting technologies.
However, the widespread adoption of fluoroantimonic acid in electrochemistry faces several significant challenges. The foremost issue is its extreme corrosiveness, which necessitates specialized handling and storage equipment. This requirement substantially increases the cost and complexity of implementing fluoroantimonic acid-based processes in industrial settings.
Safety concerns also pose a major hurdle in the practical application of fluoroantimonic acid. Its highly reactive nature demands stringent safety protocols and specialized training for personnel, limiting its use to highly controlled laboratory environments. The acid's potential for severe chemical burns and its ability to generate toxic hydrogen fluoride gas upon contact with water further complicate its handling.
Another challenge lies in the acid's incompatibility with many common materials used in electrochemical setups. Traditional electrode materials and cell components often degrade rapidly when exposed to fluoroantimonic acid, necessitating the development of new, resistant materials. This requirement has spurred research into novel electrode coatings and cell designs, but progress in this area remains limited.
The environmental impact of fluoroantimonic acid usage is also a significant concern. Its production and disposal require careful management to prevent environmental contamination. The acid's extreme reactivity with water bodies and soil poses potential ecological risks, necessitating the development of robust containment and neutralization protocols.
Despite these challenges, ongoing research continues to explore innovative applications of fluoroantimonic acid in electrochemistry. Recent studies have focused on its potential in advanced battery technologies, where its strong acidic properties could enable new electrode reactions and improve energy storage capabilities. However, translating these laboratory findings into practical, large-scale applications remains a formidable task.
In recent years, researchers have made notable progress in harnessing fluoroantimonic acid's potential for electrochemical applications. Its ability to protonate even extremely weak bases has led to its use in electrocatalysis, where it can activate inert substrates for electrochemical reactions. This property has shown particular promise in the development of novel electrocatalysts for fuel cells and water splitting technologies.
However, the widespread adoption of fluoroantimonic acid in electrochemistry faces several significant challenges. The foremost issue is its extreme corrosiveness, which necessitates specialized handling and storage equipment. This requirement substantially increases the cost and complexity of implementing fluoroantimonic acid-based processes in industrial settings.
Safety concerns also pose a major hurdle in the practical application of fluoroantimonic acid. Its highly reactive nature demands stringent safety protocols and specialized training for personnel, limiting its use to highly controlled laboratory environments. The acid's potential for severe chemical burns and its ability to generate toxic hydrogen fluoride gas upon contact with water further complicate its handling.
Another challenge lies in the acid's incompatibility with many common materials used in electrochemical setups. Traditional electrode materials and cell components often degrade rapidly when exposed to fluoroantimonic acid, necessitating the development of new, resistant materials. This requirement has spurred research into novel electrode coatings and cell designs, but progress in this area remains limited.
The environmental impact of fluoroantimonic acid usage is also a significant concern. Its production and disposal require careful management to prevent environmental contamination. The acid's extreme reactivity with water bodies and soil poses potential ecological risks, necessitating the development of robust containment and neutralization protocols.
Despite these challenges, ongoing research continues to explore innovative applications of fluoroantimonic acid in electrochemistry. Recent studies have focused on its potential in advanced battery technologies, where its strong acidic properties could enable new electrode reactions and improve energy storage capabilities. However, translating these laboratory findings into practical, large-scale applications remains a formidable task.
Existing Electrochemical Solutions Using Fluoroantimonic Acid
01 Synthesis and preparation methods
Various methods for synthesizing and preparing fluoroantimonic acid are described. These methods may involve the reaction of hydrogen fluoride with antimony pentafluoride or other precursors under specific conditions. The synthesis process often requires careful control of temperature, pressure, and reactant ratios to achieve the desired product.- Synthesis and preparation methods: Various methods for synthesizing and preparing fluoroantimonic acid are described. These methods may involve the reaction of antimony pentafluoride with hydrogen fluoride or other precursors under specific conditions. The synthesis process often requires careful control of temperature, pressure, and reactant ratios to achieve the desired product.
- Applications in catalysis: Fluoroantimonic acid is utilized as a powerful catalyst in various chemical reactions. Its strong acidity makes it effective for promoting reactions such as isomerization, alkylation, and polymerization. The acid's catalytic properties are exploited in industrial processes, particularly in the petrochemical industry.
- Use in material science and surface treatments: Fluoroantimonic acid finds applications in material science and surface treatments. It can be used for etching, cleaning, or modifying surfaces of various materials, including metals and semiconductors. The acid's strong reactivity allows for precise surface modifications and the creation of specific material properties.
- Safety and handling considerations: Due to its extreme reactivity and corrosive nature, special safety measures and handling procedures are required when working with fluoroantimonic acid. This includes the use of specialized containment materials, personal protective equipment, and strict protocols for storage and disposal. Proper training and safety precautions are essential for handling this superacid.
- Analytical and characterization techniques: Various analytical and characterization techniques are employed to study fluoroantimonic acid and its properties. These may include spectroscopic methods, electrochemical analyses, and computational studies. Such techniques help in understanding the acid's structure, reactivity, and behavior in different chemical environments.
02 Applications in catalysis
Fluoroantimonic acid is utilized as a powerful catalyst in various chemical reactions. Its super-acidic properties make it effective for promoting reactions such as isomerization, alkylation, and polymerization. The acid's catalytic activity is particularly useful in the petrochemical industry and in the synthesis of specialty chemicals.Expand Specific Solutions03 Material compatibility and handling
Due to its extreme acidity, fluoroantimonic acid requires special handling procedures and compatible materials. Research focuses on developing containers, equipment, and protective gear that can withstand its corrosive nature. This includes the use of fluoropolymers and other highly resistant materials in storage and processing equipment.Expand Specific Solutions04 Safety and environmental considerations
The use of fluoroantimonic acid presents significant safety and environmental challenges. Research in this area focuses on developing safer handling protocols, containment strategies, and methods for neutralizing or disposing of the acid. Studies also investigate the potential environmental impacts and ways to mitigate risks associated with its production and use.Expand Specific Solutions05 Analytical and characterization techniques
Various analytical and characterization techniques are employed to study fluoroantimonic acid and its properties. These may include spectroscopic methods, electrochemical analyses, and advanced imaging techniques. Such studies aim to better understand the acid's structure, reactivity, and behavior under different conditions, which is crucial for optimizing its applications and handling.Expand Specific Solutions
Key Players in Fluoroantimonic Acid and Electrochemistry
The applications of fluoroantimonic acid in electrochemistry represent an emerging field with significant potential. The market is in its early growth stage, characterized by intensive research and development activities. While the market size remains relatively small, it is expected to expand as new applications are discovered. The technology's maturity is still evolving, with leading institutions like California Institute of Technology, Centre National de la Recherche Scientifique, and Oxford University Innovation Ltd. driving fundamental research. Companies such as 3M Innovative Properties Co., Merck Patent GmbH, and BASF Corp. are actively exploring industrial applications, indicating a growing interest in commercialization. The competitive landscape is diverse, with academic institutions, established chemical companies, and specialized materials firms all contributing to advancements in this field.
BASF Corp.
Technical Solution: BASF has developed a novel electrochemical cell design utilizing fluoroantimonic acid as an electrolyte. This system employs a unique combination of fluorinated polymer electrodes and specialized containment materials resistant to the highly corrosive nature of fluoroantimonic acid. The cell architecture incorporates advanced sealing techniques to prevent electrolyte leakage and maintain long-term stability. BASF's approach enables the harnessing of fluoroantimonic acid's superacidic properties for enhanced electrochemical reactions, particularly in organic synthesis and catalysis applications.
Strengths: Exceptional reactivity and catalytic potential due to fluoroantimonic acid's superacidic nature. Weaknesses: Extreme corrosiveness requires specialized handling and containment, limiting widespread application.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a fluoroantimonic acid-based electrochemical etching technology for semiconductor manufacturing. Their system employs a carefully engineered flow cell design that allows for controlled exposure of semiconductor wafers to fluoroantimonic acid electrolyte. The technology incorporates advanced monitoring and control systems to precisely manage acid concentration, temperature, and electrical parameters. DuPont's innovation enables ultra-fine etching of complex semiconductor structures, pushing the boundaries of miniaturization in electronic devices.
Strengths: Enables extremely precise and controlled etching processes for advanced semiconductor manufacturing. Weaknesses: Requires extensive safety measures and specialized equipment, increasing production costs.
Core Innovations in Fluoroantimonic Acid Electrochemistry
Fluoride ion electrochemical cell
PatentWO2007146453A2
Innovation
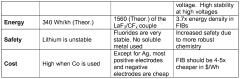
- The development of fluoride ion electrochemical cells that utilize anion charge carriers, eliminating the need for metallic lithium by using anion host materials for both electrodes, allowing for higher cell voltages, improved cycle life, and enhanced safety through the use of fluoride ions as charge carriers.
Fluoride ion electrochemical cell
PatentActiveEP1993953A2
Innovation
- The development of fluoride ion electrochemical cells using anion charge carriers like fluoride ions (F-) that eliminate the need for metallic lithium, with active electrode materials and electrolytes designed for reversible anion exchange, enabling higher cell voltages, specific capacities, and improved cycle life.
Safety and Environmental Considerations
The use of fluoroantimonic acid in electrochemistry applications presents significant safety and environmental challenges that must be carefully addressed. This superacid is extremely corrosive and reactive, posing severe risks to human health and the environment if not handled properly.
From a safety perspective, fluoroantimonic acid requires stringent containment and handling protocols. It can cause severe burns and tissue damage upon contact with skin or eyes, and its vapors are highly toxic if inhaled. Specialized personal protective equipment, including chemical-resistant suits, gloves, and respiratory protection, is essential for any personnel working with this substance. Laboratories and industrial facilities using fluoroantimonic acid must be equipped with advanced ventilation systems, emergency showers, and eyewash stations.
The extreme reactivity of fluoroantimonic acid also presents explosion and fire hazards. It reacts violently with water and many organic compounds, potentially leading to rapid heat generation and pressure buildup. Strict protocols for storage, transport, and waste disposal are necessary to prevent accidental releases or reactions.
Environmentally, fluoroantimonic acid poses severe threats to ecosystems. Its high acidity can cause widespread damage to soil and water systems if released. The fluorine and antimony components can persist in the environment, potentially bioaccumulating in food chains. Proper containment and disposal methods are crucial to prevent environmental contamination.
Waste management is a critical consideration in the use of fluoroantimonic acid. Neutralization processes must be carefully controlled, as the heat generated during neutralization can be substantial. Specialized waste treatment facilities may be required to handle the byproducts of electrochemical processes involving this superacid.
Regulatory compliance is another key aspect of safety and environmental considerations. The use of fluoroantimonic acid is subject to strict regulations in many jurisdictions, covering aspects such as transportation, storage, handling, and disposal. Companies working with this substance must maintain comprehensive documentation and safety protocols to meet these regulatory requirements.
Research into safer alternatives and improved handling techniques is ongoing. This includes the development of less hazardous superacids, enhanced containment materials, and more efficient neutralization methods. Such advancements are crucial for expanding the applications of superacids in electrochemistry while minimizing risks to human health and the environment.
From a safety perspective, fluoroantimonic acid requires stringent containment and handling protocols. It can cause severe burns and tissue damage upon contact with skin or eyes, and its vapors are highly toxic if inhaled. Specialized personal protective equipment, including chemical-resistant suits, gloves, and respiratory protection, is essential for any personnel working with this substance. Laboratories and industrial facilities using fluoroantimonic acid must be equipped with advanced ventilation systems, emergency showers, and eyewash stations.
The extreme reactivity of fluoroantimonic acid also presents explosion and fire hazards. It reacts violently with water and many organic compounds, potentially leading to rapid heat generation and pressure buildup. Strict protocols for storage, transport, and waste disposal are necessary to prevent accidental releases or reactions.
Environmentally, fluoroantimonic acid poses severe threats to ecosystems. Its high acidity can cause widespread damage to soil and water systems if released. The fluorine and antimony components can persist in the environment, potentially bioaccumulating in food chains. Proper containment and disposal methods are crucial to prevent environmental contamination.
Waste management is a critical consideration in the use of fluoroantimonic acid. Neutralization processes must be carefully controlled, as the heat generated during neutralization can be substantial. Specialized waste treatment facilities may be required to handle the byproducts of electrochemical processes involving this superacid.
Regulatory compliance is another key aspect of safety and environmental considerations. The use of fluoroantimonic acid is subject to strict regulations in many jurisdictions, covering aspects such as transportation, storage, handling, and disposal. Companies working with this substance must maintain comprehensive documentation and safety protocols to meet these regulatory requirements.
Research into safer alternatives and improved handling techniques is ongoing. This includes the development of less hazardous superacids, enhanced containment materials, and more efficient neutralization methods. Such advancements are crucial for expanding the applications of superacids in electrochemistry while minimizing risks to human health and the environment.
Economic Impact and Cost Analysis
The economic impact of fluoroantimonic acid in electrochemistry is significant, with far-reaching implications for various industries. As a super acid with exceptional proton-donating capabilities, its applications in electrochemical processes have the potential to revolutionize energy storage, catalysis, and materials processing sectors.
In the energy storage domain, fluoroantimonic acid's use in advanced battery technologies could lead to substantial cost reductions and performance improvements. By enhancing electrode materials and electrolyte compositions, it may contribute to the development of more efficient and longer-lasting batteries. This advancement could accelerate the adoption of electric vehicles and renewable energy systems, potentially saving billions in energy costs and reducing carbon emissions.
The catalysis industry stands to benefit greatly from fluoroantimonic acid's unique properties. Its ability to catalyze reactions under milder conditions could result in significant energy savings and reduced production costs for various chemical processes. This efficiency gain may translate into lower prices for end-products across multiple sectors, from pharmaceuticals to petrochemicals.
However, the cost analysis of fluoroantimonic acid implementation in electrochemistry presents a complex picture. The production of this super acid involves expensive precursors and specialized equipment, leading to high initial costs. The corrosive nature of the acid also necessitates investment in specialized containment and handling systems, further increasing capital expenditures.
Despite these upfront costs, the long-term economic benefits could be substantial. Improved process efficiencies and product yields may offset the initial investments, particularly in large-scale industrial applications. Additionally, as demand for fluoroantimonic acid in electrochemistry grows, economies of scale could drive down production costs, making it more accessible to a broader range of industries.
The environmental impact of fluoroantimonic acid use must also be considered in the economic analysis. While its application may lead to more efficient processes and reduced energy consumption, the potential environmental risks associated with its production and disposal need to be carefully managed. The costs of implementing stringent safety measures and environmental protection protocols should be factored into the overall economic assessment.
In conclusion, the economic impact of fluoroantimonic acid in electrochemistry is multifaceted. While initial costs may be high, the potential for significant efficiency gains, improved product quality, and new technological advancements could drive substantial economic benefits across multiple industries. As research progresses and applications expand, a more comprehensive cost-benefit analysis will be crucial for guiding investment decisions and policy-making in this promising field of electrochemistry.
In the energy storage domain, fluoroantimonic acid's use in advanced battery technologies could lead to substantial cost reductions and performance improvements. By enhancing electrode materials and electrolyte compositions, it may contribute to the development of more efficient and longer-lasting batteries. This advancement could accelerate the adoption of electric vehicles and renewable energy systems, potentially saving billions in energy costs and reducing carbon emissions.
The catalysis industry stands to benefit greatly from fluoroantimonic acid's unique properties. Its ability to catalyze reactions under milder conditions could result in significant energy savings and reduced production costs for various chemical processes. This efficiency gain may translate into lower prices for end-products across multiple sectors, from pharmaceuticals to petrochemicals.
However, the cost analysis of fluoroantimonic acid implementation in electrochemistry presents a complex picture. The production of this super acid involves expensive precursors and specialized equipment, leading to high initial costs. The corrosive nature of the acid also necessitates investment in specialized containment and handling systems, further increasing capital expenditures.
Despite these upfront costs, the long-term economic benefits could be substantial. Improved process efficiencies and product yields may offset the initial investments, particularly in large-scale industrial applications. Additionally, as demand for fluoroantimonic acid in electrochemistry grows, economies of scale could drive down production costs, making it more accessible to a broader range of industries.
The environmental impact of fluoroantimonic acid use must also be considered in the economic analysis. While its application may lead to more efficient processes and reduced energy consumption, the potential environmental risks associated with its production and disposal need to be carefully managed. The costs of implementing stringent safety measures and environmental protection protocols should be factored into the overall economic assessment.
In conclusion, the economic impact of fluoroantimonic acid in electrochemistry is multifaceted. While initial costs may be high, the potential for significant efficiency gains, improved product quality, and new technological advancements could drive substantial economic benefits across multiple industries. As research progresses and applications expand, a more comprehensive cost-benefit analysis will be crucial for guiding investment decisions and policy-making in this promising field of electrochemistry.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!