The Dynamics of Carbon Tetrachloride in Chemical Reactions
JUL 2, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Reaction Dynamics
Carbon tetrachloride (CCl4) plays a significant role in various chemical reactions, exhibiting complex dynamics that have been the subject of extensive research. The reaction dynamics of CCl4 are characterized by its unique molecular structure and the behavior of its chemical bonds under different conditions. Understanding these dynamics is crucial for predicting and controlling the outcomes of reactions involving this compound.
In gas-phase reactions, CCl4 molecules undergo collisions with other reactants, leading to energy transfer and potential bond breaking. The symmetrical tetrahedral structure of CCl4 influences its reactivity, as the four chlorine atoms shield the central carbon atom. This structural feature affects the accessibility of the carbon atom to nucleophilic attack and impacts the overall reaction kinetics.
The C-Cl bond dissociation energy in CCl4 is relatively low, making it susceptible to homolytic cleavage under certain conditions. This property is particularly important in radical-initiated reactions, where the formation of trichloromethyl radicals (•CCl3) can trigger chain reactions. The dynamics of these radical processes involve rapid propagation steps and can lead to the degradation of CCl4 in environmental systems.
Photochemical reactions involving CCl4 demonstrate interesting dynamics, as the molecule can absorb ultraviolet light, leading to electronic excitation. This excitation can result in the dissociation of one or more C-Cl bonds, producing reactive chlorine atoms and carbon-centered radicals. The subsequent reactions of these species contribute to the complex reaction networks observed in atmospheric chemistry.
In solution-phase reactions, the solvation of CCl4 affects its reactivity and the dynamics of its interactions with other species. The polarity of the solvent can influence the stability of reaction intermediates and transition states, thereby altering reaction rates and pathways. Additionally, the formation of solvent cages can impact the recombination probability of radical pairs generated from CCl4 dissociation.
The reaction dynamics of CCl4 in heterogeneous systems, such as those involving solid catalysts or at liquid-solid interfaces, introduce additional complexities. Surface interactions can modify the energetics of bond breaking and formation, leading to unique reaction pathways not observed in homogeneous systems. These surface-mediated processes are particularly relevant in environmental remediation efforts and industrial catalytic applications.
Understanding the reaction dynamics of CCl4 is essential for developing accurate models of atmospheric chemistry, designing effective remediation strategies for contaminated sites, and optimizing industrial processes that utilize or produce this compound. Ongoing research in this field continues to reveal new insights into the fundamental behavior of CCl4 in chemical reactions, contributing to our broader understanding of molecular dynamics and reaction kinetics.
In gas-phase reactions, CCl4 molecules undergo collisions with other reactants, leading to energy transfer and potential bond breaking. The symmetrical tetrahedral structure of CCl4 influences its reactivity, as the four chlorine atoms shield the central carbon atom. This structural feature affects the accessibility of the carbon atom to nucleophilic attack and impacts the overall reaction kinetics.
The C-Cl bond dissociation energy in CCl4 is relatively low, making it susceptible to homolytic cleavage under certain conditions. This property is particularly important in radical-initiated reactions, where the formation of trichloromethyl radicals (•CCl3) can trigger chain reactions. The dynamics of these radical processes involve rapid propagation steps and can lead to the degradation of CCl4 in environmental systems.
Photochemical reactions involving CCl4 demonstrate interesting dynamics, as the molecule can absorb ultraviolet light, leading to electronic excitation. This excitation can result in the dissociation of one or more C-Cl bonds, producing reactive chlorine atoms and carbon-centered radicals. The subsequent reactions of these species contribute to the complex reaction networks observed in atmospheric chemistry.
In solution-phase reactions, the solvation of CCl4 affects its reactivity and the dynamics of its interactions with other species. The polarity of the solvent can influence the stability of reaction intermediates and transition states, thereby altering reaction rates and pathways. Additionally, the formation of solvent cages can impact the recombination probability of radical pairs generated from CCl4 dissociation.
The reaction dynamics of CCl4 in heterogeneous systems, such as those involving solid catalysts or at liquid-solid interfaces, introduce additional complexities. Surface interactions can modify the energetics of bond breaking and formation, leading to unique reaction pathways not observed in homogeneous systems. These surface-mediated processes are particularly relevant in environmental remediation efforts and industrial catalytic applications.
Understanding the reaction dynamics of CCl4 is essential for developing accurate models of atmospheric chemistry, designing effective remediation strategies for contaminated sites, and optimizing industrial processes that utilize or produce this compound. Ongoing research in this field continues to reveal new insights into the fundamental behavior of CCl4 in chemical reactions, contributing to our broader understanding of molecular dynamics and reaction kinetics.
Industrial Applications
Carbon tetrachloride (CCl4) plays a significant role in various industrial applications due to its unique chemical properties. In the manufacturing sector, it serves as an effective solvent for oils, fats, lacquers, varnishes, rubber waxes, and resins. This versatility makes it particularly valuable in the production of semiconductors and fiber optics, where high purity and precision cleaning are essential.
The petrochemical industry utilizes carbon tetrachloride as a feedstock for the production of chlorofluorocarbons (CFCs) and their alternatives. Although the use of CFCs has been phased out due to environmental concerns, the chemical remains important in the synthesis of less harmful refrigerants and propellants. In the pharmaceutical industry, carbon tetrachloride is employed as a reagent in organic synthesis reactions, contributing to the production of various drugs and intermediates.
Fire extinguishing systems have historically incorporated carbon tetrachloride due to its fire-suppressing properties. While its use in this application has decreased due to toxicity concerns, it remains relevant in specialized fire safety equipment for certain industrial settings where alternative agents are less effective.
The textile industry benefits from carbon tetrachloride's ability to remove stains and as a dry-cleaning agent for delicate fabrics. Its non-flammability and low boiling point make it an ideal choice for these applications, although environmental regulations have led to the development of alternative cleaning methods in many regions.
In the field of analytical chemistry, carbon tetrachloride serves as a solvent in various spectroscopic techniques, including infrared spectroscopy and nuclear magnetic resonance (NMR) studies. Its transparency in certain spectral regions and ability to dissolve a wide range of organic compounds make it valuable for these analytical applications.
The agrochemical industry utilizes carbon tetrachloride in the production of pesticides and herbicides. Its role as a chemical intermediate in the synthesis of these compounds contributes to the development of more effective crop protection solutions. However, ongoing research focuses on finding more environmentally friendly alternatives to reduce the reliance on this chemical in agricultural applications.
Despite its widespread industrial applications, the use of carbon tetrachloride has been subject to increasing scrutiny and regulation due to its ozone-depleting properties and potential health hazards. This has led to a gradual reduction in its use across many sectors, with industries actively seeking safer and more sustainable alternatives. Nonetheless, its unique chemical properties continue to make it indispensable in certain specialized applications where suitable replacements have yet to be developed.
The petrochemical industry utilizes carbon tetrachloride as a feedstock for the production of chlorofluorocarbons (CFCs) and their alternatives. Although the use of CFCs has been phased out due to environmental concerns, the chemical remains important in the synthesis of less harmful refrigerants and propellants. In the pharmaceutical industry, carbon tetrachloride is employed as a reagent in organic synthesis reactions, contributing to the production of various drugs and intermediates.
Fire extinguishing systems have historically incorporated carbon tetrachloride due to its fire-suppressing properties. While its use in this application has decreased due to toxicity concerns, it remains relevant in specialized fire safety equipment for certain industrial settings where alternative agents are less effective.
The textile industry benefits from carbon tetrachloride's ability to remove stains and as a dry-cleaning agent for delicate fabrics. Its non-flammability and low boiling point make it an ideal choice for these applications, although environmental regulations have led to the development of alternative cleaning methods in many regions.
In the field of analytical chemistry, carbon tetrachloride serves as a solvent in various spectroscopic techniques, including infrared spectroscopy and nuclear magnetic resonance (NMR) studies. Its transparency in certain spectral regions and ability to dissolve a wide range of organic compounds make it valuable for these analytical applications.
The agrochemical industry utilizes carbon tetrachloride in the production of pesticides and herbicides. Its role as a chemical intermediate in the synthesis of these compounds contributes to the development of more effective crop protection solutions. However, ongoing research focuses on finding more environmentally friendly alternatives to reduce the reliance on this chemical in agricultural applications.
Despite its widespread industrial applications, the use of carbon tetrachloride has been subject to increasing scrutiny and regulation due to its ozone-depleting properties and potential health hazards. This has led to a gradual reduction in its use across many sectors, with industries actively seeking safer and more sustainable alternatives. Nonetheless, its unique chemical properties continue to make it indispensable in certain specialized applications where suitable replacements have yet to be developed.
Reaction Mechanisms
Carbon tetrachloride (CCl4) exhibits complex dynamics in chemical reactions, primarily due to its unique molecular structure and reactivity. The reaction mechanisms involving CCl4 can be broadly categorized into three main types: substitution, elimination, and radical reactions.
Substitution reactions of CCl4 typically occur through nucleophilic attack. The carbon-chlorine bonds in CCl4 are polarized, with the carbon atom bearing a partial positive charge. This makes it susceptible to nucleophilic attack, particularly in the presence of strong nucleophiles. The reaction often proceeds via an SN2 mechanism, where the nucleophile attacks the carbon atom from the opposite side of a departing chlorine atom, resulting in inversion of stereochemistry.
Elimination reactions involving CCl4 are less common but can occur under specific conditions. These reactions typically require a strong base and elevated temperatures. The base abstracts a proton from an adjacent molecule, leading to the formation of a carbene intermediate. This highly reactive species can then undergo further reactions, such as insertion into C-H bonds or rearrangement.
Radical reactions are particularly important in the environmental fate of CCl4. When exposed to UV radiation or in the presence of certain metal catalysts, CCl4 can undergo homolytic cleavage of a carbon-chlorine bond, generating a trichloromethyl radical (•CCl3) and a chlorine radical (•Cl). These radicals can then participate in chain reactions, leading to the degradation of CCl4 and the formation of various chlorinated products.
The reaction dynamics of CCl4 are significantly influenced by the reaction conditions. In aqueous environments, hydrolysis can occur, albeit slowly, leading to the formation of chloroform and hydrochloric acid. This reaction is accelerated in alkaline conditions. In the gas phase, particularly in the stratosphere, photolysis of CCl4 plays a crucial role in its decomposition and the subsequent formation of chlorine radicals, which contribute to ozone depletion.
The presence of transition metals can dramatically alter the reaction pathways of CCl4. For instance, in the presence of iron or copper, CCl4 can undergo reductive dechlorination, forming less chlorinated methanes. This process is particularly relevant in environmental remediation strategies for CCl4-contaminated sites.
Understanding these reaction mechanisms is crucial for predicting the environmental fate of CCl4, developing effective remediation strategies, and designing safer alternatives in industrial processes. The complex interplay of these mechanisms underscores the need for continued research into the dynamics of CCl4 in various chemical and environmental contexts.
Substitution reactions of CCl4 typically occur through nucleophilic attack. The carbon-chlorine bonds in CCl4 are polarized, with the carbon atom bearing a partial positive charge. This makes it susceptible to nucleophilic attack, particularly in the presence of strong nucleophiles. The reaction often proceeds via an SN2 mechanism, where the nucleophile attacks the carbon atom from the opposite side of a departing chlorine atom, resulting in inversion of stereochemistry.
Elimination reactions involving CCl4 are less common but can occur under specific conditions. These reactions typically require a strong base and elevated temperatures. The base abstracts a proton from an adjacent molecule, leading to the formation of a carbene intermediate. This highly reactive species can then undergo further reactions, such as insertion into C-H bonds or rearrangement.
Radical reactions are particularly important in the environmental fate of CCl4. When exposed to UV radiation or in the presence of certain metal catalysts, CCl4 can undergo homolytic cleavage of a carbon-chlorine bond, generating a trichloromethyl radical (•CCl3) and a chlorine radical (•Cl). These radicals can then participate in chain reactions, leading to the degradation of CCl4 and the formation of various chlorinated products.
The reaction dynamics of CCl4 are significantly influenced by the reaction conditions. In aqueous environments, hydrolysis can occur, albeit slowly, leading to the formation of chloroform and hydrochloric acid. This reaction is accelerated in alkaline conditions. In the gas phase, particularly in the stratosphere, photolysis of CCl4 plays a crucial role in its decomposition and the subsequent formation of chlorine radicals, which contribute to ozone depletion.
The presence of transition metals can dramatically alter the reaction pathways of CCl4. For instance, in the presence of iron or copper, CCl4 can undergo reductive dechlorination, forming less chlorinated methanes. This process is particularly relevant in environmental remediation strategies for CCl4-contaminated sites.
Understanding these reaction mechanisms is crucial for predicting the environmental fate of CCl4, developing effective remediation strategies, and designing safer alternatives in industrial processes. The complex interplay of these mechanisms underscores the need for continued research into the dynamics of CCl4 in various chemical and environmental contexts.
Current Methodologies
01 Production and purification of carbon tetrachloride
Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.- Production and purification of carbon tetrachloride: Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.
- Applications of carbon tetrachloride in chemical processes: Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in the production of other chemicals. Its applications span across different industries, showcasing its versatility in chemical manufacturing.
- Environmental and safety considerations: Due to its environmental impact and health hazards, research focuses on alternatives to carbon tetrachloride and methods for its safe handling, storage, and disposal. This includes developing eco-friendly substitutes and implementing safety protocols in industrial settings.
- Detection and analysis methods: Various techniques and apparatus are developed for detecting and analyzing carbon tetrachloride in different environments. These methods are crucial for monitoring air and water quality, as well as ensuring workplace safety in industries where carbon tetrachloride is used.
- Historical uses and developments: The historical applications of carbon tetrachloride, including its use as a fire extinguishing agent, cleaning solvent, and refrigerant, are documented. These patents also cover early production methods and equipment designs related to carbon tetrachloride handling.
02 Applications of carbon tetrachloride in chemical processes
Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in the production of other chemicals. Its unique properties make it valuable in specific industrial applications and chemical reactions.Expand Specific Solutions03 Environmental and safety considerations
Due to its environmental impact and health hazards, research focuses on developing alternatives to carbon tetrachloride and methods for its safe handling, storage, and disposal. This includes techniques for detecting and monitoring carbon tetrachloride in various environments.Expand Specific Solutions04 Carbon tetrachloride in analytical chemistry
Carbon tetrachloride plays a role in analytical chemistry, particularly in spectroscopic techniques and as a solvent for various analytical procedures. Its use in these applications is described, along with potential alternatives and improvements in analytical methods.Expand Specific Solutions05 Historical uses and patents related to carbon tetrachloride
Early patents and historical uses of carbon tetrachloride are documented, including its applications in fire extinguishers, dry cleaning, and as a refrigerant. These historical patents provide insight into the development and evolution of carbon tetrachloride's industrial applications.Expand Specific Solutions
Key Industry Players
The dynamics of carbon tetrachloride in chemical reactions present a competitive landscape characterized by mature technology and established players. The market is in a stable phase, with moderate growth potential due to environmental regulations limiting its use. Key players like BASF Corp., Occidental Chemical Corp., and Sumitomo Chemical Co., Ltd. dominate the industry, leveraging their extensive research capabilities and global presence. The market size remains significant, driven by industrial applications in manufacturing and chemical processes. However, the focus is shifting towards developing safer alternatives and more sustainable practices, prompting companies to invest in innovation and eco-friendly solutions.
BASF Corp.
Technical Solution: BASF has developed innovative approaches to manage carbon tetrachloride (CCl4) in chemical reactions. They have implemented a closed-loop system for CCl4 recycling, significantly reducing emissions and waste. Their process involves catalytic decomposition of CCl4 into less harmful compounds, followed by separation and purification steps [1]. BASF has also invested in advanced monitoring systems to detect and prevent CCl4 leaks, ensuring safer handling and reduced environmental impact. Additionally, they have explored alternative solvents and reaction pathways to minimize CCl4 use in various chemical processes, demonstrating a commitment to sustainable chemistry practices [3].
Strengths: Advanced recycling technology, reduced environmental impact, improved safety measures. Weaknesses: High initial investment costs, potential complexity in implementation across diverse chemical processes.
Sumitomo Chemical Co., Ltd.
Technical Solution: Sumitomo Chemical has developed a comprehensive approach to managing CCl4 dynamics in chemical reactions. They have implemented a closed-loop manufacturing system that minimizes CCl4 emissions and maximizes recovery. Their research has led to the development of novel catalysts that enable CCl4-free synthesis routes for certain chemicals traditionally produced using CCl4 as a reagent or solvent [9]. Sumitomo has also invested in advanced analytical techniques for real-time monitoring of CCl4 levels in reaction mixtures and waste streams. Additionally, they have explored the use of supercritical fluid extraction as an alternative to CCl4-based extraction processes, demonstrating potential for reducing CCl4 use in certain applications [10].
Strengths: Closed-loop manufacturing system, catalyst innovation, advanced monitoring techniques. Weaknesses: Potential challenges in scaling up alternative processes, ongoing research costs for developing CCl4-free routes.
Innovative Approaches
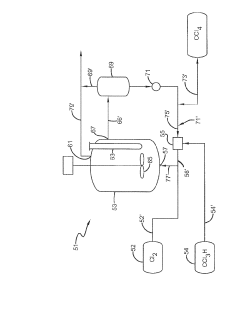
Process for the production of carbon tetrachloride
PatentInactiveEP0051236A1
Innovation
- The process involves mixing starting materials with hot reaction products immediately after entry into the reactor using a nozzle with a guide tube, achieving rapid mixing and circulation of gases to maintain the required high temperature for the reaction, eliminating the need for a pre-reaction zone and reducing excess chlorine usage.
Producing carbon tetrachloride by photochlorination of chloroform
PatentActiveJP2024069268A
Innovation
- A method involving the photochlorination of chloroform with chlorine in the presence of electromagnetic radiation, maintaining a low concentration of chloroform and a stoichiometric concentration of chlorine, to produce carbon tetrachloride with high selectivity and minimize the formation of hexachloroethane.
Environmental Impact
Carbon tetrachloride (CCl4) has been widely used in various industrial applications, but its environmental impact has become a significant concern. The release of CCl4 into the atmosphere has been linked to ozone depletion, contributing to the formation of the ozone hole. This compound is highly stable in the troposphere, with an atmospheric lifetime of approximately 26 years, allowing it to reach the stratosphere where it undergoes photolysis and releases chlorine atoms that catalyze ozone destruction.
In aquatic environments, CCl4 can persist for extended periods due to its low solubility and resistance to biodegradation. Its presence in water bodies poses risks to aquatic ecosystems, potentially accumulating in sediments and entering the food chain. Studies have shown that CCl4 can be toxic to various aquatic organisms, including fish and invertebrates, even at low concentrations.
Soil contamination by CCl4 is another critical environmental issue. The compound can leach into groundwater, potentially contaminating drinking water sources. Its persistence in soil can lead to long-term environmental and health risks, as it may volatilize and re-enter the atmosphere or be taken up by plants.
The global production and use of CCl4 have been significantly reduced under the Montreal Protocol, which classified it as an ozone-depleting substance. However, its legacy in the environment continues to be a concern. Atmospheric concentrations of CCl4 have declined more slowly than expected, suggesting ongoing emissions from unknown sources or previously unaccounted for natural processes.
Recent research has focused on the natural sources and sinks of CCl4 in the environment. Studies have identified potential natural formation mechanisms in soils and oceans, which may contribute to the observed atmospheric concentrations. Understanding these natural processes is crucial for accurately assessing the global CCl4 budget and predicting future environmental impacts.
Efforts to mitigate the environmental impact of CCl4 include improved industrial practices, stricter regulations on its production and use, and the development of alternative compounds with lower environmental risks. Remediation techniques for CCl4-contaminated sites have also been developed, including chemical oxidation, bioremediation, and thermal treatment methods.
In aquatic environments, CCl4 can persist for extended periods due to its low solubility and resistance to biodegradation. Its presence in water bodies poses risks to aquatic ecosystems, potentially accumulating in sediments and entering the food chain. Studies have shown that CCl4 can be toxic to various aquatic organisms, including fish and invertebrates, even at low concentrations.
Soil contamination by CCl4 is another critical environmental issue. The compound can leach into groundwater, potentially contaminating drinking water sources. Its persistence in soil can lead to long-term environmental and health risks, as it may volatilize and re-enter the atmosphere or be taken up by plants.
The global production and use of CCl4 have been significantly reduced under the Montreal Protocol, which classified it as an ozone-depleting substance. However, its legacy in the environment continues to be a concern. Atmospheric concentrations of CCl4 have declined more slowly than expected, suggesting ongoing emissions from unknown sources or previously unaccounted for natural processes.
Recent research has focused on the natural sources and sinks of CCl4 in the environment. Studies have identified potential natural formation mechanisms in soils and oceans, which may contribute to the observed atmospheric concentrations. Understanding these natural processes is crucial for accurately assessing the global CCl4 budget and predicting future environmental impacts.
Efforts to mitigate the environmental impact of CCl4 include improved industrial practices, stricter regulations on its production and use, and the development of alternative compounds with lower environmental risks. Remediation techniques for CCl4-contaminated sites have also been developed, including chemical oxidation, bioremediation, and thermal treatment methods.
Safety Regulations
The safety regulations surrounding the use and handling of carbon tetrachloride in chemical reactions have become increasingly stringent due to its known toxicity and environmental impact. Regulatory bodies worldwide have implemented strict guidelines to minimize exposure risks and prevent environmental contamination.
In industrial settings, the Occupational Safety and Health Administration (OSHA) has set permissible exposure limits (PEL) for carbon tetrachloride at 10 parts per million (ppm) as an 8-hour time-weighted average. Short-term exposure limits (STEL) are typically set at 25 ppm for 15-minute periods. These limits are designed to protect workers from acute and chronic health effects associated with carbon tetrachloride exposure.
Environmental protection agencies, such as the U.S. Environmental Protection Agency (EPA), have established regulations for the proper disposal and management of carbon tetrachloride waste. The substance is classified as a hazardous waste under the Resource Conservation and Recovery Act (RCRA), requiring specialized handling and disposal procedures to prevent soil and groundwater contamination.
International agreements, including the Montreal Protocol, have phased out the production and use of carbon tetrachloride due to its ozone-depleting properties. This has led to strict import and export controls, with most countries requiring special permits for any remaining essential uses.
In laboratory settings, safety protocols mandate the use of fume hoods, personal protective equipment (PPE), and proper ventilation systems when handling carbon tetrachloride. Researchers are required to undergo specific training on the safe handling and emergency response procedures related to this chemical.
Storage regulations for carbon tetrachloride require the use of tightly sealed containers in well-ventilated areas, away from sources of heat or ignition. Facilities must maintain detailed inventories and implement spill prevention and response plans to mitigate potential accidents.
Transportation of carbon tetrachloride is subject to hazardous materials regulations, including proper labeling, packaging, and documentation requirements. Carriers must adhere to specific routing and handling protocols to ensure safe transit and minimize the risk of accidental release.
As research continues into safer alternatives, regulatory bodies are continuously updating their guidelines to reflect the latest scientific understanding of carbon tetrachloride's behavior in chemical reactions and its potential environmental and health impacts. This ongoing process ensures that safety measures evolve in tandem with our growing knowledge of this compound's dynamics.
In industrial settings, the Occupational Safety and Health Administration (OSHA) has set permissible exposure limits (PEL) for carbon tetrachloride at 10 parts per million (ppm) as an 8-hour time-weighted average. Short-term exposure limits (STEL) are typically set at 25 ppm for 15-minute periods. These limits are designed to protect workers from acute and chronic health effects associated with carbon tetrachloride exposure.
Environmental protection agencies, such as the U.S. Environmental Protection Agency (EPA), have established regulations for the proper disposal and management of carbon tetrachloride waste. The substance is classified as a hazardous waste under the Resource Conservation and Recovery Act (RCRA), requiring specialized handling and disposal procedures to prevent soil and groundwater contamination.
International agreements, including the Montreal Protocol, have phased out the production and use of carbon tetrachloride due to its ozone-depleting properties. This has led to strict import and export controls, with most countries requiring special permits for any remaining essential uses.
In laboratory settings, safety protocols mandate the use of fume hoods, personal protective equipment (PPE), and proper ventilation systems when handling carbon tetrachloride. Researchers are required to undergo specific training on the safe handling and emergency response procedures related to this chemical.
Storage regulations for carbon tetrachloride require the use of tightly sealed containers in well-ventilated areas, away from sources of heat or ignition. Facilities must maintain detailed inventories and implement spill prevention and response plans to mitigate potential accidents.
Transportation of carbon tetrachloride is subject to hazardous materials regulations, including proper labeling, packaging, and documentation requirements. Carriers must adhere to specific routing and handling protocols to ensure safe transit and minimize the risk of accidental release.
As research continues into safer alternatives, regulatory bodies are continuously updating their guidelines to reflect the latest scientific understanding of carbon tetrachloride's behavior in chemical reactions and its potential environmental and health impacts. This ongoing process ensures that safety measures evolve in tandem with our growing knowledge of this compound's dynamics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!