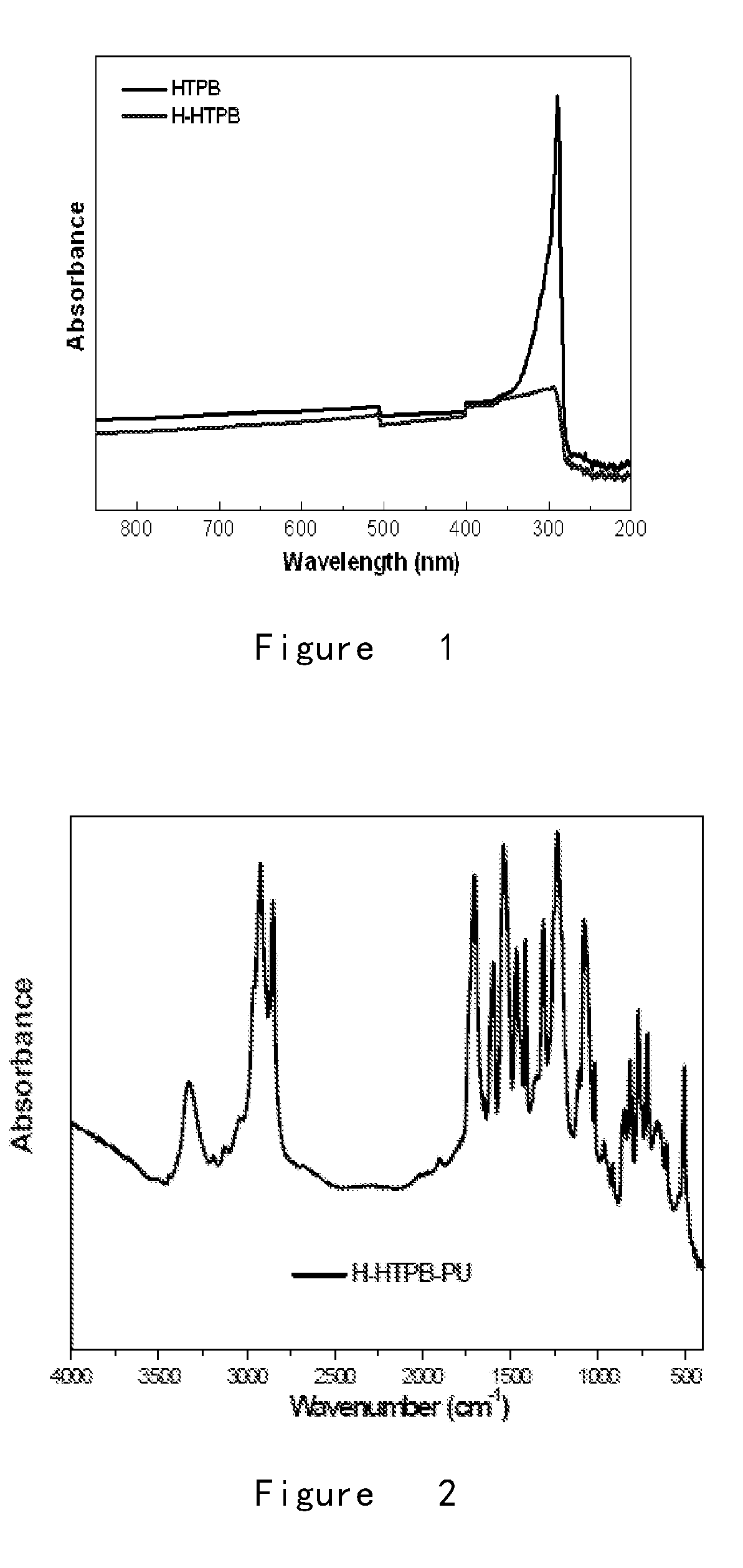
The Role of Polyurethane in Medical Device Manufacturing
JUN 25, 20255 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PU in Medical Devices: Background and Objectives
Polyurethane (PU) has emerged as a crucial material in medical device manufacturing, revolutionizing the healthcare industry with its versatile properties and biocompatibility. The evolution of PU in medical applications can be traced back to the 1960s when it was first used in catheters and artificial heart components. Since then, its role has expanded significantly, encompassing a wide range of medical devices and implants.
The primary objective of utilizing PU in medical device manufacturing is to enhance patient care and improve treatment outcomes. PU offers a unique combination of flexibility, durability, and biocompatibility, making it an ideal material for various medical applications. Its ability to be engineered with specific properties has led to its widespread adoption in the healthcare sector.
The technological evolution of PU in medical devices has been driven by the increasing demand for minimally invasive procedures, long-term implantable devices, and improved patient comfort. Researchers and manufacturers have continuously refined PU formulations to meet the stringent requirements of the medical industry, focusing on aspects such as antimicrobial properties, drug-eluting capabilities, and enhanced mechanical strength.
One of the key trends in PU development for medical devices is the creation of smart materials that can respond to physiological changes or external stimuli. This includes shape-memory polyurethanes and self-healing PU composites, which have the potential to revolutionize implantable devices and tissue engineering scaffolds.
The market demand for PU in medical device manufacturing has been steadily increasing, driven by factors such as the aging population, rising prevalence of chronic diseases, and advancements in healthcare technologies. The global market for medical-grade polyurethanes is projected to grow significantly in the coming years, with applications ranging from wound dressings and catheters to artificial organs and orthopedic implants.
As the healthcare industry continues to evolve, the role of PU in medical device manufacturing is expected to expand further. Future developments are likely to focus on improving the material's biocompatibility, enhancing its antimicrobial properties, and developing novel PU-based composites for specific medical applications. The ongoing research in this field aims to address current limitations and explore new possibilities for PU in advanced medical technologies.
The primary objective of utilizing PU in medical device manufacturing is to enhance patient care and improve treatment outcomes. PU offers a unique combination of flexibility, durability, and biocompatibility, making it an ideal material for various medical applications. Its ability to be engineered with specific properties has led to its widespread adoption in the healthcare sector.
The technological evolution of PU in medical devices has been driven by the increasing demand for minimally invasive procedures, long-term implantable devices, and improved patient comfort. Researchers and manufacturers have continuously refined PU formulations to meet the stringent requirements of the medical industry, focusing on aspects such as antimicrobial properties, drug-eluting capabilities, and enhanced mechanical strength.
One of the key trends in PU development for medical devices is the creation of smart materials that can respond to physiological changes or external stimuli. This includes shape-memory polyurethanes and self-healing PU composites, which have the potential to revolutionize implantable devices and tissue engineering scaffolds.
The market demand for PU in medical device manufacturing has been steadily increasing, driven by factors such as the aging population, rising prevalence of chronic diseases, and advancements in healthcare technologies. The global market for medical-grade polyurethanes is projected to grow significantly in the coming years, with applications ranging from wound dressings and catheters to artificial organs and orthopedic implants.
As the healthcare industry continues to evolve, the role of PU in medical device manufacturing is expected to expand further. Future developments are likely to focus on improving the material's biocompatibility, enhancing its antimicrobial properties, and developing novel PU-based composites for specific medical applications. The ongoing research in this field aims to address current limitations and explore new possibilities for PU in advanced medical technologies.
Existing PU Solutions in Medical Devices
01 Polyurethane synthesis and composition
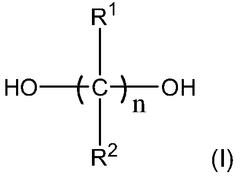
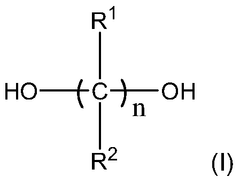
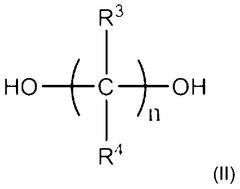
This category focuses on the development of new polyurethane compositions and synthesis methods. It includes innovations in the formulation of polyurethane materials, such as the use of novel monomers, catalysts, or additives to enhance specific properties or performance characteristics.- Polyurethane synthesis and composition: This category focuses on the development of new polyurethane compositions and synthesis methods. It includes innovations in the formulation of polyurethane materials, such as the use of novel monomers, catalysts, or additives to enhance specific properties or performance characteristics.
- Polyurethane applications in coatings and films: This point covers the use of polyurethanes in coating and film applications. It includes developments in polyurethane-based coatings for various surfaces, as well as the creation of polyurethane films with specific properties such as improved durability, flexibility, or chemical resistance.
- Polyurethane foams and insulation materials: This category focuses on innovations in polyurethane foam technology, including the development of new foam formulations, manufacturing processes, and applications. It covers advancements in insulation materials, cushioning, and structural foams for various industries.
- Polyurethane in textile and fiber applications: This point addresses the use of polyurethanes in textile and fiber-related applications. It includes developments in polyurethane-based fibers, fabrics, and finishes for textiles, as well as innovations in manufacturing processes for these materials.
- Environmentally friendly and sustainable polyurethanes: This category focuses on the development of more environmentally friendly and sustainable polyurethane materials and processes. It includes innovations in bio-based polyurethanes, recyclable or biodegradable formulations, and manufacturing methods with reduced environmental impact.
02 Polyurethane applications in coatings and adhesives
This category covers the use of polyurethanes in coating and adhesive applications. It includes developments in polyurethane-based paints, varnishes, sealants, and adhesives for various industries, focusing on improving durability, flexibility, and bonding strength.Expand Specific Solutions03 Polyurethane foam technology
This category encompasses advancements in polyurethane foam production and properties. It includes innovations in foam formulation, cell structure control, and the development of specialized foams for applications such as insulation, cushioning, and structural components.Expand Specific Solutions04 Sustainable and bio-based polyurethanes
This category focuses on the development of environmentally friendly polyurethanes. It includes research into bio-based raw materials, renewable resources for polyurethane production, and methods to improve the biodegradability or recyclability of polyurethane products.Expand Specific Solutions05 Polyurethane processing and manufacturing techniques
This category covers innovations in polyurethane processing and manufacturing methods. It includes advancements in reaction injection molding, extrusion, spray application, and other techniques used to produce polyurethane products, as well as improvements in process efficiency and quality control.Expand Specific Solutions
Innovations in PU for Medical Applications
Medical device, method for preparation thereof, and use thereof
PatentWO2018157342A1
Innovation
- Development of polyurethane materials with soft segments more resistant to oxidation reactions than polyether, such as polycarbonate and PDMS-based polyurethanes.
- Introduction of polyisobutylene soft segments in polyurethane, demonstrating excellent oxidation and hydrolytic degradation resistance.
- Tailoring of polyurethane materials to produce a range of products from soft and flexible to hard and rigid, suitable for various medical device applications.
Medical device, method for preparation thereof, and use thereof
PatentActiveUS20190111186A1
Innovation
- A method involving the production of polyolefin polyurethane using hydrogenated polyolefin diols and microwave heating to create a material with improved resistance to oxidation and hydrolysis, characterized by specific molecular weights, elongation, and mechanical properties suitable for biomedical applications.
Regulatory Framework for PU in Medical Devices
The regulatory framework for polyurethane (PU) in medical devices is a complex and evolving landscape that manufacturers must navigate to ensure compliance and patient safety. In the United States, the Food and Drug Administration (FDA) oversees the regulation of medical devices containing PU through the Center for Devices and Radiological Health (CDRH). The FDA classifies medical devices into three categories based on their risk level, with Class III devices being the most stringent in terms of regulatory requirements.
For PU-containing medical devices, manufacturers must adhere to the FDA's Quality System Regulation (QSR), which outlines good manufacturing practices. This includes rigorous testing for biocompatibility, mechanical properties, and long-term stability of PU materials. The ISO 10993 series of standards, particularly ISO 10993-1, provides guidelines for evaluating the biocompatibility of medical devices, including those made with PU.
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) govern the use of PU in medical devices. These regulations emphasize a life-cycle approach to device safety and performance, requiring manufacturers to implement robust post-market surveillance systems. The CE marking process ensures that PU-containing devices meet all applicable EU health, safety, and environmental protection requirements.
Globally, the International Medical Device Regulators Forum (IMDRF) works to harmonize regulatory standards across different regions. This effort aims to streamline the approval process for PU-based medical devices in multiple markets while maintaining high safety standards. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have also established specific requirements for PU-containing devices, often aligning with international standards but with some unique national considerations.
Manufacturers must also consider environmental regulations, such as REACH in the EU, which may impact the use of certain PU formulations or additives. As sustainability becomes increasingly important, regulations are evolving to address the entire lifecycle of medical devices, including disposal and potential environmental impacts of PU materials.
The regulatory landscape for PU in medical devices continues to evolve, with increasing focus on long-term safety, biocompatibility, and environmental impact. Manufacturers must stay abreast of these changes and invest in ongoing compliance efforts to ensure their PU-based medical devices meet global regulatory requirements.
For PU-containing medical devices, manufacturers must adhere to the FDA's Quality System Regulation (QSR), which outlines good manufacturing practices. This includes rigorous testing for biocompatibility, mechanical properties, and long-term stability of PU materials. The ISO 10993 series of standards, particularly ISO 10993-1, provides guidelines for evaluating the biocompatibility of medical devices, including those made with PU.
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) govern the use of PU in medical devices. These regulations emphasize a life-cycle approach to device safety and performance, requiring manufacturers to implement robust post-market surveillance systems. The CE marking process ensures that PU-containing devices meet all applicable EU health, safety, and environmental protection requirements.
Globally, the International Medical Device Regulators Forum (IMDRF) works to harmonize regulatory standards across different regions. This effort aims to streamline the approval process for PU-based medical devices in multiple markets while maintaining high safety standards. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have also established specific requirements for PU-containing devices, often aligning with international standards but with some unique national considerations.
Manufacturers must also consider environmental regulations, such as REACH in the EU, which may impact the use of certain PU formulations or additives. As sustainability becomes increasingly important, regulations are evolving to address the entire lifecycle of medical devices, including disposal and potential environmental impacts of PU materials.
The regulatory landscape for PU in medical devices continues to evolve, with increasing focus on long-term safety, biocompatibility, and environmental impact. Manufacturers must stay abreast of these changes and invest in ongoing compliance efforts to ensure their PU-based medical devices meet global regulatory requirements.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations are paramount in the use of polyurethane for medical device manufacturing. The interaction between polyurethane-based devices and the human body must be carefully evaluated to ensure patient safety and optimal performance.
Polyurethanes used in medical applications undergo rigorous testing to assess their biocompatibility. This includes cytotoxicity tests to evaluate potential toxic effects on cells, sensitization tests to check for allergic reactions, and irritation tests to determine if the material causes inflammation or tissue damage. Additionally, hemocompatibility tests are conducted to assess the material's interaction with blood, which is crucial for devices in contact with the circulatory system.
The safety profile of polyurethane in medical devices is influenced by its chemical composition and manufacturing process. Manufacturers must carefully select raw materials and additives to minimize the risk of harmful leachables or degradation products. The use of catalysts, chain extenders, and other additives must be closely monitored to ensure they do not compromise the material's biocompatibility.
Long-term implantable devices made from polyurethane require extensive evaluation of their degradation behavior in the physiological environment. This includes assessing the potential for hydrolysis, oxidation, and enzymatic degradation, which could lead to the release of potentially harmful compounds or alter the device's mechanical properties over time.
Surface modifications of polyurethane can enhance its biocompatibility and safety profile. Techniques such as plasma treatment, grafting of bioactive molecules, or the incorporation of antimicrobial agents can improve the material's interaction with biological tissues and reduce the risk of infection or adverse reactions.
Regulatory bodies, such as the FDA and European Medicines Agency, have established guidelines for the evaluation of medical-grade polyurethanes. Manufacturers must comply with standards like ISO 10993 for biological evaluation of medical devices, which outlines a series of tests to assess the biocompatibility and safety of materials used in medical applications.
The sterilization process for polyurethane medical devices is another critical safety consideration. Common methods like ethylene oxide treatment, gamma irradiation, or steam sterilization must be carefully selected and validated to ensure they do not compromise the material's properties or introduce harmful residues.
In conclusion, while polyurethane offers numerous advantages in medical device manufacturing, its use necessitates a comprehensive approach to biocompatibility and safety. Continuous research and development efforts are focused on improving the long-term stability and biological performance of polyurethane-based medical devices, ensuring their safe and effective use in various medical applications.
Polyurethanes used in medical applications undergo rigorous testing to assess their biocompatibility. This includes cytotoxicity tests to evaluate potential toxic effects on cells, sensitization tests to check for allergic reactions, and irritation tests to determine if the material causes inflammation or tissue damage. Additionally, hemocompatibility tests are conducted to assess the material's interaction with blood, which is crucial for devices in contact with the circulatory system.
The safety profile of polyurethane in medical devices is influenced by its chemical composition and manufacturing process. Manufacturers must carefully select raw materials and additives to minimize the risk of harmful leachables or degradation products. The use of catalysts, chain extenders, and other additives must be closely monitored to ensure they do not compromise the material's biocompatibility.
Long-term implantable devices made from polyurethane require extensive evaluation of their degradation behavior in the physiological environment. This includes assessing the potential for hydrolysis, oxidation, and enzymatic degradation, which could lead to the release of potentially harmful compounds or alter the device's mechanical properties over time.
Surface modifications of polyurethane can enhance its biocompatibility and safety profile. Techniques such as plasma treatment, grafting of bioactive molecules, or the incorporation of antimicrobial agents can improve the material's interaction with biological tissues and reduce the risk of infection or adverse reactions.
Regulatory bodies, such as the FDA and European Medicines Agency, have established guidelines for the evaluation of medical-grade polyurethanes. Manufacturers must comply with standards like ISO 10993 for biological evaluation of medical devices, which outlines a series of tests to assess the biocompatibility and safety of materials used in medical applications.
The sterilization process for polyurethane medical devices is another critical safety consideration. Common methods like ethylene oxide treatment, gamma irradiation, or steam sterilization must be carefully selected and validated to ensure they do not compromise the material's properties or introduce harmful residues.
In conclusion, while polyurethane offers numerous advantages in medical device manufacturing, its use necessitates a comprehensive approach to biocompatibility and safety. Continuous research and development efforts are focused on improving the long-term stability and biological performance of polyurethane-based medical devices, ensuring their safe and effective use in various medical applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!