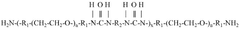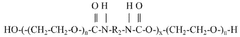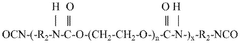What PU Bio‑Inks Advance 3D Bioprinting Applications?
JUN 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PU Bio-Inks Background
Polyurethane (PU) bio-inks have emerged as a promising material in the field of 3D bioprinting, offering unique properties that advance various biomedical applications. The development of PU bio-inks stems from the need for biocompatible, printable materials that can mimic the mechanical and biological properties of natural tissues.
PU bio-inks are derived from polyurethane, a versatile polymer known for its elasticity, durability, and biocompatibility. These characteristics make PU an ideal candidate for bioprinting applications, as it can be tailored to match the mechanical properties of different tissues while supporting cell growth and function.
The evolution of PU bio-inks can be traced back to the early 2000s when researchers began exploring the potential of polyurethane in tissue engineering. Initial studies focused on developing PU scaffolds for tissue regeneration, which laid the foundation for their use in 3D bioprinting.
As 3D bioprinting technology advanced, the demand for suitable bio-inks grew. PU bio-inks gained attention due to their ability to be easily modified and functionalized, allowing for the incorporation of various bioactive molecules and growth factors. This versatility enables the creation of complex, multi-functional structures that can better mimic the native tissue environment.
One of the key advantages of PU bio-inks is their tunable mechanical properties. By adjusting the chemical composition and crosslinking density, researchers can create bio-inks with a wide range of stiffness and elasticity. This flexibility allows for the fabrication of tissues with varying mechanical requirements, from soft tissues like cartilage to more rigid structures like bone.
The biocompatibility of PU bio-inks has been extensively studied, demonstrating their ability to support cell adhesion, proliferation, and differentiation. This is crucial for creating functional tissue constructs that can integrate with the host tissue upon implantation.
Recent advancements in PU bio-ink technology have focused on improving their printability and resolution. Researchers have developed novel formulations that exhibit shear-thinning behavior, allowing for smooth extrusion during the printing process while maintaining shape fidelity post-printing.
The application of PU bio-inks in 3D bioprinting has expanded to various fields, including tissue engineering, drug delivery, and regenerative medicine. They have been successfully used to create complex tissue constructs, such as vascularized tissues, cartilage, and bone scaffolds.
As the field of 3D bioprinting continues to evolve, PU bio-inks are expected to play an increasingly important role in advancing biomedical applications. Ongoing research aims to further enhance their properties, develop new functionalization strategies, and explore their potential in creating more complex, multi-tissue structures.
PU bio-inks are derived from polyurethane, a versatile polymer known for its elasticity, durability, and biocompatibility. These characteristics make PU an ideal candidate for bioprinting applications, as it can be tailored to match the mechanical properties of different tissues while supporting cell growth and function.
The evolution of PU bio-inks can be traced back to the early 2000s when researchers began exploring the potential of polyurethane in tissue engineering. Initial studies focused on developing PU scaffolds for tissue regeneration, which laid the foundation for their use in 3D bioprinting.
As 3D bioprinting technology advanced, the demand for suitable bio-inks grew. PU bio-inks gained attention due to their ability to be easily modified and functionalized, allowing for the incorporation of various bioactive molecules and growth factors. This versatility enables the creation of complex, multi-functional structures that can better mimic the native tissue environment.
One of the key advantages of PU bio-inks is their tunable mechanical properties. By adjusting the chemical composition and crosslinking density, researchers can create bio-inks with a wide range of stiffness and elasticity. This flexibility allows for the fabrication of tissues with varying mechanical requirements, from soft tissues like cartilage to more rigid structures like bone.
The biocompatibility of PU bio-inks has been extensively studied, demonstrating their ability to support cell adhesion, proliferation, and differentiation. This is crucial for creating functional tissue constructs that can integrate with the host tissue upon implantation.
Recent advancements in PU bio-ink technology have focused on improving their printability and resolution. Researchers have developed novel formulations that exhibit shear-thinning behavior, allowing for smooth extrusion during the printing process while maintaining shape fidelity post-printing.
The application of PU bio-inks in 3D bioprinting has expanded to various fields, including tissue engineering, drug delivery, and regenerative medicine. They have been successfully used to create complex tissue constructs, such as vascularized tissues, cartilage, and bone scaffolds.
As the field of 3D bioprinting continues to evolve, PU bio-inks are expected to play an increasingly important role in advancing biomedical applications. Ongoing research aims to further enhance their properties, develop new functionalization strategies, and explore their potential in creating more complex, multi-tissue structures.
3D Bioprinting Market
The 3D bioprinting market has experienced significant growth in recent years, driven by advancements in technology and increasing applications in healthcare and research. This market segment is poised for continued expansion, with projections indicating substantial growth over the next decade.
The healthcare sector remains the primary driver of the 3D bioprinting market, with applications spanning tissue engineering, regenerative medicine, and drug discovery. The ability to create complex, three-dimensional biological structures has opened new avenues for personalized medicine and organ transplantation alternatives.
Pharmaceutical companies are increasingly adopting 3D bioprinting technologies for drug screening and toxicity testing, reducing the reliance on animal models and potentially accelerating the drug development process. This shift is expected to contribute significantly to market growth in the coming years.
Academic and research institutions continue to be major contributors to the 3D bioprinting market, driving innovation and exploring new applications. Collaborations between academia and industry are becoming more prevalent, fostering the development of novel bioinks and printing techniques.
Geographically, North America currently leads the 3D bioprinting market, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by increasing investments in healthcare infrastructure and research facilities.
The market is characterized by a mix of established players and innovative startups. Key companies in the space are focusing on developing advanced bioinks, improving printing resolution, and expanding the range of printable biomaterials. Strategic partnerships and acquisitions are becoming common as companies seek to strengthen their market positions and expand their technological capabilities.
Challenges in the 3D bioprinting market include regulatory hurdles, particularly for clinical applications, and the need for standardization in bioink formulations and printing processes. Overcoming these challenges will be crucial for widespread adoption and commercialization of 3D bioprinted products.
Despite these challenges, the potential applications of 3D bioprinting continue to expand. Emerging areas of interest include the development of in vitro disease models, personalized implants, and bioprinted meat alternatives, indicating diverse growth opportunities beyond traditional healthcare applications.
The healthcare sector remains the primary driver of the 3D bioprinting market, with applications spanning tissue engineering, regenerative medicine, and drug discovery. The ability to create complex, three-dimensional biological structures has opened new avenues for personalized medicine and organ transplantation alternatives.
Pharmaceutical companies are increasingly adopting 3D bioprinting technologies for drug screening and toxicity testing, reducing the reliance on animal models and potentially accelerating the drug development process. This shift is expected to contribute significantly to market growth in the coming years.
Academic and research institutions continue to be major contributors to the 3D bioprinting market, driving innovation and exploring new applications. Collaborations between academia and industry are becoming more prevalent, fostering the development of novel bioinks and printing techniques.
Geographically, North America currently leads the 3D bioprinting market, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by increasing investments in healthcare infrastructure and research facilities.
The market is characterized by a mix of established players and innovative startups. Key companies in the space are focusing on developing advanced bioinks, improving printing resolution, and expanding the range of printable biomaterials. Strategic partnerships and acquisitions are becoming common as companies seek to strengthen their market positions and expand their technological capabilities.
Challenges in the 3D bioprinting market include regulatory hurdles, particularly for clinical applications, and the need for standardization in bioink formulations and printing processes. Overcoming these challenges will be crucial for widespread adoption and commercialization of 3D bioprinted products.
Despite these challenges, the potential applications of 3D bioprinting continue to expand. Emerging areas of interest include the development of in vitro disease models, personalized implants, and bioprinted meat alternatives, indicating diverse growth opportunities beyond traditional healthcare applications.
PU Bio-Inks Challenges
Despite the promising potential of PU bio-inks in 3D bioprinting applications, several significant challenges hinder their widespread adoption and optimal performance. These challenges span across material properties, processing techniques, and biological considerations.
One of the primary obstacles is achieving the ideal balance between printability and biocompatibility. PU bio-inks must possess suitable rheological properties for extrusion-based printing while maintaining a cell-friendly environment. The viscosity and shear-thinning behavior of the ink need to be carefully tuned to ensure smooth extrusion without compromising cell viability.
Another critical challenge lies in the crosslinking mechanisms of PU bio-inks. While rapid crosslinking is desirable for shape fidelity, it can potentially harm encapsulated cells. Conversely, slow crosslinking may lead to poor structural integrity. Developing PU bio-inks with controllable crosslinking kinetics that allow for both efficient printing and cell survival remains a significant hurdle.
The mechanical properties of printed PU constructs also present challenges. Achieving the right balance between strength and elasticity to mimic native tissue properties is crucial. Many PU bio-inks struggle to provide adequate mechanical support while maintaining the necessary flexibility for tissue-like behavior.
Biodegradation rate control is another complex issue. The degradation of PU scaffolds should ideally match the rate of new tissue formation. However, fine-tuning this process to align with specific tissue regeneration timelines proves challenging, especially considering the variability in tissue types and patient-specific factors.
Long-term biocompatibility and potential toxicity of degradation products remain concerns. While PUs are generally considered biocompatible, the long-term effects of their degradation byproducts on cellular behavior and tissue function are not fully understood. This uncertainty poses risks for clinical translation.
Scalability and reproducibility in manufacturing PU bio-inks present additional hurdles. Ensuring consistent quality and properties across different batches is crucial for standardization and regulatory approval. The complex chemistry of PUs makes this particularly challenging.
Furthermore, the integration of bioactive molecules and growth factors into PU bio-inks without compromising their functionality during the printing process is a significant challenge. Preserving the bioactivity of these molecules throughout the ink preparation, printing, and crosslinking stages is critical for promoting desired cellular responses.
Lastly, the optimization of print parameters for PU bio-inks remains a complex task. Factors such as print speed, extrusion pressure, and environmental conditions significantly impact print quality and cell viability. Developing standardized protocols that can be adapted to various PU formulations and printing setups is an ongoing challenge in the field.
One of the primary obstacles is achieving the ideal balance between printability and biocompatibility. PU bio-inks must possess suitable rheological properties for extrusion-based printing while maintaining a cell-friendly environment. The viscosity and shear-thinning behavior of the ink need to be carefully tuned to ensure smooth extrusion without compromising cell viability.
Another critical challenge lies in the crosslinking mechanisms of PU bio-inks. While rapid crosslinking is desirable for shape fidelity, it can potentially harm encapsulated cells. Conversely, slow crosslinking may lead to poor structural integrity. Developing PU bio-inks with controllable crosslinking kinetics that allow for both efficient printing and cell survival remains a significant hurdle.
The mechanical properties of printed PU constructs also present challenges. Achieving the right balance between strength and elasticity to mimic native tissue properties is crucial. Many PU bio-inks struggle to provide adequate mechanical support while maintaining the necessary flexibility for tissue-like behavior.
Biodegradation rate control is another complex issue. The degradation of PU scaffolds should ideally match the rate of new tissue formation. However, fine-tuning this process to align with specific tissue regeneration timelines proves challenging, especially considering the variability in tissue types and patient-specific factors.
Long-term biocompatibility and potential toxicity of degradation products remain concerns. While PUs are generally considered biocompatible, the long-term effects of their degradation byproducts on cellular behavior and tissue function are not fully understood. This uncertainty poses risks for clinical translation.
Scalability and reproducibility in manufacturing PU bio-inks present additional hurdles. Ensuring consistent quality and properties across different batches is crucial for standardization and regulatory approval. The complex chemistry of PUs makes this particularly challenging.
Furthermore, the integration of bioactive molecules and growth factors into PU bio-inks without compromising their functionality during the printing process is a significant challenge. Preserving the bioactivity of these molecules throughout the ink preparation, printing, and crosslinking stages is critical for promoting desired cellular responses.
Lastly, the optimization of print parameters for PU bio-inks remains a complex task. Factors such as print speed, extrusion pressure, and environmental conditions significantly impact print quality and cell viability. Developing standardized protocols that can be adapted to various PU formulations and printing setups is an ongoing challenge in the field.
Current PU Bio-Inks
01 Composition of PU Bio-Inks
PU bio-inks are formulated using biodegradable polyurethane materials combined with biocompatible additives. These inks are designed to be environmentally friendly and suitable for various bioprinting applications. The composition may include natural polymers, synthetic polymers, and bioactive components to enhance printability and biological performance.- Composition of PU Bio-Inks: PU bio-inks are composed of polyurethane-based materials combined with biocompatible components. These inks are designed to be used in 3D bioprinting applications, offering a balance between mechanical properties and biocompatibility. The formulation may include natural polymers, growth factors, and cells to enhance biological functionality.
- 3D Bioprinting Applications: PU bio-inks are utilized in various 3D bioprinting applications, including tissue engineering, organ-on-a-chip models, and drug screening platforms. The inks can be precisely deposited to create complex, three-dimensional structures that mimic natural tissue architecture, supporting cell growth and differentiation.
- Mechanical and Biological Properties: The development of PU bio-inks focuses on optimizing both mechanical and biological properties. This includes tuning the elasticity, strength, and degradation rate of the printed structures while maintaining cell viability and promoting tissue formation. Advanced formulations may incorporate nanoparticles or other additives to enhance specific properties.
- Crosslinking and Curing Methods: Various crosslinking and curing methods are employed to solidify PU bio-inks after printing. These may include UV-initiated polymerization, thermal curing, or ionic crosslinking. The choice of method affects the final properties of the printed structure and must be compatible with cell survival and function.
- Biocompatibility and Biodegradability: Ensuring the biocompatibility and controlled biodegradability of PU bio-inks is crucial for their successful application in tissue engineering and regenerative medicine. Research focuses on developing formulations that support long-term cell viability, promote tissue integration, and degrade at rates matching tissue regeneration.
02 3D Bioprinting Applications
PU bio-inks are utilized in 3D bioprinting for tissue engineering and regenerative medicine. These inks enable the fabrication of complex tissue-like structures with controlled porosity and mechanical properties. They can be used to create scaffolds for cell growth, drug delivery systems, and personalized medical implants.Expand Specific Solutions03 Crosslinking and Curing Methods
Various crosslinking and curing methods are employed to solidify PU bio-inks after printing. These may include UV-initiated photopolymerization, thermal curing, or chemical crosslinking. The choice of curing method affects the final properties of the printed structures, such as mechanical strength and degradation rate.Expand Specific Solutions04 Incorporation of Bioactive Molecules
PU bio-inks can be modified to incorporate bioactive molecules such as growth factors, drugs, or cell-adhesion peptides. This enhances the biological functionality of the printed constructs, promoting cell growth, differentiation, and tissue formation. The controlled release of these molecules can be tailored for specific therapeutic applications.Expand Specific Solutions05 Mechanical and Rheological Properties
The mechanical and rheological properties of PU bio-inks are crucial for successful 3D printing and the performance of the final constructs. Researchers focus on optimizing viscosity, shear-thinning behavior, and elasticity to ensure good printability and shape fidelity. Post-printing, the mechanical strength and elasticity of the cured structures are tailored to match the target tissue properties.Expand Specific Solutions
Bioprinting Industry
The 3D bioprinting market utilizing PU bio-inks is in a growth phase, with increasing applications in tissue engineering and regenerative medicine. The global market size for 3D bioprinting is projected to reach $1.9 billion by 2028, driven by advancements in biomaterials and printing technologies. The technology's maturity varies across applications, with companies like BICO Group AB and Aspect Biosystems Ltd. leading commercial development. Academic institutions such as Texas A&M University and Sun Yat-Sen University are pushing the boundaries of PU bio-ink research, while collaborations between industry and academia, exemplified by UVic Industry Partnerships, Inc., are accelerating innovation in this field. The diverse range of players, from established biotech firms to emerging startups, indicates a competitive and rapidly evolving landscape.
BICO Group AB
Technical Solution: BICO Group AB has developed advanced PU bio-inks for 3D bioprinting applications. Their proprietary formulations incorporate biocompatible polyurethane materials with tunable mechanical properties. These bio-inks can be precisely extruded to create complex tissue-like structures with high resolution and shape fidelity. The PU-based bio-inks are designed to mimic the extracellular matrix and provide a supportive environment for cell growth and differentiation. BICO's bio-inks are compatible with various cell types and can be customized for specific tissue engineering applications.
Strengths: Highly customizable formulations, excellent printability, and good biocompatibility. Weaknesses: May have limited biodegradability compared to natural polymers.
Aspect Biosystems Ltd.
Technical Solution: Aspect Biosystems has developed a proprietary Lab-on-a-Printer™ 3D bioprinting platform that utilizes advanced PU bio-inks. Their technology combines microfluidic printheads with tailored bio-ink formulations to create complex tissue structures. The PU-based bio-inks are engineered to provide optimal mechanical support and allow for the incorporation of multiple cell types and biomolecules. Aspect's bio-inks can be rapidly crosslinked during the printing process, enabling the creation of intricate 3D geometries with high precision. The company's bio-inks are particularly well-suited for applications in regenerative medicine and drug discovery.
Strengths: High-precision printing capabilities, versatile bio-ink formulations. Weaknesses: May require specialized printing equipment.
Key PU Bio-Ink Patents
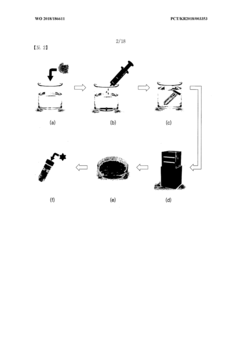
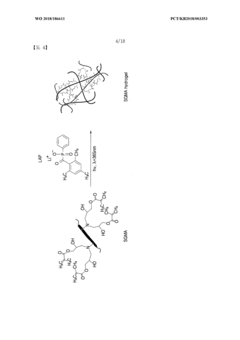
Water-soluble, biocompatible, biodegradable, 3d-printable, and radiation-curable polymeric products used as bioink
PatentWO2025095908A1
Innovation
- Development of synthetic, water-soluble, biocompatible, biodegradable, and radiation-curable bioinks based on PEO containing reactive functionally terminated polyurethane and polyurea oligomers, which can be tailored for specific mechanical and printing needs by adjusting molecular weight, viscosity, and cross-linking density.
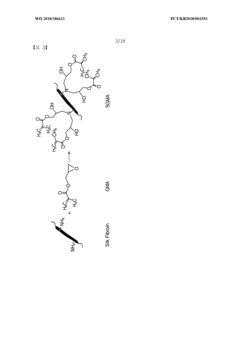
Bioink and preparation method therefor
PatentWO2018186611A2
Innovation
- A bio-ink composed of silk fibroin and methacrylate-based compounds, polymerized with a photoinitiator, which forms a hydrogel upon light exposure, offering enhanced mechanical properties and biocompatibility, suitable for 3D printing using methods like DLP, enabling the creation of structures with improved cytocompatibility and physical properties.
Biocompatibility Tests
Biocompatibility testing is a critical aspect of developing PU bio-inks for 3D bioprinting applications. These tests are essential to ensure that the printed constructs are safe and suitable for use in biological systems. The evaluation of biocompatibility typically involves a series of in vitro and in vivo studies designed to assess the material's interactions with living tissues and cells.
In vitro biocompatibility tests for PU bio-inks often begin with cytotoxicity assays. These tests evaluate the potential toxic effects of the bio-ink on cells, typically using standardized cell lines. Common methods include the MTT assay, which measures cell metabolic activity, and the Live/Dead assay, which distinguishes between viable and non-viable cells. Additionally, cell adhesion and proliferation studies are conducted to assess how well cells attach to and grow on the printed PU constructs.
Further in vitro tests may include genotoxicity assays to evaluate potential DNA damage, and hemolysis tests to assess the bio-ink's compatibility with blood components. These tests provide valuable insights into the material's safety profile and its potential for use in specific biomedical applications.
In vivo biocompatibility testing of PU bio-inks involves implanting the printed constructs into animal models. These studies assess the host response to the implanted material over time, including inflammation, immune response, and tissue integration. Histological analysis of the surrounding tissues is performed to evaluate cell infiltration, vascularization, and potential adverse reactions.
Long-term biocompatibility studies are crucial for understanding the degradation behavior of PU bio-inks and their impact on surrounding tissues. These studies typically span several weeks to months and involve periodic assessments of the implant site. Researchers evaluate factors such as the rate of material degradation, the formation of new tissue, and any potential long-term adverse effects.
Specific biocompatibility tests may be tailored to the intended application of the PU bio-ink. For example, if the bio-ink is designed for use in bone tissue engineering, osteoconductivity and osteoinductivity tests may be performed to assess its ability to support bone formation. Similarly, for cardiovascular applications, tests may focus on hemocompatibility and endothelialization.
Regulatory compliance is a crucial consideration in biocompatibility testing. Researchers must adhere to established guidelines, such as those outlined in ISO 10993, which provides a framework for the biological evaluation of medical devices. Compliance with these standards ensures that the biocompatibility testing is comprehensive and meets regulatory requirements for potential clinical translation.
In vitro biocompatibility tests for PU bio-inks often begin with cytotoxicity assays. These tests evaluate the potential toxic effects of the bio-ink on cells, typically using standardized cell lines. Common methods include the MTT assay, which measures cell metabolic activity, and the Live/Dead assay, which distinguishes between viable and non-viable cells. Additionally, cell adhesion and proliferation studies are conducted to assess how well cells attach to and grow on the printed PU constructs.
Further in vitro tests may include genotoxicity assays to evaluate potential DNA damage, and hemolysis tests to assess the bio-ink's compatibility with blood components. These tests provide valuable insights into the material's safety profile and its potential for use in specific biomedical applications.
In vivo biocompatibility testing of PU bio-inks involves implanting the printed constructs into animal models. These studies assess the host response to the implanted material over time, including inflammation, immune response, and tissue integration. Histological analysis of the surrounding tissues is performed to evaluate cell infiltration, vascularization, and potential adverse reactions.
Long-term biocompatibility studies are crucial for understanding the degradation behavior of PU bio-inks and their impact on surrounding tissues. These studies typically span several weeks to months and involve periodic assessments of the implant site. Researchers evaluate factors such as the rate of material degradation, the formation of new tissue, and any potential long-term adverse effects.
Specific biocompatibility tests may be tailored to the intended application of the PU bio-ink. For example, if the bio-ink is designed for use in bone tissue engineering, osteoconductivity and osteoinductivity tests may be performed to assess its ability to support bone formation. Similarly, for cardiovascular applications, tests may focus on hemocompatibility and endothelialization.
Regulatory compliance is a crucial consideration in biocompatibility testing. Researchers must adhere to established guidelines, such as those outlined in ISO 10993, which provides a framework for the biological evaluation of medical devices. Compliance with these standards ensures that the biocompatibility testing is comprehensive and meets regulatory requirements for potential clinical translation.
Regulatory Framework
The regulatory framework surrounding PU bio-inks and 3D bioprinting applications is complex and evolving, reflecting the rapid advancements in this field. Regulatory bodies worldwide are working to establish guidelines that ensure the safety and efficacy of bioprinted products while fostering innovation.
In the United States, the Food and Drug Administration (FDA) has taken a leading role in developing regulatory approaches for 3D-printed medical devices and bioprinted tissues. The FDA has issued guidance documents outlining the technical considerations for additive manufactured devices, including those using PU bio-inks. These guidelines address aspects such as material characterization, process validation, and quality control measures.
The European Medicines Agency (EMA) and the European Commission have also been actively developing regulatory frameworks for advanced therapy medicinal products (ATMPs), which include bioprinted tissues and organs. Their approach emphasizes risk-based assessments and the implementation of good manufacturing practices (GMP) throughout the production process.
In Asia, countries like Japan and Singapore have implemented fast-track approval processes for regenerative medicine products, potentially accelerating the path to market for certain bioprinted applications. These regulatory pathways aim to balance patient safety with the need for rapid innovation in the field.
International standardization efforts are underway to harmonize regulatory approaches across different regions. Organizations such as the International Organization for Standardization (ISO) and the American Society for Testing and Materials (ASTM) are developing standards specific to bioprinting processes and materials, including PU bio-inks.
Key regulatory considerations for PU bio-inks in 3D bioprinting applications include biocompatibility testing, sterilization validation, and long-term stability assessments. Regulators are particularly focused on ensuring the consistency and reproducibility of bioprinted constructs, given the complexity of the manufacturing process and the variability of biological materials.
As the field continues to advance, regulatory frameworks are expected to evolve to address emerging challenges, such as the integration of artificial intelligence in bioprinting processes and the development of more complex, multi-material constructs. Ongoing dialogue between researchers, industry stakeholders, and regulatory agencies will be crucial in shaping a regulatory landscape that promotes innovation while safeguarding public health.
In the United States, the Food and Drug Administration (FDA) has taken a leading role in developing regulatory approaches for 3D-printed medical devices and bioprinted tissues. The FDA has issued guidance documents outlining the technical considerations for additive manufactured devices, including those using PU bio-inks. These guidelines address aspects such as material characterization, process validation, and quality control measures.
The European Medicines Agency (EMA) and the European Commission have also been actively developing regulatory frameworks for advanced therapy medicinal products (ATMPs), which include bioprinted tissues and organs. Their approach emphasizes risk-based assessments and the implementation of good manufacturing practices (GMP) throughout the production process.
In Asia, countries like Japan and Singapore have implemented fast-track approval processes for regenerative medicine products, potentially accelerating the path to market for certain bioprinted applications. These regulatory pathways aim to balance patient safety with the need for rapid innovation in the field.
International standardization efforts are underway to harmonize regulatory approaches across different regions. Organizations such as the International Organization for Standardization (ISO) and the American Society for Testing and Materials (ASTM) are developing standards specific to bioprinting processes and materials, including PU bio-inks.
Key regulatory considerations for PU bio-inks in 3D bioprinting applications include biocompatibility testing, sterilization validation, and long-term stability assessments. Regulators are particularly focused on ensuring the consistency and reproducibility of bioprinted constructs, given the complexity of the manufacturing process and the variability of biological materials.
As the field continues to advance, regulatory frameworks are expected to evolve to address emerging challenges, such as the integration of artificial intelligence in bioprinting processes and the development of more complex, multi-material constructs. Ongoing dialogue between researchers, industry stakeholders, and regulatory agencies will be crucial in shaping a regulatory landscape that promotes innovation while safeguarding public health.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!