The Science Behind Carbon Tetrachloride's Solvent Characteristics
JUL 2, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Solvent Properties
Carbon tetrachloride (CCl4) is a colorless, volatile liquid with exceptional solvent properties that have made it a valuable compound in various industrial and laboratory applications. Its unique characteristics stem from its molecular structure and intermolecular forces, which contribute to its ability to dissolve a wide range of substances.
The solvent properties of CCl4 are primarily attributed to its non-polar nature. The molecule's symmetrical tetrahedral structure, with four chlorine atoms surrounding a central carbon atom, results in a uniform distribution of electron density. This symmetry leads to the cancellation of dipole moments, making CCl4 a non-polar molecule. As a result, it excels at dissolving other non-polar substances, following the principle of "like dissolves like."
One of the key features that make CCl4 an effective solvent is its low dielectric constant. This property indicates a weak ability to stabilize charge separation, which enhances its capacity to dissolve non-polar compounds. The low dielectric constant also contributes to CCl4's immiscibility with water, a characteristic that is exploited in various separation techniques.
CCl4's high volatility is another crucial aspect of its solvent properties. The relatively weak intermolecular forces between CCl4 molecules allow for easy vaporization, making it ideal for applications where rapid evaporation is desired. This property is particularly useful in cleaning and degreasing processes, as well as in certain extraction methods.
The density of CCl4 is higher than that of water, which enables its use in density-based separations. This property, combined with its immiscibility with water, makes it valuable in liquid-liquid extractions and as a solvent in organic synthesis reactions where phase separation is required.
Carbon tetrachloride's ability to dissolve a wide range of organic compounds, including fats, oils, and many synthetic polymers, has made it a versatile solvent in various industries. Its inertness towards many organic reactions also contributes to its usefulness in organic synthesis, where it can serve as a non-reactive medium for chemical transformations.
However, it is important to note that the use of CCl4 has been significantly restricted due to its toxicity and environmental impact. Its ozone-depleting properties and potential carcinogenicity have led to the development of alternative solvents in many applications. Despite these limitations, understanding the science behind CCl4's solvent characteristics remains valuable for developing safer and more environmentally friendly solvents with similar properties.
The solvent properties of CCl4 are primarily attributed to its non-polar nature. The molecule's symmetrical tetrahedral structure, with four chlorine atoms surrounding a central carbon atom, results in a uniform distribution of electron density. This symmetry leads to the cancellation of dipole moments, making CCl4 a non-polar molecule. As a result, it excels at dissolving other non-polar substances, following the principle of "like dissolves like."
One of the key features that make CCl4 an effective solvent is its low dielectric constant. This property indicates a weak ability to stabilize charge separation, which enhances its capacity to dissolve non-polar compounds. The low dielectric constant also contributes to CCl4's immiscibility with water, a characteristic that is exploited in various separation techniques.
CCl4's high volatility is another crucial aspect of its solvent properties. The relatively weak intermolecular forces between CCl4 molecules allow for easy vaporization, making it ideal for applications where rapid evaporation is desired. This property is particularly useful in cleaning and degreasing processes, as well as in certain extraction methods.
The density of CCl4 is higher than that of water, which enables its use in density-based separations. This property, combined with its immiscibility with water, makes it valuable in liquid-liquid extractions and as a solvent in organic synthesis reactions where phase separation is required.
Carbon tetrachloride's ability to dissolve a wide range of organic compounds, including fats, oils, and many synthetic polymers, has made it a versatile solvent in various industries. Its inertness towards many organic reactions also contributes to its usefulness in organic synthesis, where it can serve as a non-reactive medium for chemical transformations.
However, it is important to note that the use of CCl4 has been significantly restricted due to its toxicity and environmental impact. Its ozone-depleting properties and potential carcinogenicity have led to the development of alternative solvents in many applications. Despite these limitations, understanding the science behind CCl4's solvent characteristics remains valuable for developing safer and more environmentally friendly solvents with similar properties.
Industrial Demand Analysis
Carbon tetrachloride's unique solvent characteristics have driven significant industrial demand across various sectors. The chemical's ability to dissolve a wide range of organic compounds, coupled with its non-flammability and stability, has made it a valuable solvent in numerous applications. Historically, carbon tetrachloride found extensive use in dry cleaning, fire extinguishers, and as a precursor in the production of refrigerants.
In the chemical industry, carbon tetrachloride has been widely employed as a solvent for oils, fats, lacquers, varnishes, rubber waxes, and resins. Its effectiveness in dissolving these substances has made it an essential component in many manufacturing processes. The pharmaceutical sector has also utilized carbon tetrachloride in drug synthesis and as a solvent for extracting and purifying active pharmaceutical ingredients.
The electronics industry has benefited from carbon tetrachloride's solvent properties in the production of semiconductors and in cleaning electronic components. Its ability to remove oils and greases without leaving residues has made it valuable in precision cleaning applications. Additionally, the agrochemical sector has used carbon tetrachloride in the formulation of pesticides and herbicides, taking advantage of its solvent capabilities to enhance the effectiveness of active ingredients.
However, the demand for carbon tetrachloride has faced significant challenges in recent years due to environmental and health concerns. The Montreal Protocol, which aims to protect the ozone layer, has phased out the production and consumption of ozone-depleting substances, including carbon tetrachloride. This has led to a sharp decline in its use in many applications, particularly in developed countries.
Despite these restrictions, there remains a limited demand for carbon tetrachloride in certain industrial processes where suitable alternatives are not readily available. Some countries still use it as a feedstock for the production of other chemicals, taking advantage of its unique properties while implementing strict controls to prevent environmental release.
The market for carbon tetrachloride has shifted towards more specialized and controlled applications. Research and development efforts are ongoing to find safer alternatives that can match its solvent effectiveness. This has created opportunities for innovation in green chemistry and sustainable solvent technologies. As industries adapt to regulatory changes and environmental concerns, the demand for carbon tetrachloride continues to evolve, with a focus on essential uses where its solvent characteristics remain unmatched by safer alternatives.
In the chemical industry, carbon tetrachloride has been widely employed as a solvent for oils, fats, lacquers, varnishes, rubber waxes, and resins. Its effectiveness in dissolving these substances has made it an essential component in many manufacturing processes. The pharmaceutical sector has also utilized carbon tetrachloride in drug synthesis and as a solvent for extracting and purifying active pharmaceutical ingredients.
The electronics industry has benefited from carbon tetrachloride's solvent properties in the production of semiconductors and in cleaning electronic components. Its ability to remove oils and greases without leaving residues has made it valuable in precision cleaning applications. Additionally, the agrochemical sector has used carbon tetrachloride in the formulation of pesticides and herbicides, taking advantage of its solvent capabilities to enhance the effectiveness of active ingredients.
However, the demand for carbon tetrachloride has faced significant challenges in recent years due to environmental and health concerns. The Montreal Protocol, which aims to protect the ozone layer, has phased out the production and consumption of ozone-depleting substances, including carbon tetrachloride. This has led to a sharp decline in its use in many applications, particularly in developed countries.
Despite these restrictions, there remains a limited demand for carbon tetrachloride in certain industrial processes where suitable alternatives are not readily available. Some countries still use it as a feedstock for the production of other chemicals, taking advantage of its unique properties while implementing strict controls to prevent environmental release.
The market for carbon tetrachloride has shifted towards more specialized and controlled applications. Research and development efforts are ongoing to find safer alternatives that can match its solvent effectiveness. This has created opportunities for innovation in green chemistry and sustainable solvent technologies. As industries adapt to regulatory changes and environmental concerns, the demand for carbon tetrachloride continues to evolve, with a focus on essential uses where its solvent characteristics remain unmatched by safer alternatives.
Current Challenges
Carbon tetrachloride (CCl4) has long been recognized for its exceptional solvent properties, particularly in industrial applications. However, the current challenges surrounding its use are multifaceted and significant, encompassing environmental, health, and regulatory concerns.
One of the primary challenges is the severe environmental impact of carbon tetrachloride. It is a potent ozone-depleting substance, contributing to the depletion of the Earth's protective ozone layer. This has led to strict regulations and phase-outs under international agreements such as the Montreal Protocol. The persistence of CCl4 in the atmosphere, with a lifetime of approximately 26 years, exacerbates its long-term environmental effects.
The toxicity of carbon tetrachloride poses another major challenge. Exposure to CCl4 can cause serious health issues, including liver and kidney damage, central nervous system depression, and potential carcinogenicity. These health risks have resulted in stringent occupational safety regulations and a significant reduction in its use in consumer products.
Regulatory restrictions present a substantial hurdle for industries that have traditionally relied on carbon tetrachloride as a solvent. Many countries have banned or severely limited its production and use, forcing companies to seek alternative solvents or develop new processes. This transition often requires significant investment in research and development, as well as modifications to existing manufacturing infrastructure.
The search for suitable replacements that match carbon tetrachloride's solvent characteristics without its negative impacts remains an ongoing challenge. While some alternatives have been developed, they often fall short in terms of effectiveness, cost-efficiency, or have their own environmental and health concerns.
Another challenge lies in the remediation of sites contaminated with carbon tetrachloride. Its high stability and low water solubility make it difficult to remove from soil and groundwater, necessitating complex and costly cleanup efforts. This legacy contamination continues to pose environmental and health risks long after the cessation of CCl4 use in many areas.
The scientific community faces the challenge of fully understanding the atmospheric chemistry of carbon tetrachloride. Recent studies have revealed discrepancies between observed atmospheric concentrations and estimated emissions, suggesting there may be unknown sources or incomplete understanding of its environmental fate and transport mechanisms.
Lastly, the global nature of carbon tetrachloride's impact requires coordinated international efforts for effective control and mitigation. Ensuring compliance with regulations across different countries and addressing illegal production and trade remain ongoing challenges in the global management of this potent solvent.
One of the primary challenges is the severe environmental impact of carbon tetrachloride. It is a potent ozone-depleting substance, contributing to the depletion of the Earth's protective ozone layer. This has led to strict regulations and phase-outs under international agreements such as the Montreal Protocol. The persistence of CCl4 in the atmosphere, with a lifetime of approximately 26 years, exacerbates its long-term environmental effects.
The toxicity of carbon tetrachloride poses another major challenge. Exposure to CCl4 can cause serious health issues, including liver and kidney damage, central nervous system depression, and potential carcinogenicity. These health risks have resulted in stringent occupational safety regulations and a significant reduction in its use in consumer products.
Regulatory restrictions present a substantial hurdle for industries that have traditionally relied on carbon tetrachloride as a solvent. Many countries have banned or severely limited its production and use, forcing companies to seek alternative solvents or develop new processes. This transition often requires significant investment in research and development, as well as modifications to existing manufacturing infrastructure.
The search for suitable replacements that match carbon tetrachloride's solvent characteristics without its negative impacts remains an ongoing challenge. While some alternatives have been developed, they often fall short in terms of effectiveness, cost-efficiency, or have their own environmental and health concerns.
Another challenge lies in the remediation of sites contaminated with carbon tetrachloride. Its high stability and low water solubility make it difficult to remove from soil and groundwater, necessitating complex and costly cleanup efforts. This legacy contamination continues to pose environmental and health risks long after the cessation of CCl4 use in many areas.
The scientific community faces the challenge of fully understanding the atmospheric chemistry of carbon tetrachloride. Recent studies have revealed discrepancies between observed atmospheric concentrations and estimated emissions, suggesting there may be unknown sources or incomplete understanding of its environmental fate and transport mechanisms.
Lastly, the global nature of carbon tetrachloride's impact requires coordinated international efforts for effective control and mitigation. Ensuring compliance with regulations across different countries and addressing illegal production and trade remain ongoing challenges in the global management of this potent solvent.
Existing Applications
01 Chemical properties and reactivity
Carbon tetrachloride is a colorless, volatile liquid with a characteristic odor. It is non-flammable and highly stable, making it useful in various industrial applications. However, it can react with some metals and strong bases, producing toxic gases. Its stability and reactivity characteristics make it suitable for certain chemical processes but also pose environmental and health risks.- Physical properties of carbon tetrachloride: Carbon tetrachloride is a colorless, volatile liquid with a characteristic odor. It has a high density and low boiling point, making it an effective solvent for various organic compounds. Its non-flammability and stability at room temperature contribute to its usefulness in certain industrial applications.
- Solvent capabilities and applications: Carbon tetrachloride is an excellent solvent for fats, oils, waxes, and resins. It has been widely used in dry cleaning, as a degreasing agent in metal industries, and as a solvent in various chemical processes. Its ability to dissolve a wide range of organic compounds makes it valuable in laboratory and industrial settings.
- Safety and environmental concerns: Despite its effectiveness as a solvent, carbon tetrachloride has been largely phased out due to its toxicity and environmental impact. It is known to be harmful to the ozone layer and can cause severe health effects upon exposure. Many countries have restricted or banned its use, leading to the development of safer alternatives in various applications.
- Chemical reactivity and stability: Carbon tetrachloride is generally stable under normal conditions but can react violently with some metals, especially when heated. It can decompose to form toxic phosgene gas when exposed to high temperatures or flames. Its chemical stability makes it useful in certain reactions, but precautions must be taken to prevent unwanted decomposition.
- Analytical and extraction applications: In analytical chemistry, carbon tetrachloride has been used as a solvent for spectroscopic studies due to its transparency in certain wavelength ranges. It has also been employed in liquid-liquid extraction processes and as a medium for chemical reactions. Its unique properties make it suitable for specific analytical techniques and separation processes.
02 Solvent capabilities and applications
Carbon tetrachloride is an excellent solvent for organic compounds, oils, fats, and resins. It has been widely used in dry cleaning, fire extinguishers, and as a degreasing agent in industrial processes. Its high solvency power makes it effective in extracting various substances, but its use has been restricted due to environmental concerns.Expand Specific Solutions03 Environmental impact and regulations
Due to its ozone-depleting properties and potential health hazards, the use of carbon tetrachloride has been heavily regulated and phased out in many applications. It is classified as a hazardous air pollutant and its production and use are controlled under international agreements. Alternative solvents and processes have been developed to replace carbon tetrachloride in various industries.Expand Specific Solutions04 Analytical and laboratory uses
Despite restrictions, carbon tetrachloride still finds limited use in analytical chemistry and laboratory applications. It serves as a solvent in spectroscopy, a reagent in organic synthesis, and a standard in some analytical methods. Its unique properties make it valuable in certain scientific procedures, although safer alternatives are preferred when possible.Expand Specific Solutions05 Physical properties and handling
Carbon tetrachloride has a high density, low viscosity, and low surface tension, which contribute to its effectiveness as a solvent. It is immiscible with water but miscible with many organic solvents. Due to its toxicity and environmental impact, special handling and disposal procedures are required. Proper storage and use of protective equipment are essential when working with this compound.Expand Specific Solutions
Key Industry Players
The competitive landscape for carbon tetrachloride's solvent characteristics is in a mature phase, with established players and well-understood applications. The global market size for carbon tetrachloride is relatively stable, primarily driven by its use in industrial processes and as a feedstock for other chemicals. Technologically, the field is well-developed, with companies like Evonik Operations GmbH, Air Liquide SA, and PetroChina Co., Ltd. leading in production and application. Research institutions such as Central South University and Changzhou University contribute to ongoing refinements in understanding and utilizing carbon tetrachloride's properties. While innovation continues, major breakthroughs are less frequent, focusing more on optimizing existing processes and exploring safer alternatives due to environmental concerns.
Air Liquide SA
Technical Solution: Air Liquide SA has focused on developing safe and sustainable methods for carbon tetrachloride production and utilization. Their approach combines innovative synthesis routes with advanced purification techniques to produce high-purity carbon tetrachloride with minimal environmental impact. Air Liquide has invested in research to understand the thermodynamic and kinetic properties of carbon tetrachloride under various conditions, enabling them to optimize its use in industrial processes[7]. They have also explored alternative applications that capitalize on carbon tetrachloride's unique solvent properties while minimizing its environmental footprint. This includes developing novel extraction and separation processes that leverage carbon tetrachloride's selectivity and efficiency[8].
Strengths: Sustainable production methods and optimized industrial applications. Weaknesses: Ongoing challenges in completely eliminating environmental risks.
Beijing University of Chemical Technology
Technical Solution: Beijing University of Chemical Technology has conducted extensive research on the molecular dynamics of carbon tetrachloride as a solvent. Their studies have employed advanced computational techniques to simulate the behavior of carbon tetrachloride molecules in various environments and with different solutes. This research has provided insights into the solvent's structure-property relationships and its interactions with both polar and non-polar substances[9]. The university has also investigated the role of carbon tetrachloride in supercritical fluid applications, exploring its potential as a green solvent alternative in certain processes. Additionally, their work has contributed to understanding the environmental fate and transport of carbon tetrachloride, informing strategies for its responsible use and remediation[10].
Strengths: Deep understanding of molecular dynamics and potential for green chemistry applications. Weaknesses: Challenges in translating theoretical insights into practical industrial solutions.
Molecular Interactions
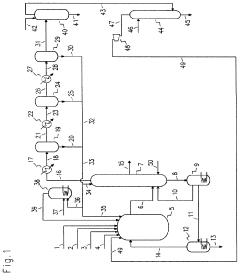
Chlorinolysis process for producing carbon tetrachloride
PatentActiveUS20210130266A1
Innovation
- A process involving a chlorination zone with chlorine, a C1 chlorinated compound, and a carbon/second chlorine source to produce a reaction mixture that favors the formation of carbon tetrachloride over perchloroethylene, using waste products as the carbon/second chlorine source to enhance efficiency and reduce impurity formation.
Treatment of water effluent
PatentPendingUS20190092658A1
Innovation
- A method and system using a filter material comprising a porous carbon support layer and silicate wool or glass wool with distributed halogens or halides, where an electric current is passed through the filter material to adsorb and decompose hormonal contaminants from aqueous effluent streams.
Environmental Impact
Carbon tetrachloride, once widely used as a solvent, has been recognized for its significant environmental impact. This compound's persistence in the environment and its ability to deplete the ozone layer have led to severe restrictions on its use globally.
When released into the atmosphere, carbon tetrachloride contributes to the depletion of the ozone layer. It breaks down in the stratosphere, releasing chlorine atoms that catalyze the destruction of ozone molecules. This process reduces the Earth's protection against harmful ultraviolet radiation, potentially increasing the risk of skin cancer and other health issues for humans and wildlife.
In aquatic environments, carbon tetrachloride can persist for long periods due to its low solubility in water and resistance to biodegradation. It can accumulate in sediments and bioaccumulate in aquatic organisms, potentially causing long-term ecological damage. The compound's toxicity to aquatic life, even at low concentrations, poses a threat to the balance of marine and freshwater ecosystems.
Soil contamination by carbon tetrachloride is another significant concern. Its high mobility in soil allows it to migrate easily, potentially contaminating groundwater sources. This contamination can persist for decades, affecting drinking water quality and posing risks to human health and agricultural activities.
The production and use of carbon tetrachloride also contribute to air pollution. Its volatile nature means it can easily evaporate and disperse in the air, contributing to the formation of smog and potentially affecting air quality in urban and industrial areas.
Given its environmental persistence and toxicity, the global community has taken steps to phase out the use of carbon tetrachloride under the Montreal Protocol. This international treaty, designed to protect the ozone layer, has significantly reduced the production and consumption of ozone-depleting substances, including carbon tetrachloride.
Despite these efforts, the environmental legacy of past carbon tetrachloride use continues to be a concern. Remediation of contaminated sites and ongoing monitoring of environmental levels remain important challenges. The scientific community continues to study the long-term effects of carbon tetrachloride on ecosystems and global atmospheric chemistry to better understand and mitigate its environmental impact.
When released into the atmosphere, carbon tetrachloride contributes to the depletion of the ozone layer. It breaks down in the stratosphere, releasing chlorine atoms that catalyze the destruction of ozone molecules. This process reduces the Earth's protection against harmful ultraviolet radiation, potentially increasing the risk of skin cancer and other health issues for humans and wildlife.
In aquatic environments, carbon tetrachloride can persist for long periods due to its low solubility in water and resistance to biodegradation. It can accumulate in sediments and bioaccumulate in aquatic organisms, potentially causing long-term ecological damage. The compound's toxicity to aquatic life, even at low concentrations, poses a threat to the balance of marine and freshwater ecosystems.
Soil contamination by carbon tetrachloride is another significant concern. Its high mobility in soil allows it to migrate easily, potentially contaminating groundwater sources. This contamination can persist for decades, affecting drinking water quality and posing risks to human health and agricultural activities.
The production and use of carbon tetrachloride also contribute to air pollution. Its volatile nature means it can easily evaporate and disperse in the air, contributing to the formation of smog and potentially affecting air quality in urban and industrial areas.
Given its environmental persistence and toxicity, the global community has taken steps to phase out the use of carbon tetrachloride under the Montreal Protocol. This international treaty, designed to protect the ozone layer, has significantly reduced the production and consumption of ozone-depleting substances, including carbon tetrachloride.
Despite these efforts, the environmental legacy of past carbon tetrachloride use continues to be a concern. Remediation of contaminated sites and ongoing monitoring of environmental levels remain important challenges. The scientific community continues to study the long-term effects of carbon tetrachloride on ecosystems and global atmospheric chemistry to better understand and mitigate its environmental impact.
Regulatory Framework
The regulatory framework surrounding carbon tetrachloride has evolved significantly over the past few decades due to its environmental and health impacts. In the United States, the Environmental Protection Agency (EPA) has implemented strict regulations on the production, use, and disposal of carbon tetrachloride under the Toxic Substances Control Act (TSCA) and the Clean Air Act. The substance is classified as a hazardous air pollutant and is subject to stringent emission controls.
Internationally, the Montreal Protocol on Substances that Deplete the Ozone Layer has played a crucial role in phasing out the production and consumption of carbon tetrachloride. As a result, its use as a solvent in many applications has been severely restricted or banned in numerous countries. The European Union, through its REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation, has also imposed strict controls on the use of carbon tetrachloride.
Despite these regulations, carbon tetrachloride continues to be produced in limited quantities for specific industrial applications, such as the manufacture of certain pharmaceuticals and agrochemicals. In these cases, stringent safety protocols and containment measures are required to minimize environmental release and worker exposure.
The regulatory landscape also extends to the handling and disposal of carbon tetrachloride. Many countries have implemented comprehensive waste management regulations that classify carbon tetrachloride as a hazardous waste, requiring specialized treatment and disposal methods. This includes strict guidelines for storage, transportation, and incineration of carbon tetrachloride-containing materials.
Research institutions and laboratories working with carbon tetrachloride must adhere to specific safety protocols and obtain necessary permits. These regulations often mandate the use of fume hoods, personal protective equipment, and proper ventilation systems to minimize exposure risks.
As scientific understanding of the environmental and health impacts of carbon tetrachloride continues to evolve, regulatory frameworks are likely to be further refined. This may include more stringent emission controls, enhanced monitoring requirements, and potentially the development of safer alternatives for remaining industrial applications.
The global nature of environmental concerns has led to increased international cooperation in regulating carbon tetrachloride. Initiatives such as the Strategic Approach to International Chemicals Management (SAICM) aim to promote chemical safety worldwide, including the management of substances like carbon tetrachloride.
Internationally, the Montreal Protocol on Substances that Deplete the Ozone Layer has played a crucial role in phasing out the production and consumption of carbon tetrachloride. As a result, its use as a solvent in many applications has been severely restricted or banned in numerous countries. The European Union, through its REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation, has also imposed strict controls on the use of carbon tetrachloride.
Despite these regulations, carbon tetrachloride continues to be produced in limited quantities for specific industrial applications, such as the manufacture of certain pharmaceuticals and agrochemicals. In these cases, stringent safety protocols and containment measures are required to minimize environmental release and worker exposure.
The regulatory landscape also extends to the handling and disposal of carbon tetrachloride. Many countries have implemented comprehensive waste management regulations that classify carbon tetrachloride as a hazardous waste, requiring specialized treatment and disposal methods. This includes strict guidelines for storage, transportation, and incineration of carbon tetrachloride-containing materials.
Research institutions and laboratories working with carbon tetrachloride must adhere to specific safety protocols and obtain necessary permits. These regulations often mandate the use of fume hoods, personal protective equipment, and proper ventilation systems to minimize exposure risks.
As scientific understanding of the environmental and health impacts of carbon tetrachloride continues to evolve, regulatory frameworks are likely to be further refined. This may include more stringent emission controls, enhanced monitoring requirements, and potentially the development of safer alternatives for remaining industrial applications.
The global nature of environmental concerns has led to increased international cooperation in regulating carbon tetrachloride. Initiatives such as the Strategic Approach to International Chemicals Management (SAICM) aim to promote chemical safety worldwide, including the management of substances like carbon tetrachloride.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!