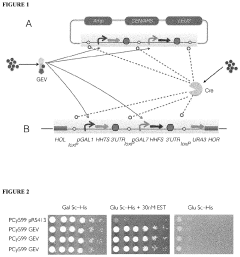
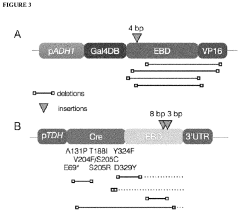
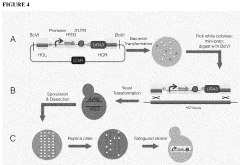
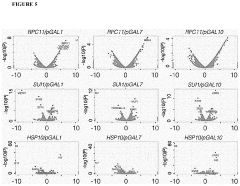
The Use Of Auxotrophic Microbes For Biocontainment.
SEP 4, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Auxotrophic Biocontainment Background and Objectives
Biocontainment has emerged as a critical concern in the field of synthetic biology and genetic engineering, particularly as genetically modified organisms (GMOs) increasingly find applications in diverse environments. Auxotrophic biocontainment represents one of the earliest and most fundamental approaches to addressing these safety concerns. This strategy leverages the principle of auxotrophy - the inability of an organism to synthesize a compound necessary for its growth - as a mechanism to restrict the survival of engineered microbes outside controlled environments.
The concept of auxotrophic biocontainment dates back to the 1970s when concerns about recombinant DNA technology first emerged. The historical progression of this technology has evolved from simple single-gene auxotrophy systems to sophisticated multi-layered containment strategies that incorporate multiple dependencies and fail-safe mechanisms. This evolution reflects the growing understanding of microbial adaptation and the potential ecological impacts of engineered organisms.
Current technological trends in auxotrophic biocontainment focus on developing more robust systems that can withstand evolutionary pressures. These include the creation of synthetic auxotrophs dependent on non-natural compounds, the engineering of multiple auxotrophies to reduce escape frequency, and the integration of auxotrophy with other containment strategies such as kill switches and genetic firewalls.
The primary objective of auxotrophic biocontainment research is to develop systems that effectively balance functionality with safety. This involves creating microbes that can perform their intended functions efficiently in controlled settings while maintaining negligible survival rates when released into unintended environments. Quantitatively, the field aims to achieve escape frequencies below 10^-8 per cell, which is considered the standard threshold for effective biocontainment.
Another critical objective is addressing the challenge of horizontal gene transfer, whereby contained genetic elements might be transferred to wild microorganisms. Auxotrophic systems must be designed to minimize this risk through mechanisms that prevent the rescue of engineered strains by environmental metabolites or genetic exchange with native organisms.
The field is also moving toward developing standardized frameworks for evaluating biocontainment efficacy across different applications and environments. This includes establishing protocols for testing containment under various stress conditions and developing predictive models for long-term containment stability.
As synthetic biology applications expand into areas such as bioremediation, agricultural biocontrol, and medical therapeutics, the objectives of auxotrophic biocontainment are increasingly focused on creating application-specific solutions that address the unique challenges and requirements of each deployment context while maintaining rigorous safety standards.
The concept of auxotrophic biocontainment dates back to the 1970s when concerns about recombinant DNA technology first emerged. The historical progression of this technology has evolved from simple single-gene auxotrophy systems to sophisticated multi-layered containment strategies that incorporate multiple dependencies and fail-safe mechanisms. This evolution reflects the growing understanding of microbial adaptation and the potential ecological impacts of engineered organisms.
Current technological trends in auxotrophic biocontainment focus on developing more robust systems that can withstand evolutionary pressures. These include the creation of synthetic auxotrophs dependent on non-natural compounds, the engineering of multiple auxotrophies to reduce escape frequency, and the integration of auxotrophy with other containment strategies such as kill switches and genetic firewalls.
The primary objective of auxotrophic biocontainment research is to develop systems that effectively balance functionality with safety. This involves creating microbes that can perform their intended functions efficiently in controlled settings while maintaining negligible survival rates when released into unintended environments. Quantitatively, the field aims to achieve escape frequencies below 10^-8 per cell, which is considered the standard threshold for effective biocontainment.
Another critical objective is addressing the challenge of horizontal gene transfer, whereby contained genetic elements might be transferred to wild microorganisms. Auxotrophic systems must be designed to minimize this risk through mechanisms that prevent the rescue of engineered strains by environmental metabolites or genetic exchange with native organisms.
The field is also moving toward developing standardized frameworks for evaluating biocontainment efficacy across different applications and environments. This includes establishing protocols for testing containment under various stress conditions and developing predictive models for long-term containment stability.
As synthetic biology applications expand into areas such as bioremediation, agricultural biocontrol, and medical therapeutics, the objectives of auxotrophic biocontainment are increasingly focused on creating application-specific solutions that address the unique challenges and requirements of each deployment context while maintaining rigorous safety standards.
Market Analysis for Biosafety Solutions
The biosafety solutions market is experiencing significant growth driven by increasing regulatory scrutiny and public concern over genetically modified organisms (GMOs) and synthetic biology applications. The global biosafety market was valued at approximately $3.2 billion in 2022 and is projected to reach $5.7 billion by 2028, growing at a CAGR of 10.1% during the forecast period. This growth is particularly evident in regions with advanced biotechnology sectors such as North America, Europe, and increasingly in Asia-Pacific.
Auxotrophic microbial biocontainment solutions represent a specialized but rapidly expanding segment within this market. These solutions address critical needs in various sectors including pharmaceutical manufacturing, agricultural biotechnology, environmental remediation, and academic research. The pharmaceutical and biotechnology sectors currently account for the largest market share at approximately 45%, followed by academic and research institutions at 30%.
Market demand is primarily driven by stringent biosafety regulations implemented by agencies such as the FDA, EPA, and their international counterparts. The NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules specifically address containment measures, creating a compliance-driven market for auxotrophic biocontainment technologies.
Customer segments can be categorized into three main groups: large pharmaceutical and biotechnology companies seeking robust containment solutions for large-scale production; academic and research institutions requiring flexible biocontainment for diverse research applications; and emerging synthetic biology startups developing novel engineered organisms for various applications.
Regional analysis reveals North America dominates the market with approximately 40% share, followed by Europe at 35% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to witness the highest growth rate over the next five years due to increasing biotechnology investments in China, Japan, and South Korea.
Key market trends include the integration of auxotrophic biocontainment with other containment strategies to create multi-layered safety systems, growing demand for standardized biocontainment solutions that can receive regulatory pre-approval, and increasing interest in customizable auxotrophic systems for specific application environments.
The market faces challenges including high development costs, technical limitations in designing robust auxotrophic systems that prevent escape mutations, and varying regulatory requirements across different jurisdictions. However, opportunities exist in developing auxotrophic systems for emerging applications such as microbiome therapeutics, engineered probiotics, and environmental biosensors, which are expected to drive market expansion in the coming years.
Auxotrophic microbial biocontainment solutions represent a specialized but rapidly expanding segment within this market. These solutions address critical needs in various sectors including pharmaceutical manufacturing, agricultural biotechnology, environmental remediation, and academic research. The pharmaceutical and biotechnology sectors currently account for the largest market share at approximately 45%, followed by academic and research institutions at 30%.
Market demand is primarily driven by stringent biosafety regulations implemented by agencies such as the FDA, EPA, and their international counterparts. The NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules specifically address containment measures, creating a compliance-driven market for auxotrophic biocontainment technologies.
Customer segments can be categorized into three main groups: large pharmaceutical and biotechnology companies seeking robust containment solutions for large-scale production; academic and research institutions requiring flexible biocontainment for diverse research applications; and emerging synthetic biology startups developing novel engineered organisms for various applications.
Regional analysis reveals North America dominates the market with approximately 40% share, followed by Europe at 35% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to witness the highest growth rate over the next five years due to increasing biotechnology investments in China, Japan, and South Korea.
Key market trends include the integration of auxotrophic biocontainment with other containment strategies to create multi-layered safety systems, growing demand for standardized biocontainment solutions that can receive regulatory pre-approval, and increasing interest in customizable auxotrophic systems for specific application environments.
The market faces challenges including high development costs, technical limitations in designing robust auxotrophic systems that prevent escape mutations, and varying regulatory requirements across different jurisdictions. However, opportunities exist in developing auxotrophic systems for emerging applications such as microbiome therapeutics, engineered probiotics, and environmental biosensors, which are expected to drive market expansion in the coming years.
Current Auxotrophic Biocontainment Challenges
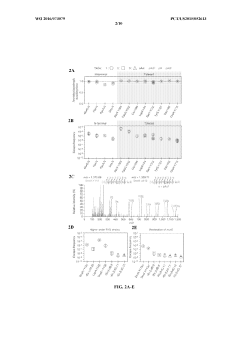
Despite significant advancements in auxotrophic biocontainment strategies, several critical challenges persist that limit their widespread implementation and reliability. The fundamental challenge remains the inherent genetic instability of auxotrophic systems. Engineered microorganisms can undergo spontaneous mutations that restore their ability to synthesize essential compounds, effectively bypassing the intended containment mechanism. Studies have documented escape frequencies ranging from 10^-6 to 10^-8 per cell generation, which remains unacceptably high for many applications, particularly those involving environmental release.
Metabolic redundancy presents another significant obstacle. Many microorganisms possess alternative metabolic pathways or can utilize unexpected environmental resources to compensate for engineered deficiencies. This redundancy makes it difficult to create truly dependent auxotrophs, as organisms may adapt to utilize alternative substrates or develop cross-feeding relationships with other microbes in non-sterile environments.
The environmental availability of complementary compounds further complicates biocontainment efforts. Natural environments often contain trace amounts of the very compounds that auxotrophs are engineered to require, such as amino acids, nucleotides, or vitamins. These compounds may originate from decaying organic matter or be produced by indigenous microorganisms, potentially allowing engineered auxotrophs to survive and proliferate outside controlled settings.
Technical limitations in genetic engineering also pose challenges. Creating multiple auxotrophies to enhance containment reliability often results in severely compromised cellular fitness and reduced industrial performance. This fitness burden creates a practical trade-off between containment security and the organism's utility for intended applications, limiting commercial viability.
Regulatory frameworks present additional hurdles. Current regulations governing genetically modified organisms vary significantly across jurisdictions and often lack specific provisions for auxotrophic containment strategies. This regulatory uncertainty complicates the development and deployment of auxotrophic biocontainment systems, particularly for applications requiring environmental release.
Standardization and validation methodologies remain underdeveloped. There is no universally accepted protocol for testing the effectiveness of auxotrophic containment systems across different environmental conditions, making it difficult to compare different approaches or establish minimum safety thresholds. This lack of standardization impedes both regulatory approval and public acceptance.
Horizontal gene transfer represents a persistent concern, as auxotrophic modifications could potentially be transferred to wild-type organisms, or conversely, genes restoring prototrophy could be acquired by engineered strains. While the probability of such events is low, their potential consequences necessitate careful consideration in risk assessment frameworks.
Metabolic redundancy presents another significant obstacle. Many microorganisms possess alternative metabolic pathways or can utilize unexpected environmental resources to compensate for engineered deficiencies. This redundancy makes it difficult to create truly dependent auxotrophs, as organisms may adapt to utilize alternative substrates or develop cross-feeding relationships with other microbes in non-sterile environments.
The environmental availability of complementary compounds further complicates biocontainment efforts. Natural environments often contain trace amounts of the very compounds that auxotrophs are engineered to require, such as amino acids, nucleotides, or vitamins. These compounds may originate from decaying organic matter or be produced by indigenous microorganisms, potentially allowing engineered auxotrophs to survive and proliferate outside controlled settings.
Technical limitations in genetic engineering also pose challenges. Creating multiple auxotrophies to enhance containment reliability often results in severely compromised cellular fitness and reduced industrial performance. This fitness burden creates a practical trade-off between containment security and the organism's utility for intended applications, limiting commercial viability.
Regulatory frameworks present additional hurdles. Current regulations governing genetically modified organisms vary significantly across jurisdictions and often lack specific provisions for auxotrophic containment strategies. This regulatory uncertainty complicates the development and deployment of auxotrophic biocontainment systems, particularly for applications requiring environmental release.
Standardization and validation methodologies remain underdeveloped. There is no universally accepted protocol for testing the effectiveness of auxotrophic containment systems across different environmental conditions, making it difficult to compare different approaches or establish minimum safety thresholds. This lack of standardization impedes both regulatory approval and public acceptance.
Horizontal gene transfer represents a persistent concern, as auxotrophic modifications could potentially be transferred to wild-type organisms, or conversely, genes restoring prototrophy could be acquired by engineered strains. While the probability of such events is low, their potential consequences necessitate careful consideration in risk assessment frameworks.
Established Auxotrophic Containment Methodologies
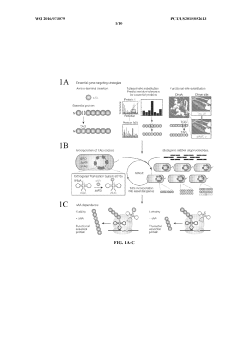
01 Auxotrophic microorganisms for biological containment
Auxotrophic microorganisms are engineered to require specific nutrients for survival that are not available in the environment, creating an effective biocontainment strategy. These microbes are genetically modified to be dependent on exogenous supply of essential compounds such as amino acids, nucleotides, or vitamins. When these nutrients are absent, the microorganisms cannot survive, preventing their uncontrolled spread in natural environments.- Auxotrophic microorganisms for environmental biocontainment: Auxotrophic microorganisms that require specific nutrients for growth can be engineered for biocontainment purposes. By creating dependencies on nutrients that are not available in the natural environment, these microbes cannot survive outside controlled conditions. This approach involves genetic modifications that disable the microorganism's ability to synthesize essential compounds, ensuring they can only grow when supplemented with specific nutrients in laboratory or industrial settings.
- Genetic kill switches and conditional survival mechanisms: Engineered genetic circuits that function as kill switches provide an additional layer of biocontainment for modified microorganisms. These systems can be designed to trigger cell death when specific environmental conditions are detected or when certain molecules are absent. Conditional survival mechanisms ensure that the microorganisms can only proliferate under precisely defined conditions, preventing their spread in unintended environments.
- Synthetic amino acid dependency for biocontainment: Creating microorganisms dependent on non-canonical or synthetic amino acids represents an advanced biocontainment strategy. These engineered microbes have modified genetic codes requiring amino acids not found in nature, ensuring they cannot survive without human intervention. This approach involves recoding essential genes to incorporate synthetic amino acids, creating a robust containment system that prevents horizontal gene transfer and environmental escape.
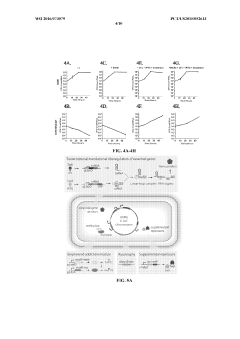
- Multiple containment barriers and redundant systems: Implementing multiple, orthogonal biocontainment strategies in a single organism creates redundant safety systems that significantly reduce escape risk. By combining different auxotrophies, kill switches, and conditional survival mechanisms, the probability of simultaneous mutations that would overcome all containment barriers becomes statistically negligible. This multi-layered approach addresses regulatory concerns and enhances the safety profile of engineered microorganisms for industrial and environmental applications.
- Metabolic engineering for controlled growth and application: Metabolic engineering techniques can create auxotrophic microbes with specific growth requirements while optimizing them for industrial applications. These engineered strains can be designed to perform desired functions such as bioremediation, biofuel production, or pharmaceutical synthesis while maintaining strict biocontainment. The metabolic dependencies ensure that the microorganisms can only function in controlled environments where specific nutrients or cofactors are provided.
02 Genetic kill switches for enhanced biocontainment
Genetic kill switches provide an additional layer of biocontainment for genetically modified microorganisms. These systems are designed to trigger cell death or growth arrest when specific environmental conditions occur or when certain molecules are absent. Kill switches can be based on toxin-antitoxin systems, where the antitoxin is only produced under controlled conditions, or on essential genes that are conditionally expressed. This approach ensures that engineered microbes cannot survive outside their intended environment.Expand Specific Solutions03 Synthetic amino acid dependency for biocontainment
Engineered microorganisms can be designed to depend on synthetic or non-canonical amino acids that do not exist in nature. By replacing essential genes with versions that require these synthetic amino acids, the microbes cannot survive without human supplementation. This creates a robust biocontainment strategy as the likelihood of environmental acquisition of these non-natural compounds is extremely low, effectively preventing horizontal gene transfer and environmental escape.Expand Specific Solutions04 Multi-layered biocontainment systems
Multi-layered biocontainment approaches combine different containment strategies to minimize the risk of environmental escape. These systems typically integrate auxotrophy for multiple nutrients, genetic kill switches, and other containment mechanisms. By requiring multiple simultaneous mutations to overcome the containment, the probability of escape becomes statistically negligible. This redundancy ensures that even if one containment mechanism fails, others remain effective.Expand Specific Solutions05 Environmental applications of contained auxotrophic microbes
Auxotrophic microorganisms with biocontainment features are particularly valuable for environmental applications such as bioremediation, agricultural improvements, and waste treatment. These engineered microbes can be designed to perform specific functions like degrading pollutants or producing beneficial compounds while being unable to persist in the environment once their task is complete. This controlled release approach balances the benefits of microbial engineering with ecological safety concerns.Expand Specific Solutions
Leading Organizations in Biocontainment Research
The biocontainment of auxotrophic microbes represents an emerging field at the intersection of synthetic biology and biosafety, currently in its early growth phase. The market is expanding rapidly with increasing regulatory focus on biosafety in biotechnology applications, estimated to reach significant scale as engineered microbes gain commercial traction. Technical maturity varies considerably among key players, with academic institutions like Yale University, Vanderbilt University, and Johns Hopkins University leading fundamental research, while companies including LanzaTech, Calysta, and Deinove are advancing commercial applications. Research organizations such as Centre National de la Recherche Scientifique and Battelle Memorial Institute are bridging theoretical concepts with practical implementations. The field is characterized by collaborative development across academia, government, and industry, with increasing patent activity signaling growing commercial interest in auxotrophic containment strategies.
Yale University
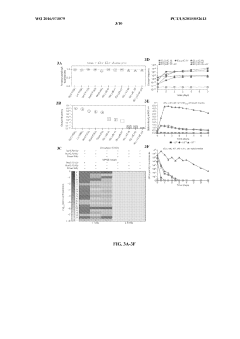
Technical Solution: Yale University has developed advanced auxotrophic biocontainment systems based on engineered genetic safeguards. Their approach involves creating microorganisms with multiple engineered auxotrophies that require synthetic compounds not found in nature for survival. The technology employs genomically recoded organisms (GROs) with reassigned codons and synthetic amino acids, creating dependency on non-canonical amino acids that must be supplied exogenously. Yale researchers have demonstrated systems with escape frequencies below 10^-12, representing one of the most secure biocontainment strategies available[1]. Their multi-layered approach combines auxotrophy with genetic firewalls that prevent horizontal gene transfer, addressing both environmental escape and genetic transfer concerns simultaneously[2].
Strengths: Extremely low escape frequencies (below 10^-12); multiple redundant containment mechanisms; prevents horizontal gene transfer. Weaknesses: Requires continuous supply of synthetic compounds; potential metabolic burden on engineered organisms may reduce performance in industrial applications; complex system may be challenging to implement across diverse microbial species.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has pioneered innovative auxotrophic biocontainment strategies focusing on metabolic dependencies engineered through CRISPR-Cas9 genome editing. Their approach creates microorganisms with multiple engineered auxotrophies for essential metabolites that cannot be scavenged from the environment. CNRS researchers have developed systems requiring synthetic thiamine analogs and modified amino acids simultaneously, creating a dual-dependency system. Their technology incorporates genetic circuits that actively degrade the organism upon detection of environmental escape conditions, providing an additional layer of security[3]. The CNRS system has been demonstrated in both prokaryotic and eukaryotic microorganisms, showing versatility across different biological platforms while maintaining containment efficiency of approximately 10^-8 to 10^-10 escape frequency[4].
Strengths: Versatile application across multiple microbial species; combines passive auxotrophy with active kill-switch mechanisms; relatively simple implementation using established genome editing techniques. Weaknesses: Higher escape frequencies compared to some competing technologies; potential for evolutionary adaptation to overcome auxotrophic requirements over time; may require regulatory approval processes that vary by country.
Key Patents and Innovations in Auxotrophic Systems
Compositions and methods for biocontainment of microorganisms
PatentWO2016073079A2
Innovation
- The development of genetically modified organisms (GMOs) with modular, robust genetic safeguards that restrict growth to synthetic environments, using genomically recoded organisms (GROs) and engineered riboregulation, auxotrophy, and addiction systems to ensure low escape frequencies and maintain viability only in defined conditions.
Compositions and methods for controlling microbial growth
PatentActiveUS20200024582A1
Innovation
- Development of modified microorganisms that require exogenously provided compounds for growth, using inducible promoters and site-specific recombinase systems to control genetic alterations, ensuring biocontainment without adverse effects on fitness.
Regulatory Framework for Engineered Microbe Release
The regulatory landscape governing the release of engineered microbes, particularly auxotrophic strains designed for biocontainment, has evolved significantly over the past decade. Currently, multiple international and national regulatory bodies oversee the deliberate release of genetically modified microorganisms (GMMs) into the environment, each with varying approaches to risk assessment and management.
In the United States, the Coordinated Framework for Regulation of Biotechnology involves three primary agencies: the Environmental Protection Agency (EPA), the Food and Drug Administration (FDA), and the United States Department of Agriculture (USDA). The EPA regulates microbial products under the Toxic Substances Control Act (TSCA), requiring comprehensive risk assessments for environmental applications of engineered microbes, including those with auxotrophic containment systems.
The European Union implements a more precautionary approach through Directive 2001/18/EC on the deliberate release of GMOs into the environment. This framework mandates extensive environmental risk assessment, post-release monitoring plans, and public consultation processes. Notably, the EU regulatory system places particular emphasis on containment strategies, making auxotrophic biocontainment systems especially relevant for regulatory compliance.
Internationally, the Cartagena Protocol on Biosafety provides guidelines for the transboundary movement of living modified organisms (LMOs), including engineered microbes. This protocol has been ratified by 173 countries and establishes risk assessment procedures and information-sharing mechanisms through the Biosafety Clearing-House.
Recent regulatory developments have begun to specifically address auxotrophic containment strategies. The NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules now include provisions for biological containment using auxotrophic microorganisms, classifying them based on their escape frequency and environmental persistence potential.
Regulatory challenges specific to auxotrophic biocontainment include standardizing methodologies for measuring containment efficacy, establishing acceptable escape frequency thresholds, and developing protocols for monitoring potential horizontal gene transfer events. The lack of harmonized international standards for evaluating auxotrophic containment systems presents a significant hurdle for global deployment of these technologies.
Industry stakeholders and academic researchers have advocated for a tiered regulatory approach that considers both the nature of the genetic modifications and the intended application environment. This risk-based framework would potentially streamline approval processes for well-characterized auxotrophic systems while maintaining appropriate safeguards for novel applications.
Moving forward, regulatory frameworks will likely evolve toward more nuanced, case-by-case evaluations of engineered microbes with biocontainment features, potentially creating specialized pathways for auxotrophic systems with demonstrated safety records.
In the United States, the Coordinated Framework for Regulation of Biotechnology involves three primary agencies: the Environmental Protection Agency (EPA), the Food and Drug Administration (FDA), and the United States Department of Agriculture (USDA). The EPA regulates microbial products under the Toxic Substances Control Act (TSCA), requiring comprehensive risk assessments for environmental applications of engineered microbes, including those with auxotrophic containment systems.
The European Union implements a more precautionary approach through Directive 2001/18/EC on the deliberate release of GMOs into the environment. This framework mandates extensive environmental risk assessment, post-release monitoring plans, and public consultation processes. Notably, the EU regulatory system places particular emphasis on containment strategies, making auxotrophic biocontainment systems especially relevant for regulatory compliance.
Internationally, the Cartagena Protocol on Biosafety provides guidelines for the transboundary movement of living modified organisms (LMOs), including engineered microbes. This protocol has been ratified by 173 countries and establishes risk assessment procedures and information-sharing mechanisms through the Biosafety Clearing-House.
Recent regulatory developments have begun to specifically address auxotrophic containment strategies. The NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules now include provisions for biological containment using auxotrophic microorganisms, classifying them based on their escape frequency and environmental persistence potential.
Regulatory challenges specific to auxotrophic biocontainment include standardizing methodologies for measuring containment efficacy, establishing acceptable escape frequency thresholds, and developing protocols for monitoring potential horizontal gene transfer events. The lack of harmonized international standards for evaluating auxotrophic containment systems presents a significant hurdle for global deployment of these technologies.
Industry stakeholders and academic researchers have advocated for a tiered regulatory approach that considers both the nature of the genetic modifications and the intended application environment. This risk-based framework would potentially streamline approval processes for well-characterized auxotrophic systems while maintaining appropriate safeguards for novel applications.
Moving forward, regulatory frameworks will likely evolve toward more nuanced, case-by-case evaluations of engineered microbes with biocontainment features, potentially creating specialized pathways for auxotrophic systems with demonstrated safety records.
Environmental Impact Assessment of Auxotrophic Systems
The environmental impact of auxotrophic microbial biocontainment systems represents a critical consideration in their deployment for various applications. These engineered microorganisms, designed with nutritional dependencies that restrict their growth outside controlled environments, offer significant advantages over traditional containment methods but also present unique ecological considerations.
Assessment of auxotrophic biocontainment systems reveals minimal direct environmental impact when properly implemented. Unlike chemical containment strategies that may introduce toxic compounds into ecosystems, auxotrophic dependencies rely on natural metabolic limitations. The absence of essential nutrients in natural environments effectively prevents proliferation without introducing novel pollutants. This represents a substantial improvement over physical containment methods that may fail catastrophically or chemical methods with persistent environmental effects.
Ecological risk modeling of auxotrophic escape scenarios demonstrates significantly reduced survival probability compared to conventional genetically modified organisms. Studies tracking auxotrophic E. coli and yeast strains in simulated environmental conditions show rapid population decline within 24-72 hours without supplementation. This inherent safety mechanism provides a passive containment strategy that continues functioning regardless of physical containment breaches.
However, horizontal gene transfer remains a theoretical concern. While auxotrophy itself cannot be transferred to wild populations through this mechanism, other engineered genetic elements within the same organism might be. Recent research indicates this risk can be mitigated through strategic placement of auxotrophic modifications and additional genetic safeguards that prevent functional gene transfer.
Long-term environmental monitoring of test sites utilizing auxotrophic microbes has not detected persistent populations or ecological disruption. This aligns with theoretical predictions but requires continued verification across diverse ecosystems and conditions. Standardized environmental impact assessment protocols specifically designed for auxotrophic systems are being developed by regulatory agencies to ensure comprehensive evaluation.
Biodiversity impact studies suggest minimal interference with native microbial communities when auxotrophic strains are properly contained. The metabolic disadvantage prevents competitive displacement of indigenous species, addressing a common concern with introduced microorganisms. This characteristic makes auxotrophic systems particularly suitable for applications in sensitive ecological zones where traditional GMOs might pose greater risks.
Future environmental assessment frameworks must incorporate specific considerations for auxotrophic systems, including evaluation of potential adaptation mechanisms, interaction with environmental nutrient profiles, and ecosystem-specific containment efficacy. These specialized protocols will ensure responsible deployment while maximizing the safety advantages inherent to auxotrophic biocontainment strategies.
Assessment of auxotrophic biocontainment systems reveals minimal direct environmental impact when properly implemented. Unlike chemical containment strategies that may introduce toxic compounds into ecosystems, auxotrophic dependencies rely on natural metabolic limitations. The absence of essential nutrients in natural environments effectively prevents proliferation without introducing novel pollutants. This represents a substantial improvement over physical containment methods that may fail catastrophically or chemical methods with persistent environmental effects.
Ecological risk modeling of auxotrophic escape scenarios demonstrates significantly reduced survival probability compared to conventional genetically modified organisms. Studies tracking auxotrophic E. coli and yeast strains in simulated environmental conditions show rapid population decline within 24-72 hours without supplementation. This inherent safety mechanism provides a passive containment strategy that continues functioning regardless of physical containment breaches.
However, horizontal gene transfer remains a theoretical concern. While auxotrophy itself cannot be transferred to wild populations through this mechanism, other engineered genetic elements within the same organism might be. Recent research indicates this risk can be mitigated through strategic placement of auxotrophic modifications and additional genetic safeguards that prevent functional gene transfer.
Long-term environmental monitoring of test sites utilizing auxotrophic microbes has not detected persistent populations or ecological disruption. This aligns with theoretical predictions but requires continued verification across diverse ecosystems and conditions. Standardized environmental impact assessment protocols specifically designed for auxotrophic systems are being developed by regulatory agencies to ensure comprehensive evaluation.
Biodiversity impact studies suggest minimal interference with native microbial communities when auxotrophic strains are properly contained. The metabolic disadvantage prevents competitive displacement of indigenous species, addressing a common concern with introduced microorganisms. This characteristic makes auxotrophic systems particularly suitable for applications in sensitive ecological zones where traditional GMOs might pose greater risks.
Future environmental assessment frameworks must incorporate specific considerations for auxotrophic systems, including evaluation of potential adaptation mechanisms, interaction with environmental nutrient profiles, and ecosystem-specific containment efficacy. These specialized protocols will ensure responsible deployment while maximizing the safety advantages inherent to auxotrophic biocontainment strategies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!