Understanding Carbon Tetrachloride's Chemical Behavior in Industry
JUL 2, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Industrial Background and Objectives
Carbon tetrachloride (CCl4) has played a significant role in various industrial applications since its discovery in the mid-19th century. Initially synthesized by Henri Victor Regnault in 1839, this colorless, non-flammable liquid quickly gained prominence due to its unique chemical properties. The industrial journey of CCl4 began in the early 20th century when it found widespread use as a solvent, cleaning agent, and fire extinguishing medium.
The evolution of CCl4's industrial applications has been marked by both innovation and controversy. Its excellent solvency and non-flammability made it a preferred choice in dry cleaning, metal degreasing, and as a precursor in the production of refrigerants. However, the discovery of its ozone-depleting properties and potential health hazards led to a significant shift in its usage patterns and regulatory landscape.
In recent decades, the focus on CCl4 has shifted from widespread industrial use to understanding its environmental impact and developing safer alternatives. This transition has been driven by international agreements such as the Montreal Protocol, which aimed to phase out substances that deplete the ozone layer. As a result, industries have been compelled to reevaluate their reliance on CCl4 and explore more sustainable options.
The current technological landscape surrounding CCl4 is characterized by efforts to mitigate its environmental impact while still leveraging its beneficial properties in controlled settings. Research is ongoing to develop processes that can effectively destroy or convert CCl4 into less harmful substances. Additionally, there is a growing interest in understanding the long-term effects of historical CCl4 emissions and developing remediation strategies for contaminated sites.
The objectives of current research and industrial practices related to CCl4 are multifaceted. Firstly, there is a pressing need to fully comprehend the chemical behavior of CCl4 in various industrial processes, particularly its reactions and degradation pathways under different conditions. This understanding is crucial for developing more efficient containment and disposal methods. Secondly, industries are focusing on finding sustainable alternatives that can match the performance of CCl4 without its environmental drawbacks.
Furthermore, there is an increasing emphasis on developing advanced detection and monitoring technologies to track CCl4 emissions and environmental concentrations. This is essential for ensuring compliance with regulatory standards and assessing the effectiveness of phase-out efforts. Lastly, the industrial sector is actively exploring innovative technologies for the remediation of CCl4-contaminated soil and groundwater, aiming to address the legacy of its past widespread use.
The evolution of CCl4's industrial applications has been marked by both innovation and controversy. Its excellent solvency and non-flammability made it a preferred choice in dry cleaning, metal degreasing, and as a precursor in the production of refrigerants. However, the discovery of its ozone-depleting properties and potential health hazards led to a significant shift in its usage patterns and regulatory landscape.
In recent decades, the focus on CCl4 has shifted from widespread industrial use to understanding its environmental impact and developing safer alternatives. This transition has been driven by international agreements such as the Montreal Protocol, which aimed to phase out substances that deplete the ozone layer. As a result, industries have been compelled to reevaluate their reliance on CCl4 and explore more sustainable options.
The current technological landscape surrounding CCl4 is characterized by efforts to mitigate its environmental impact while still leveraging its beneficial properties in controlled settings. Research is ongoing to develop processes that can effectively destroy or convert CCl4 into less harmful substances. Additionally, there is a growing interest in understanding the long-term effects of historical CCl4 emissions and developing remediation strategies for contaminated sites.
The objectives of current research and industrial practices related to CCl4 are multifaceted. Firstly, there is a pressing need to fully comprehend the chemical behavior of CCl4 in various industrial processes, particularly its reactions and degradation pathways under different conditions. This understanding is crucial for developing more efficient containment and disposal methods. Secondly, industries are focusing on finding sustainable alternatives that can match the performance of CCl4 without its environmental drawbacks.
Furthermore, there is an increasing emphasis on developing advanced detection and monitoring technologies to track CCl4 emissions and environmental concentrations. This is essential for ensuring compliance with regulatory standards and assessing the effectiveness of phase-out efforts. Lastly, the industrial sector is actively exploring innovative technologies for the remediation of CCl4-contaminated soil and groundwater, aiming to address the legacy of its past widespread use.
Market Demand Analysis for CCl4
Carbon tetrachloride (CCl4) has experienced significant shifts in market demand over the past decades, primarily due to environmental concerns and regulatory changes. The global market for CCl4 has been steadily declining since the 1980s when it was widely used as a solvent, cleaning agent, and refrigerant. This decline is largely attributed to the Montreal Protocol, which phased out the production of ozone-depleting substances, including CCl4.
Despite the overall decrease in demand, CCl4 still maintains a presence in certain industrial applications. The chemical industry continues to use CCl4 as a feedstock for the production of other chlorinated compounds, particularly hydrofluorocarbons (HFCs) and hydrochlorofluorocarbons (HCFCs). These substances are used as alternatives to chlorofluorocarbons (CFCs) in refrigeration and air conditioning systems.
The pharmaceutical sector represents another area of sustained demand for CCl4. It serves as a precursor in the synthesis of various pharmaceutical compounds and as a solvent in drug manufacturing processes. However, this demand is relatively small compared to its historical use and is subject to strict regulations and control measures.
In the agrochemical industry, CCl4 finds limited application in the production of certain pesticides and herbicides. While alternatives are increasingly being sought, some specialized formulations still rely on CCl4 as an intermediate compound.
The analytical and research sectors continue to use small quantities of CCl4 for laboratory applications, including spectroscopy and as a solvent for specific chemical reactions. This demand, while persistent, represents a minor fraction of the overall market.
Geographically, the demand for CCl4 varies significantly. Developed countries have largely phased out its use in most applications, adhering to strict environmental regulations. However, some developing nations still permit limited use of CCl4 in certain industrial processes, contributing to a small but notable market demand.
Looking forward, the market for CCl4 is expected to face further constraints. Ongoing international efforts to reduce emissions of ozone-depleting substances and greenhouse gases are likely to result in additional restrictions on CCl4 production and use. Industries currently relying on CCl4 are actively seeking alternatives, which will further impact market demand.
In conclusion, while the overall market demand for CCl4 has significantly diminished, niche applications in chemical manufacturing, pharmaceuticals, and research ensure a continued, albeit limited, demand for this compound. The future market trajectory will largely depend on the development of suitable alternatives and the evolution of global environmental policies.
Despite the overall decrease in demand, CCl4 still maintains a presence in certain industrial applications. The chemical industry continues to use CCl4 as a feedstock for the production of other chlorinated compounds, particularly hydrofluorocarbons (HFCs) and hydrochlorofluorocarbons (HCFCs). These substances are used as alternatives to chlorofluorocarbons (CFCs) in refrigeration and air conditioning systems.
The pharmaceutical sector represents another area of sustained demand for CCl4. It serves as a precursor in the synthesis of various pharmaceutical compounds and as a solvent in drug manufacturing processes. However, this demand is relatively small compared to its historical use and is subject to strict regulations and control measures.
In the agrochemical industry, CCl4 finds limited application in the production of certain pesticides and herbicides. While alternatives are increasingly being sought, some specialized formulations still rely on CCl4 as an intermediate compound.
The analytical and research sectors continue to use small quantities of CCl4 for laboratory applications, including spectroscopy and as a solvent for specific chemical reactions. This demand, while persistent, represents a minor fraction of the overall market.
Geographically, the demand for CCl4 varies significantly. Developed countries have largely phased out its use in most applications, adhering to strict environmental regulations. However, some developing nations still permit limited use of CCl4 in certain industrial processes, contributing to a small but notable market demand.
Looking forward, the market for CCl4 is expected to face further constraints. Ongoing international efforts to reduce emissions of ozone-depleting substances and greenhouse gases are likely to result in additional restrictions on CCl4 production and use. Industries currently relying on CCl4 are actively seeking alternatives, which will further impact market demand.
In conclusion, while the overall market demand for CCl4 has significantly diminished, niche applications in chemical manufacturing, pharmaceuticals, and research ensure a continued, albeit limited, demand for this compound. The future market trajectory will largely depend on the development of suitable alternatives and the evolution of global environmental policies.
CCl4 Technical Challenges
Carbon tetrachloride (CCl4) presents several technical challenges in industrial applications, primarily due to its unique chemical properties and environmental concerns. One of the main difficulties lies in its high volatility and low boiling point, which makes containment and handling problematic in many industrial processes. This characteristic also contributes to its potential for atmospheric release, raising significant environmental and health concerns.
The compound's strong solvency properties, while beneficial in certain applications, pose challenges in terms of material compatibility. CCl4 can degrade many common materials used in industrial equipment, including certain plastics and rubbers, necessitating careful selection of containment materials and seals. This corrosive nature not only impacts equipment longevity but also raises safety concerns in industrial settings.
Another significant technical challenge is the compound's reactivity under certain conditions. When exposed to high temperatures or in the presence of specific metals, CCl4 can decompose to form highly toxic phosgene gas. This reactivity necessitates stringent control measures in industrial processes, particularly those involving elevated temperatures or potential catalytic surfaces.
The environmental persistence of CCl4 presents a substantial challenge for its use and disposal. Its long atmospheric lifetime and ozone-depleting potential have led to strict regulations on its production and use. This regulatory environment creates technical challenges in finding suitable alternatives for processes where CCl4 has traditionally been employed, such as in the production of chlorofluorocarbons and as a feedstock for various chemical syntheses.
In the realm of analytical chemistry, while CCl4's properties make it useful as a solvent, its toxicity and environmental impact create challenges in developing safer analytical methods. This has spurred research into alternative solvents and methodologies, which often require significant re-optimization of established procedures.
The remediation of CCl4 contamination in soil and groundwater presents another set of technical challenges. Its low water solubility and tendency to form dense non-aqueous phase liquids (DNAPLs) make it difficult to remove from contaminated sites. Innovative remediation technologies, such as in-situ chemical oxidation and bioremediation, face obstacles due to CCl4's recalcitrance to degradation.
These technical challenges collectively drive ongoing research and development efforts aimed at finding safer alternatives, improving handling and containment technologies, and developing more effective remediation strategies for CCl4 contamination. The industrial sector continues to grapple with balancing the compound's useful properties against its significant environmental and health risks.
The compound's strong solvency properties, while beneficial in certain applications, pose challenges in terms of material compatibility. CCl4 can degrade many common materials used in industrial equipment, including certain plastics and rubbers, necessitating careful selection of containment materials and seals. This corrosive nature not only impacts equipment longevity but also raises safety concerns in industrial settings.
Another significant technical challenge is the compound's reactivity under certain conditions. When exposed to high temperatures or in the presence of specific metals, CCl4 can decompose to form highly toxic phosgene gas. This reactivity necessitates stringent control measures in industrial processes, particularly those involving elevated temperatures or potential catalytic surfaces.
The environmental persistence of CCl4 presents a substantial challenge for its use and disposal. Its long atmospheric lifetime and ozone-depleting potential have led to strict regulations on its production and use. This regulatory environment creates technical challenges in finding suitable alternatives for processes where CCl4 has traditionally been employed, such as in the production of chlorofluorocarbons and as a feedstock for various chemical syntheses.
In the realm of analytical chemistry, while CCl4's properties make it useful as a solvent, its toxicity and environmental impact create challenges in developing safer analytical methods. This has spurred research into alternative solvents and methodologies, which often require significant re-optimization of established procedures.
The remediation of CCl4 contamination in soil and groundwater presents another set of technical challenges. Its low water solubility and tendency to form dense non-aqueous phase liquids (DNAPLs) make it difficult to remove from contaminated sites. Innovative remediation technologies, such as in-situ chemical oxidation and bioremediation, face obstacles due to CCl4's recalcitrance to degradation.
These technical challenges collectively drive ongoing research and development efforts aimed at finding safer alternatives, improving handling and containment technologies, and developing more effective remediation strategies for CCl4 contamination. The industrial sector continues to grapple with balancing the compound's useful properties against its significant environmental and health risks.
Current CCl4 Applications
01 Chemical reactions and transformations
Carbon tetrachloride undergoes various chemical reactions and transformations. These may include decomposition, substitution reactions, and interactions with other compounds. The behavior of carbon tetrachloride in different chemical environments is studied to understand its reactivity and potential applications.- Chemical reactions and transformations: Carbon tetrachloride undergoes various chemical reactions and transformations. These include decomposition, substitution reactions, and interactions with other compounds. The behavior of carbon tetrachloride in different chemical environments is studied to understand its reactivity and potential applications.
- Industrial applications and processes: Carbon tetrachloride is used in various industrial applications and processes. Its chemical behavior is exploited in manufacturing, synthesis, and as a solvent. The compound's properties make it suitable for specific industrial uses, although environmental concerns have led to restrictions on its use in some areas.
- Environmental impact and degradation: The chemical behavior of carbon tetrachloride in the environment is of significant interest. Studies focus on its persistence, degradation pathways, and potential ecological effects. Understanding these aspects is crucial for assessing environmental risks and developing remediation strategies.
- Analytical methods and detection: Various analytical methods are employed to study the chemical behavior of carbon tetrachloride. These include spectroscopic techniques, chromatography, and other detection methods. Such analyses are important for monitoring its presence in different matrices and understanding its interactions with other substances.
- Safety and handling considerations: The chemical behavior of carbon tetrachloride necessitates specific safety and handling considerations. Its reactivity, volatility, and potential health hazards require appropriate precautions in laboratory and industrial settings. Understanding its behavior is crucial for developing safe handling protocols and protective measures.
02 Industrial applications and processes
Carbon tetrachloride is used in various industrial applications and processes. Its chemical behavior is exploited in manufacturing, synthesis, and as a solvent. Understanding its properties and reactions is crucial for optimizing industrial processes and developing new applications.Expand Specific Solutions03 Environmental impact and degradation
The chemical behavior of carbon tetrachloride in the environment is of significant interest. This includes its persistence, degradation pathways, and potential impact on ecosystems. Research focuses on understanding its fate in different environmental compartments and developing strategies for remediation.Expand Specific Solutions04 Analytical methods and detection
Various analytical methods are employed to study the chemical behavior of carbon tetrachloride. These techniques are used for detection, quantification, and characterization of carbon tetrachloride in different matrices. Developing sensitive and accurate analytical methods is crucial for monitoring and research purposes.Expand Specific Solutions05 Interactions with other substances
Carbon tetrachloride's chemical behavior is influenced by its interactions with other substances. This includes its behavior in mixtures, solutions, and when in contact with different materials. Understanding these interactions is important for predicting its behavior in complex systems and developing new applications or safety measures.Expand Specific Solutions
Key Players in CCl4 Industry
The carbon tetrachloride industry is in a mature phase, with a global market size estimated at around $30-40 million annually. The technology for production and handling of carbon tetrachloride is well-established, with major players like DuPont, Occidental Chemical, and The Chemours Company having decades of experience. However, due to environmental concerns and regulations, the market has been declining. Companies are focusing on developing alternatives and safer handling methods. Research institutions like the Institute of Process Engineering, CAS and universities such as Beijing University of Chemical Technology are contributing to technological advancements in this field.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed advanced technologies for the safe handling and use of carbon tetrachloride in industrial processes. Their approach includes innovative containment systems that minimize exposure risks and potential environmental impacts. DuPont's method involves using specialized catalysts to convert carbon tetrachloride into less harmful compounds during chemical reactions, reducing its overall environmental footprint[1]. They have also implemented sophisticated monitoring systems to detect and prevent leaks, ensuring worker safety and environmental protection. DuPont's research has led to the development of alternative compounds that can replace carbon tetrachloride in some applications, demonstrating their commitment to sustainable chemistry practices[2].
Strengths: Extensive experience in chemical engineering, strong focus on safety and environmental protection. Weaknesses: Potential high costs associated with advanced containment and conversion technologies.
Occidental Chemical Corp.
Technical Solution: Occidental Chemical Corp. has focused on developing closed-loop systems for carbon tetrachloride use in industrial processes. Their approach aims to minimize emissions and maximize recycling of the compound. They have implemented advanced distillation techniques to purify and recover carbon tetrachloride from process streams, significantly reducing waste and environmental impact[3]. Occidental has also invested in research to understand the long-term effects of carbon tetrachloride on various materials, leading to improved storage and handling protocols. Their work includes the development of specialized coatings and alloys resistant to carbon tetrachloride corrosion, extending the lifespan of equipment and reducing the risk of leaks[4].
Strengths: Expertise in large-scale chemical processing, focus on efficiency and waste reduction. Weaknesses: Potential limitations in completely eliminating carbon tetrachloride use in certain processes.
CCl4 Reaction Mechanisms
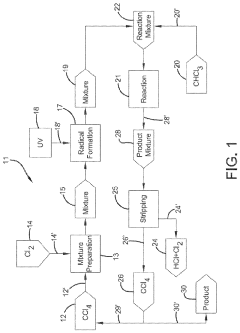
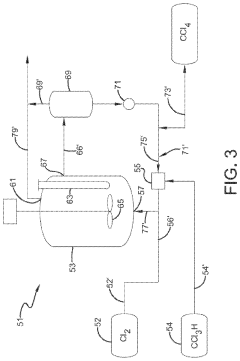
Photochlorination of partially-chlorinated chloromethanes to carbon tetrachloride
PatentActiveUS20240025823A1
Innovation
- A method involving the photochlorination of a chloromethanes stream containing chloroform, methyl chloride, and methylene chloride, combined with chlorine and additional carbon tetrachloride, and subjected to electromagnetic radiation to form carbon tetrachloride, achieving high conversion rates with reduced levels of unwanted chlorinated hydrocarbons.
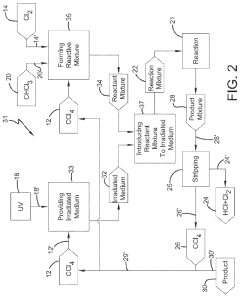
Chlorinolysis process for producing carbon tetrachloride
PatentActiveUS20210130266A1
Innovation
- A process involving a chlorination zone with chlorine, a C1 chlorinated compound, and a carbon/second chlorine source to produce a reaction mixture that favors the formation of carbon tetrachloride over perchloroethylene, using waste products as the carbon/second chlorine source to enhance efficiency and reduce impurity formation.
Environmental Impact of CCl4
Carbon tetrachloride (CCl4) has been widely used in various industrial applications for decades, but its environmental impact has become a significant concern in recent years. The release of CCl4 into the atmosphere has been shown to have severe consequences for the Earth's ozone layer, contributing to its depletion and potentially exacerbating global climate change.
When released into the air, CCl4 can persist for many years due to its chemical stability. It slowly migrates to the stratosphere, where it undergoes photolysis by ultraviolet radiation, releasing chlorine atoms. These chlorine atoms catalyze the breakdown of ozone molecules, leading to the thinning of the ozone layer. This process not only increases the amount of harmful UV radiation reaching the Earth's surface but also affects the delicate balance of atmospheric chemistry.
In aquatic environments, CCl4 can contaminate both surface and groundwater sources. Its low solubility in water and high density cause it to sink and form dense non-aqueous phase liquids (DNAPLs) in groundwater systems. This makes remediation efforts particularly challenging and costly. Aquatic organisms exposed to CCl4 may suffer from various toxic effects, including liver damage and potential carcinogenic outcomes.
Soil contamination by CCl4 is another significant environmental concern. The compound can persist in soil for extended periods, slowly volatilizing or leaching into groundwater. This persistence leads to long-term exposure risks for soil-dwelling organisms and plants, potentially disrupting ecosystem functions and biodiversity.
The global efforts to phase out CCl4 production and use, as mandated by the Montreal Protocol, have significantly reduced its environmental impact. However, legacy contamination and ongoing emissions from various sources continue to pose challenges. Recent studies have identified unexpected sources of CCl4 emissions, including industrial processes that were not previously considered significant contributors.
Addressing the environmental impact of CCl4 requires a multifaceted approach. This includes continued monitoring of atmospheric concentrations, implementation of stricter regulations on its production and use, development of more effective remediation technologies for contaminated sites, and research into environmentally friendly alternatives for industrial processes that still rely on CCl4.
When released into the air, CCl4 can persist for many years due to its chemical stability. It slowly migrates to the stratosphere, where it undergoes photolysis by ultraviolet radiation, releasing chlorine atoms. These chlorine atoms catalyze the breakdown of ozone molecules, leading to the thinning of the ozone layer. This process not only increases the amount of harmful UV radiation reaching the Earth's surface but also affects the delicate balance of atmospheric chemistry.
In aquatic environments, CCl4 can contaminate both surface and groundwater sources. Its low solubility in water and high density cause it to sink and form dense non-aqueous phase liquids (DNAPLs) in groundwater systems. This makes remediation efforts particularly challenging and costly. Aquatic organisms exposed to CCl4 may suffer from various toxic effects, including liver damage and potential carcinogenic outcomes.
Soil contamination by CCl4 is another significant environmental concern. The compound can persist in soil for extended periods, slowly volatilizing or leaching into groundwater. This persistence leads to long-term exposure risks for soil-dwelling organisms and plants, potentially disrupting ecosystem functions and biodiversity.
The global efforts to phase out CCl4 production and use, as mandated by the Montreal Protocol, have significantly reduced its environmental impact. However, legacy contamination and ongoing emissions from various sources continue to pose challenges. Recent studies have identified unexpected sources of CCl4 emissions, including industrial processes that were not previously considered significant contributors.
Addressing the environmental impact of CCl4 requires a multifaceted approach. This includes continued monitoring of atmospheric concentrations, implementation of stricter regulations on its production and use, development of more effective remediation technologies for contaminated sites, and research into environmentally friendly alternatives for industrial processes that still rely on CCl4.
CCl4 Safety Regulations
Carbon tetrachloride (CCl4) safety regulations have evolved significantly over the years due to the compound's known toxicity and environmental impact. The Occupational Safety and Health Administration (OSHA) has established strict exposure limits for workers, setting the permissible exposure limit (PEL) at 10 ppm as an 8-hour time-weighted average. The National Institute for Occupational Safety and Health (NIOSH) recommends an even lower exposure limit of 2 ppm for up to a 60-minute period.
In industrial settings, proper ventilation systems are mandatory when handling CCl4. Local exhaust ventilation must be designed to capture and remove vapors at the source, preventing their dispersion into the work environment. Personal protective equipment (PPE) requirements include impervious gloves, chemical-resistant clothing, and appropriate respiratory protection, such as a full-face respirator with organic vapor cartridges for concentrations up to 50 ppm.
Storage regulations dictate that CCl4 must be kept in tightly sealed containers in a cool, well-ventilated area away from incompatible materials. Secondary containment is often required to prevent spills from spreading. Facilities must also have proper spill response equipment and procedures in place, including absorbent materials and decontamination supplies.
The Environmental Protection Agency (EPA) regulates CCl4 under the Clean Air Act as a hazardous air pollutant. Emissions from industrial processes must be carefully controlled and monitored. The EPA has also classified CCl4 as a probable human carcinogen, leading to stringent regulations on its use and disposal. Under the Resource Conservation and Recovery Act (RCRA), CCl4 is considered a hazardous waste and must be disposed of according to specific guidelines.
International regulations, such as the Montreal Protocol, have phased out the production and consumption of CCl4 due to its ozone-depleting properties. This has led to a significant reduction in its industrial use globally. However, some countries still permit limited use for specific applications under strict controls.
Transportation of CCl4 is regulated by the Department of Transportation (DOT) as a hazardous material. It must be properly labeled, packaged, and transported in accordance with DOT regulations. Shipping documents must include appropriate hazard information, and carriers must be trained in handling hazardous materials.
Regular employee training on CCl4 hazards, safe handling procedures, and emergency response is mandated by various regulatory bodies. This includes instruction on proper use of PPE, understanding safety data sheets (SDS), and recognizing symptoms of exposure. Employers are required to maintain detailed records of employee training and exposure monitoring.
In industrial settings, proper ventilation systems are mandatory when handling CCl4. Local exhaust ventilation must be designed to capture and remove vapors at the source, preventing their dispersion into the work environment. Personal protective equipment (PPE) requirements include impervious gloves, chemical-resistant clothing, and appropriate respiratory protection, such as a full-face respirator with organic vapor cartridges for concentrations up to 50 ppm.
Storage regulations dictate that CCl4 must be kept in tightly sealed containers in a cool, well-ventilated area away from incompatible materials. Secondary containment is often required to prevent spills from spreading. Facilities must also have proper spill response equipment and procedures in place, including absorbent materials and decontamination supplies.
The Environmental Protection Agency (EPA) regulates CCl4 under the Clean Air Act as a hazardous air pollutant. Emissions from industrial processes must be carefully controlled and monitored. The EPA has also classified CCl4 as a probable human carcinogen, leading to stringent regulations on its use and disposal. Under the Resource Conservation and Recovery Act (RCRA), CCl4 is considered a hazardous waste and must be disposed of according to specific guidelines.
International regulations, such as the Montreal Protocol, have phased out the production and consumption of CCl4 due to its ozone-depleting properties. This has led to a significant reduction in its industrial use globally. However, some countries still permit limited use for specific applications under strict controls.
Transportation of CCl4 is regulated by the Department of Transportation (DOT) as a hazardous material. It must be properly labeled, packaged, and transported in accordance with DOT regulations. Shipping documents must include appropriate hazard information, and carriers must be trained in handling hazardous materials.
Regular employee training on CCl4 hazards, safe handling procedures, and emergency response is mandated by various regulatory bodies. This includes instruction on proper use of PPE, understanding safety data sheets (SDS), and recognizing symptoms of exposure. Employers are required to maintain detailed records of employee training and exposure monitoring.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!