Vascularization Strategies For Thick Engineered Living Tissues.
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Vascularization Challenges and Objectives
The field of tissue engineering has made remarkable strides in recent decades, yet the vascularization of thick engineered tissues remains one of the most significant challenges. Tissues exceeding 100-200 micrometers in thickness face diffusion limitations that prevent adequate oxygen and nutrient delivery to cells in their core, resulting in necrosis and functional failure. This fundamental constraint has severely limited the clinical translation of engineered tissues for applications such as organ replacement, regenerative medicine, and drug testing platforms.
The primary objective of vascularization strategies is to establish functional microvascular networks capable of supporting cell viability throughout thick tissue constructs. These networks must not only deliver oxygen and nutrients but also remove metabolic waste products, mirroring the complex hierarchical architecture of native vasculature. Additionally, these vascular structures must maintain their integrity and functionality over extended periods to ensure long-term tissue viability.
Current vascularization approaches can be broadly categorized into three strategic directions: in vitro pre-vascularization, in vivo vascularization, and scaffold-based approaches. Each strategy presents unique advantages and limitations that must be carefully considered in the context of specific tissue engineering applications. The integration of these approaches with advanced biomaterials and cell sources represents a promising avenue for overcoming current limitations.
Beyond the biological challenges, technical hurdles in manufacturing precision, scalability, and reproducibility must be addressed. The creation of hierarchical vascular networks with vessels ranging from large conduits to capillary-sized channels requires unprecedented precision in fabrication techniques. Furthermore, the temporal coordination of vascular development with tissue maturation presents additional complexity that current technologies struggle to manage effectively.
Regulatory considerations also pose significant challenges, as vascularized tissues represent complex biological products with multiple components and dynamic properties. Establishing standardized protocols for quality control, safety assessment, and efficacy evaluation will be crucial for clinical translation. The development of appropriate animal models and in vitro testing platforms specifically designed to evaluate vascularization efficiency represents another critical objective.
The ultimate goal extends beyond merely creating perfusable channels within engineered tissues. Rather, it aims to develop intelligent, responsive vascular networks capable of adapting to changing metabolic demands, integrating seamlessly with host vasculature upon implantation, and supporting tissue homeostasis throughout the lifespan of the implant. Achieving this vision will require interdisciplinary collaboration spanning tissue engineering, vascular biology, materials science, and advanced manufacturing technologies.
The primary objective of vascularization strategies is to establish functional microvascular networks capable of supporting cell viability throughout thick tissue constructs. These networks must not only deliver oxygen and nutrients but also remove metabolic waste products, mirroring the complex hierarchical architecture of native vasculature. Additionally, these vascular structures must maintain their integrity and functionality over extended periods to ensure long-term tissue viability.
Current vascularization approaches can be broadly categorized into three strategic directions: in vitro pre-vascularization, in vivo vascularization, and scaffold-based approaches. Each strategy presents unique advantages and limitations that must be carefully considered in the context of specific tissue engineering applications. The integration of these approaches with advanced biomaterials and cell sources represents a promising avenue for overcoming current limitations.
Beyond the biological challenges, technical hurdles in manufacturing precision, scalability, and reproducibility must be addressed. The creation of hierarchical vascular networks with vessels ranging from large conduits to capillary-sized channels requires unprecedented precision in fabrication techniques. Furthermore, the temporal coordination of vascular development with tissue maturation presents additional complexity that current technologies struggle to manage effectively.
Regulatory considerations also pose significant challenges, as vascularized tissues represent complex biological products with multiple components and dynamic properties. Establishing standardized protocols for quality control, safety assessment, and efficacy evaluation will be crucial for clinical translation. The development of appropriate animal models and in vitro testing platforms specifically designed to evaluate vascularization efficiency represents another critical objective.
The ultimate goal extends beyond merely creating perfusable channels within engineered tissues. Rather, it aims to develop intelligent, responsive vascular networks capable of adapting to changing metabolic demands, integrating seamlessly with host vasculature upon implantation, and supporting tissue homeostasis throughout the lifespan of the implant. Achieving this vision will require interdisciplinary collaboration spanning tissue engineering, vascular biology, materials science, and advanced manufacturing technologies.
Market Analysis for Engineered Tissue Applications
The engineered tissue market is experiencing significant growth, driven by increasing demand for organ transplants and the limitations of traditional donor systems. The global market for engineered tissues was valued at approximately $9.7 billion in 2022 and is projected to reach $25.3 billion by 2030, growing at a CAGR of 12.8%. Vascularized thick tissues represent a particularly promising segment within this broader market.
Healthcare applications dominate the current market landscape, with regenerative medicine accounting for nearly 40% of the total market share. Engineered skin substitutes currently lead commercial applications, followed by bone, cartilage, and cardiovascular tissues. However, the demand for more complex vascularized organs such as liver, kidney, and heart tissues is rapidly accelerating due to transplant waiting lists that continue to grow by 5-7% annually in developed countries.
Pharmaceutical companies constitute another significant market segment, utilizing vascularized tissue models for drug testing and toxicology studies. This application reduces animal testing requirements while providing more physiologically relevant human tissue models, potentially saving pharmaceutical companies between $300-500 million per successful drug development cycle by identifying failures earlier.
Regional analysis reveals North America holds the largest market share (approximately 45%), followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and Japan, demonstrates the fastest growth rate at 15.2% annually, driven by increasing healthcare expenditure and government initiatives supporting regenerative medicine research.
Market penetration faces several barriers including high production costs, with current vascularized tissue constructs costing between $1,000-5,000 per square centimeter depending on complexity. Regulatory hurdles also present significant challenges, with approval pathways varying substantially across different regions and typically requiring 3-7 years for novel tissue products.
Consumer adoption trends indicate increasing acceptance among clinicians, with survey data showing 72% of surgeons expressing willingness to use engineered tissues when available. Patient awareness and acceptance are also growing, with 65% of patients surveyed indicating preference for engineered tissue solutions over traditional treatments when presented with comparable efficacy data.
The reimbursement landscape remains complex, with coverage varying widely across different healthcare systems. In the United States, Medicare and private insurers have begun establishing reimbursement codes for certain engineered tissue products, primarily for wound care and orthopedic applications, though comprehensive coverage for more complex vascularized tissues remains limited.
Healthcare applications dominate the current market landscape, with regenerative medicine accounting for nearly 40% of the total market share. Engineered skin substitutes currently lead commercial applications, followed by bone, cartilage, and cardiovascular tissues. However, the demand for more complex vascularized organs such as liver, kidney, and heart tissues is rapidly accelerating due to transplant waiting lists that continue to grow by 5-7% annually in developed countries.
Pharmaceutical companies constitute another significant market segment, utilizing vascularized tissue models for drug testing and toxicology studies. This application reduces animal testing requirements while providing more physiologically relevant human tissue models, potentially saving pharmaceutical companies between $300-500 million per successful drug development cycle by identifying failures earlier.
Regional analysis reveals North America holds the largest market share (approximately 45%), followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and Japan, demonstrates the fastest growth rate at 15.2% annually, driven by increasing healthcare expenditure and government initiatives supporting regenerative medicine research.
Market penetration faces several barriers including high production costs, with current vascularized tissue constructs costing between $1,000-5,000 per square centimeter depending on complexity. Regulatory hurdles also present significant challenges, with approval pathways varying substantially across different regions and typically requiring 3-7 years for novel tissue products.
Consumer adoption trends indicate increasing acceptance among clinicians, with survey data showing 72% of surgeons expressing willingness to use engineered tissues when available. Patient awareness and acceptance are also growing, with 65% of patients surveyed indicating preference for engineered tissue solutions over traditional treatments when presented with comparable efficacy data.
The reimbursement landscape remains complex, with coverage varying widely across different healthcare systems. In the United States, Medicare and private insurers have begun establishing reimbursement codes for certain engineered tissue products, primarily for wound care and orthopedic applications, though comprehensive coverage for more complex vascularized tissues remains limited.
Current Vascularization Techniques and Limitations
Current vascularization techniques for thick engineered tissues can be broadly categorized into three approaches: scaffold-based methods, cell-based strategies, and biofabrication techniques. Each approach offers distinct advantages but also faces significant limitations that hinder clinical translation.
Scaffold-based vascularization methods utilize pre-fabricated channels or porous structures to facilitate blood vessel formation. Techniques such as sacrificial molding, where temporary structures are embedded in hydrogels and later dissolved, create vessel-like networks. However, these methods often struggle with achieving physiologically relevant vessel hierarchies and typically produce vessels with diameters larger than capillaries (>100 μm), limiting effective nutrient exchange at the cellular level.
Cell-based strategies leverage the natural vessel-forming capabilities of endothelial cells, either alone or in combination with supporting cells like pericytes and fibroblasts. While these approaches can generate capillary-sized vessels through self-assembly, they face challenges in creating organized, perfusable networks. The vessels formed often lack sufficient mechanical stability and take considerable time to develop (typically 7-14 days), during which tissue necrosis can occur in the construct core.
Biofabrication techniques, including bioprinting and microfluidics, offer precise spatial control over vessel architecture. 3D bioprinting can position cells and materials with micrometer precision, while microfluidic devices can create perfusable channels that mimic native vasculature. Despite these advantages, bioprinted vessels frequently suffer from low resolution (typically limited to 100-200 μm) and poor mechanical properties, while microfluidic approaches face scalability challenges for clinically relevant tissue sizes.
A critical limitation across all current techniques is the difficulty in achieving multi-scale vasculature that mimics the hierarchical organization found in native tissues—from large vessels to capillaries. This hierarchical structure is essential for efficient nutrient delivery and waste removal throughout thick tissues.
Temporal considerations also present significant challenges. Most engineered vascular networks require extended maturation periods before becoming fully functional, creating a critical window during which tissue viability is compromised. Additionally, the integration of engineered vasculature with host circulation remains problematic, with anastomosis (connection between engineered and host vessels) often being slow and inefficient.
Regulatory and manufacturing hurdles further complicate clinical translation. Complex vascularization strategies often involve multiple cell types and biomaterials, creating challenges for quality control, scalability, and regulatory approval. The lack of standardized methods for assessing vascular function in engineered tissues also impedes progress in the field.
Scaffold-based vascularization methods utilize pre-fabricated channels or porous structures to facilitate blood vessel formation. Techniques such as sacrificial molding, where temporary structures are embedded in hydrogels and later dissolved, create vessel-like networks. However, these methods often struggle with achieving physiologically relevant vessel hierarchies and typically produce vessels with diameters larger than capillaries (>100 μm), limiting effective nutrient exchange at the cellular level.
Cell-based strategies leverage the natural vessel-forming capabilities of endothelial cells, either alone or in combination with supporting cells like pericytes and fibroblasts. While these approaches can generate capillary-sized vessels through self-assembly, they face challenges in creating organized, perfusable networks. The vessels formed often lack sufficient mechanical stability and take considerable time to develop (typically 7-14 days), during which tissue necrosis can occur in the construct core.
Biofabrication techniques, including bioprinting and microfluidics, offer precise spatial control over vessel architecture. 3D bioprinting can position cells and materials with micrometer precision, while microfluidic devices can create perfusable channels that mimic native vasculature. Despite these advantages, bioprinted vessels frequently suffer from low resolution (typically limited to 100-200 μm) and poor mechanical properties, while microfluidic approaches face scalability challenges for clinically relevant tissue sizes.
A critical limitation across all current techniques is the difficulty in achieving multi-scale vasculature that mimics the hierarchical organization found in native tissues—from large vessels to capillaries. This hierarchical structure is essential for efficient nutrient delivery and waste removal throughout thick tissues.
Temporal considerations also present significant challenges. Most engineered vascular networks require extended maturation periods before becoming fully functional, creating a critical window during which tissue viability is compromised. Additionally, the integration of engineered vasculature with host circulation remains problematic, with anastomosis (connection between engineered and host vessels) often being slow and inefficient.
Regulatory and manufacturing hurdles further complicate clinical translation. Complex vascularization strategies often involve multiple cell types and biomaterials, creating challenges for quality control, scalability, and regulatory approval. The lack of standardized methods for assessing vascular function in engineered tissues also impedes progress in the field.
Established Vascularization Approaches for Thick Tissues
01 Angiogenic factors for promoting vascularization in thick tissues
Various angiogenic factors can be incorporated into tissue engineering constructs to promote blood vessel formation in thick tissues. These factors stimulate endothelial cell migration, proliferation, and tube formation, which are essential for creating functional vascular networks. By enhancing vascularization, these factors help overcome the diffusion limitations in thick engineered tissues, ensuring adequate oxygen and nutrient supply to cells throughout the construct.- Angiogenic growth factors for vascularization: Angiogenic growth factors can be incorporated into tissue engineering scaffolds to promote vascularization in thick tissue constructs. These bioactive molecules stimulate the formation of new blood vessels, enhancing oxygen and nutrient delivery throughout the tissue. By strategically delivering growth factors such as VEGF, FGF, and PDGF, the vascularization process can be accelerated, allowing for the development of thicker, more viable engineered tissues.
- Scaffold design for vascular network formation: Advanced scaffold designs with specific architectural features can facilitate vascularization in thick tissues. These scaffolds may incorporate microchannels, porous structures, or hierarchical designs that mimic natural vascular networks. The physical properties of the scaffold, including pore size, interconnectivity, and degradation rate, can be optimized to support cell infiltration and blood vessel formation, ultimately enabling the development of thicker tissue constructs with adequate vascularization.
- Co-culture systems for enhanced vascularization: Co-culture systems involving endothelial cells with supporting cell types can significantly improve vascularization in thick tissue constructs. By combining endothelial cells with fibroblasts, mesenchymal stem cells, or pericytes, the formation of stable and functional blood vessels is promoted. These co-culture approaches create a more physiologically relevant microenvironment that supports the development of vascular networks capable of sustaining thicker tissue constructs.
- Prevascularization techniques for thick tissues: Prevascularization techniques involve creating vascular networks within tissue constructs prior to implantation. These methods may include in vitro cultivation of vascular structures or the use of existing vascular beds as a foundation for tissue development. By establishing a vascular network before implantation, the critical limitation of oxygen diffusion in thick tissues can be overcome, allowing for the successful integration and survival of engineered tissues with substantial thickness.
- Mechanical and electrical stimulation for vascularization: Mechanical and electrical stimulation methods can enhance vascularization in thick tissue constructs. These approaches include applying controlled mechanical forces, such as cyclic strain or fluid flow, or electrical stimulation to promote angiogenesis and vascular maturation. Such stimulation techniques can activate mechanotransduction pathways and upregulate angiogenic factors, leading to improved vascular network formation and increased tissue thickness capacity.
02 Scaffold design for optimal vascularization in thick tissues
Advanced scaffold designs with specific architectural features can enhance vascularization in thick tissue constructs. These designs include controlled porosity, interconnected channels, and bioactive surface modifications that facilitate cell infiltration and blood vessel ingrowth. The physical properties of scaffolds, such as pore size and interconnectivity, significantly influence the extent and rate of vascularization, which is crucial for maintaining cell viability in thick tissue constructs.Expand Specific Solutions03 Co-culture systems for enhancing vascularization
Co-culturing endothelial cells with supporting cell types, such as fibroblasts, mesenchymal stem cells, or pericytes, can significantly improve vascularization in thick tissue constructs. These supporting cells provide essential paracrine factors and physical cues that promote endothelial cell organization into stable vascular networks. The strategic combination of different cell types creates a more physiologically relevant microenvironment that supports robust vascularization throughout thick tissue constructs.Expand Specific Solutions04 Microfluidic approaches for vascularization of thick tissues
Microfluidic technologies can be employed to create pre-defined vascular networks within thick tissue constructs. These approaches involve the fabrication of microchannels that can be lined with endothelial cells to form perfusable blood vessels. Microfluidic systems allow for controlled flow of culture medium, mimicking blood circulation and enabling efficient nutrient and oxygen delivery throughout thick tissue constructs. This technology is particularly valuable for engineering complex tissues that require immediate perfusion upon implantation.Expand Specific Solutions05 In vivo vascularization strategies for thick tissue integration
Various in vivo approaches can be used to enhance vascularization of thick tissue constructs after implantation. These include pre-vascularization techniques, where vascular networks are partially developed in vitro before implantation, and the use of vascular pedicles or arteriovenous loops to provide immediate blood supply to the implanted construct. Additionally, staged implantation approaches allow for gradual vascularization of thick tissues, improving their integration with host tissue and long-term survival.Expand Specific Solutions
Leading Research Groups and Companies in Vascularization
The vascularization of thick engineered tissues represents a critical challenge in tissue engineering, currently in an early-to-mid development stage. The market is expanding rapidly, projected to reach significant growth as regenerative medicine advances. Leading academic institutions like MIT, Tsinghua University, and Cornell University are driving fundamental research, while companies such as Advanced Solutions Life Sciences and Encellin are developing commercial applications. Established medical corporations including Ethicon and Cardinal Health are investing in translational technologies. The field exhibits varying technical maturity, with microfabrication approaches pioneered by MIT and Carnegie Mellon showing promise alongside bioprinting techniques from Advanced Solutions. Collaborative efforts between academic institutions and industry partners are accelerating progress toward clinically viable vascularized tissue constructs.
The Charles Stark Draper Laboratory, Inc.
Technical Solution: Draper Laboratory has developed an innovative organ-on-chip platform with integrated vascularization for thick engineered tissues. Their approach utilizes microfluidic technology to create perfusable vascular networks within 3D tissue constructs. The laboratory's proprietary PREDICT96 platform incorporates 96 individually addressable tissue chambers with integrated vascular channels, enabling high-throughput testing of vascularized tissue constructs. Draper's technology employs a combination of photolithography and soft lithography techniques to create multi-layered microfluidic devices with precisely controlled vascular geometries. Their system allows for real-time monitoring of oxygen gradients and metabolic activities within the engineered tissues through integrated biosensors[4][7]. Draper has demonstrated successful long-term culture of vascularized liver and kidney tissue constructs with maintained functionality for over 28 days. Their approach also includes computational fluid dynamics modeling to optimize flow parameters and ensure physiologically relevant shear stress within the vascular networks. The laboratory has recently developed techniques for creating anastomoses between engineered vascular networks and host vasculature to facilitate rapid integration upon implantation.
Strengths: Exceptional microfluidic engineering capabilities; high-throughput screening potential; sophisticated integrated sensing technologies for real-time monitoring of vascular function. Weaknesses: Limited scalability for creating larger tissue constructs; primarily focused on in vitro applications rather than implantable tissues; complex manufacturing processes requiring specialized cleanroom facilities.
The Regents of the University of California
Technical Solution: The University of California system has developed multiple innovative approaches to vascularization of thick engineered tissues. Their primary strategy involves the use of decellularized extracellular matrix (ECM) scaffolds with preserved vascular architecture that can be recellularized with patient-specific cells. UC researchers have pioneered the development of bioprinting techniques that incorporate sacrificial materials to create perfusable channels within hydrogel-based tissues. Their approach also includes the use of microfluidic devices with integrated vascular networks that can sustain thick tissue constructs over extended culture periods. The UC system has demonstrated successful vascularization of various tissue types including cardiac, hepatic, and neural tissues with improved functionality compared to avascular constructs[9][11]. Their technology incorporates spatially controlled delivery of angiogenic factors to guide vascular development in specific patterns. Recent advancements include the development of light-based bioprinting techniques that allow for the creation of complex vascular geometries with micrometer-scale precision. UC researchers have also developed innovative approaches to promote anastomosis between engineered vascular networks and host vasculature, facilitating rapid integration upon implantation.
Strengths: Diverse portfolio of complementary vascularization approaches; strong focus on translational applications; excellent integration with host vasculature. Weaknesses: Variability in outcomes across different tissue types; challenges in standardization due to multiple approaches being developed simultaneously; complex regulatory pathway for clinical translation.
Key Innovations in Vascular Network Formation
Bioengineered vascular network
PatentWO2018106652A1
Innovation
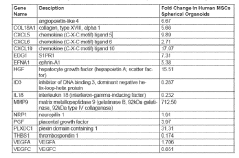
- Development of an in vitro vasculogenic technology using spherical organoids composed of endothelial cells and mesenchymal stem cells embedded in a hydrogel, which promotes the formation of a multiscale and multiphenotype vascular network, including arterial, microvascular, and venous components, capable of supporting thick engineered tissues.
Biomaterials and Scaffolds for Vascular Support
The development of biomaterials and scaffolds for vascular support represents a critical component in addressing the challenge of vascularization in thick engineered tissues. Traditional biomaterials have proven inadequate for supporting complex vascular networks, necessitating the development of specialized materials with properties conducive to blood vessel formation and maintenance.
Hydrogels have emerged as promising candidates due to their tunable mechanical properties and high water content, mimicking the natural extracellular matrix. Particularly, natural hydrogels such as collagen, fibrin, and alginate provide excellent biocompatibility and cell adhesion properties. Synthetic alternatives like poly(ethylene glycol) (PEG) and poly(lactic-co-glycolic acid) (PLGA) offer greater control over degradation rates and mechanical strength, though they may lack inherent bioactive properties.
Recent advances have focused on composite biomaterials that combine the advantages of both natural and synthetic materials. These hybrid systems can be engineered with specific degradation profiles that match the rate of neovascularization, ensuring structural support during initial stages while allowing for tissue remodeling over time. Additionally, incorporation of growth factor delivery systems within these scaffolds has shown significant enhancement in vascular network formation.
Microarchitecture of scaffolds plays a crucial role in guiding vascular development. Techniques such as 3D bioprinting, electrospinning, and microfabrication have enabled the creation of scaffolds with precise geometries and controlled porosity. Studies have demonstrated that scaffolds with interconnected pores of 150-200 μm diameter optimize cell migration and vascular ingrowth. Furthermore, the incorporation of pre-defined channels within scaffolds can serve as templates for vessel formation, facilitating the development of hierarchical vascular structures.
Surface modification strategies have also advanced significantly, with biomaterials now routinely functionalized with peptides such as RGD (Arg-Gly-Asp) to enhance endothelial cell adhesion and proliferation. More sophisticated approaches include immobilization of angiogenic growth factors like VEGF and bFGF in spatial gradients to direct vessel growth in specific patterns.
Biodegradable polymers with shape-memory properties represent another frontier, allowing for minimally invasive delivery followed by expansion in situ to form complex vascular structures. These materials can be programmed to respond to physiological stimuli such as temperature or pH, facilitating dynamic scaffold remodeling that mimics natural vascular development processes.
The integration of conductive materials such as carbon nanotubes and gold nanoparticles into vascular scaffolds has opened new possibilities for electrical stimulation of angiogenesis, with preliminary studies showing enhanced endothelial cell alignment and tubulogenesis under appropriate electrical fields.
Hydrogels have emerged as promising candidates due to their tunable mechanical properties and high water content, mimicking the natural extracellular matrix. Particularly, natural hydrogels such as collagen, fibrin, and alginate provide excellent biocompatibility and cell adhesion properties. Synthetic alternatives like poly(ethylene glycol) (PEG) and poly(lactic-co-glycolic acid) (PLGA) offer greater control over degradation rates and mechanical strength, though they may lack inherent bioactive properties.
Recent advances have focused on composite biomaterials that combine the advantages of both natural and synthetic materials. These hybrid systems can be engineered with specific degradation profiles that match the rate of neovascularization, ensuring structural support during initial stages while allowing for tissue remodeling over time. Additionally, incorporation of growth factor delivery systems within these scaffolds has shown significant enhancement in vascular network formation.
Microarchitecture of scaffolds plays a crucial role in guiding vascular development. Techniques such as 3D bioprinting, electrospinning, and microfabrication have enabled the creation of scaffolds with precise geometries and controlled porosity. Studies have demonstrated that scaffolds with interconnected pores of 150-200 μm diameter optimize cell migration and vascular ingrowth. Furthermore, the incorporation of pre-defined channels within scaffolds can serve as templates for vessel formation, facilitating the development of hierarchical vascular structures.
Surface modification strategies have also advanced significantly, with biomaterials now routinely functionalized with peptides such as RGD (Arg-Gly-Asp) to enhance endothelial cell adhesion and proliferation. More sophisticated approaches include immobilization of angiogenic growth factors like VEGF and bFGF in spatial gradients to direct vessel growth in specific patterns.
Biodegradable polymers with shape-memory properties represent another frontier, allowing for minimally invasive delivery followed by expansion in situ to form complex vascular structures. These materials can be programmed to respond to physiological stimuli such as temperature or pH, facilitating dynamic scaffold remodeling that mimics natural vascular development processes.
The integration of conductive materials such as carbon nanotubes and gold nanoparticles into vascular scaffolds has opened new possibilities for electrical stimulation of angiogenesis, with preliminary studies showing enhanced endothelial cell alignment and tubulogenesis under appropriate electrical fields.
Regulatory Pathways for Engineered Living Tissues
The regulatory landscape for engineered living tissues with vascularization strategies presents a complex and evolving framework that developers must navigate carefully. In the United States, the FDA's regulatory approach typically categorizes these advanced therapies under combination products, often regulated through the Center for Biologics Evaluation and Research (CBER) or the Center for Devices and Radiological Health (CDRH), depending on the primary mode of action.
For vascularized engineered tissues, the regulatory pathway generally follows a risk-based approach, with higher scrutiny applied to products intended for implantation or those containing significant cellular components. The FDA's guidance on Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps) provides the foundation, with vascularized tissues typically falling under Section 351 of the Public Health Service Act, requiring premarket approval.
The European Medicines Agency (EMA) classifies these products under the Advanced Therapy Medicinal Products (ATMP) framework, specifically as Tissue Engineered Products or Combined ATMPs. The presence of vascular networks within engineered tissues adds complexity to the classification and approval process, often requiring extensive preclinical and clinical data demonstrating the safety and functionality of the vascular components.
Japan's regulatory framework, revised under the Act on the Safety of Regenerative Medicine, offers an accelerated pathway for regenerative medicine products, potentially beneficial for vascularized tissue constructs that demonstrate preliminary safety and efficacy. This conditional approval system allows market entry while continuing to collect clinical evidence.
Regulatory considerations specific to vascularization strategies include demonstrating the stability and functionality of the vascular networks, addressing concerns about potential thrombogenicity, and ensuring appropriate integration with host vasculature. Quality control measures must verify consistent vascular formation throughout the engineered tissue.
Manufacturing standards present significant regulatory challenges, particularly regarding Good Manufacturing Practice (GMP) compliance for complex three-dimensional vascularized constructs. Regulators increasingly require standardized protocols for vascular network formation, whether through bioprinting, scaffold-based approaches, or self-organization methods.
Clinical trial designs for vascularized engineered tissues typically follow phased approaches, with initial focus on safety and vascular integration, followed by efficacy assessments. Regulatory agencies often require specialized endpoints that demonstrate functional perfusion and tissue viability maintained through the engineered vascular networks.
Global harmonization efforts, including those through the International Council for Harmonisation (ICH), are working to standardize requirements for these advanced therapies, though significant regional differences remain in the regulatory pathways for vascularized engineered tissues.
For vascularized engineered tissues, the regulatory pathway generally follows a risk-based approach, with higher scrutiny applied to products intended for implantation or those containing significant cellular components. The FDA's guidance on Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps) provides the foundation, with vascularized tissues typically falling under Section 351 of the Public Health Service Act, requiring premarket approval.
The European Medicines Agency (EMA) classifies these products under the Advanced Therapy Medicinal Products (ATMP) framework, specifically as Tissue Engineered Products or Combined ATMPs. The presence of vascular networks within engineered tissues adds complexity to the classification and approval process, often requiring extensive preclinical and clinical data demonstrating the safety and functionality of the vascular components.
Japan's regulatory framework, revised under the Act on the Safety of Regenerative Medicine, offers an accelerated pathway for regenerative medicine products, potentially beneficial for vascularized tissue constructs that demonstrate preliminary safety and efficacy. This conditional approval system allows market entry while continuing to collect clinical evidence.
Regulatory considerations specific to vascularization strategies include demonstrating the stability and functionality of the vascular networks, addressing concerns about potential thrombogenicity, and ensuring appropriate integration with host vasculature. Quality control measures must verify consistent vascular formation throughout the engineered tissue.
Manufacturing standards present significant regulatory challenges, particularly regarding Good Manufacturing Practice (GMP) compliance for complex three-dimensional vascularized constructs. Regulators increasingly require standardized protocols for vascular network formation, whether through bioprinting, scaffold-based approaches, or self-organization methods.
Clinical trial designs for vascularized engineered tissues typically follow phased approaches, with initial focus on safety and vascular integration, followed by efficacy assessments. Regulatory agencies often require specialized endpoints that demonstrate functional perfusion and tissue viability maintained through the engineered vascular networks.
Global harmonization efforts, including those through the International Council for Harmonisation (ICH), are working to standardize requirements for these advanced therapies, though significant regional differences remain in the regulatory pathways for vascularized engineered tissues.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!