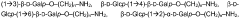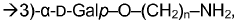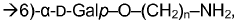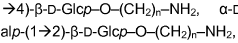What Researchers Need to Know About Carbon Tetrachloride's Structure?
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Structure Background and Research Objectives
Carbon tetrachloride (CCl4) has been a subject of scientific interest for over a century, with its structure playing a crucial role in understanding molecular geometry and bonding. The tetrahedral arrangement of chlorine atoms around a central carbon atom in CCl4 has made it a model compound for studying symmetry and electron distribution in molecules.
The evolution of our understanding of CCl4's structure parallels the development of chemical bonding theories and analytical techniques. Early studies in the late 19th and early 20th centuries relied on chemical reactivity and physical properties to infer structural information. The advent of X-ray crystallography and spectroscopic methods in the mid-20th century provided more direct evidence of CCl4's tetrahedral geometry.
Recent technological advancements have allowed for more precise measurements and theoretical calculations of CCl4's structure. High-resolution spectroscopy, neutron diffraction, and advanced computational methods have refined our knowledge of bond lengths, angles, and electron density distribution within the molecule.
The primary objective of current research on CCl4's structure is to achieve a comprehensive understanding of its electronic and geometric properties at the atomic level. This includes investigating the nature of carbon-chlorine bonds, the influence of electron correlation on molecular geometry, and the behavior of CCl4 under various conditions such as high pressure or in different solvents.
Another key research goal is to explore the relationship between CCl4's structure and its reactivity. Understanding how the tetrahedral arrangement affects the molecule's chemical behavior is crucial for predicting its environmental impact and potential applications in chemical synthesis.
Researchers are also focusing on the dynamic aspects of CCl4's structure, including vibrational modes and conformational changes. This information is vital for interpreting spectroscopic data and understanding the molecule's behavior in different physical states and chemical environments.
The study of CCl4's structure continues to be relevant in various fields, including atmospheric chemistry, materials science, and theoretical chemistry. Its simple yet symmetrical structure makes it an ideal model for testing new analytical techniques and theoretical models, contributing to the broader understanding of molecular structure and bonding.
The evolution of our understanding of CCl4's structure parallels the development of chemical bonding theories and analytical techniques. Early studies in the late 19th and early 20th centuries relied on chemical reactivity and physical properties to infer structural information. The advent of X-ray crystallography and spectroscopic methods in the mid-20th century provided more direct evidence of CCl4's tetrahedral geometry.
Recent technological advancements have allowed for more precise measurements and theoretical calculations of CCl4's structure. High-resolution spectroscopy, neutron diffraction, and advanced computational methods have refined our knowledge of bond lengths, angles, and electron density distribution within the molecule.
The primary objective of current research on CCl4's structure is to achieve a comprehensive understanding of its electronic and geometric properties at the atomic level. This includes investigating the nature of carbon-chlorine bonds, the influence of electron correlation on molecular geometry, and the behavior of CCl4 under various conditions such as high pressure or in different solvents.
Another key research goal is to explore the relationship between CCl4's structure and its reactivity. Understanding how the tetrahedral arrangement affects the molecule's chemical behavior is crucial for predicting its environmental impact and potential applications in chemical synthesis.
Researchers are also focusing on the dynamic aspects of CCl4's structure, including vibrational modes and conformational changes. This information is vital for interpreting spectroscopic data and understanding the molecule's behavior in different physical states and chemical environments.
The study of CCl4's structure continues to be relevant in various fields, including atmospheric chemistry, materials science, and theoretical chemistry. Its simple yet symmetrical structure makes it an ideal model for testing new analytical techniques and theoretical models, contributing to the broader understanding of molecular structure and bonding.
Industrial Applications and Market Demand
Carbon tetrachloride, a compound with the molecular formula CCl4, has played a significant role in various industrial applications throughout its history. Despite its declining use due to environmental concerns, it continues to have niche applications in certain sectors. The market demand for carbon tetrachloride has evolved significantly over the years, reflecting changes in regulatory policies and technological advancements.
Historically, carbon tetrachloride was widely used as a solvent in dry cleaning, fire extinguishers, and as a precursor in the production of refrigerants. However, its use in these applications has been largely phased out due to its ozone-depleting properties and potential health hazards. This shift has led to a substantial decrease in global demand for carbon tetrachloride in traditional consumer and industrial applications.
Despite this decline, carbon tetrachloride still finds use in specific industrial processes. One of the primary current applications is in the production of chlorofluorocarbons (CFCs) and their alternatives, such as hydrofluorocarbons (HFCs). While CFCs themselves are being phased out, the transition to alternative compounds still requires carbon tetrachloride in some manufacturing processes.
The chemical industry continues to utilize carbon tetrachloride as a feedstock for the production of other chlorinated compounds. It serves as an important intermediate in the synthesis of various chemicals, including pharmaceuticals and agrochemicals. This sector represents a stable, albeit limited, demand for carbon tetrachloride.
In the semiconductor industry, high-purity carbon tetrachloride is used in the production of silicon tetrachloride, which is essential for manufacturing optical fibers and high-purity silicon for electronic components. As the demand for advanced electronics and telecommunications infrastructure grows, this application area may see a modest increase in carbon tetrachloride consumption.
The market for carbon tetrachloride is heavily influenced by regulatory frameworks, particularly those aimed at protecting the ozone layer and reducing greenhouse gas emissions. The Montreal Protocol and subsequent amendments have significantly restricted the production and consumption of carbon tetrachloride, leading to a constrained supply and higher prices for legitimate uses.
Looking ahead, the market for carbon tetrachloride is expected to remain relatively stable, with potential for slight growth in specialized applications. Research into alternative compounds and processes that can replace carbon tetrachloride in its remaining applications continues, which may further impact future demand. The emphasis on sustainable and environmentally friendly industrial processes is likely to drive innovation in this area, potentially leading to the development of new markets for alternative compounds that can match or exceed the performance of carbon tetrachloride in specific applications.
Historically, carbon tetrachloride was widely used as a solvent in dry cleaning, fire extinguishers, and as a precursor in the production of refrigerants. However, its use in these applications has been largely phased out due to its ozone-depleting properties and potential health hazards. This shift has led to a substantial decrease in global demand for carbon tetrachloride in traditional consumer and industrial applications.
Despite this decline, carbon tetrachloride still finds use in specific industrial processes. One of the primary current applications is in the production of chlorofluorocarbons (CFCs) and their alternatives, such as hydrofluorocarbons (HFCs). While CFCs themselves are being phased out, the transition to alternative compounds still requires carbon tetrachloride in some manufacturing processes.
The chemical industry continues to utilize carbon tetrachloride as a feedstock for the production of other chlorinated compounds. It serves as an important intermediate in the synthesis of various chemicals, including pharmaceuticals and agrochemicals. This sector represents a stable, albeit limited, demand for carbon tetrachloride.
In the semiconductor industry, high-purity carbon tetrachloride is used in the production of silicon tetrachloride, which is essential for manufacturing optical fibers and high-purity silicon for electronic components. As the demand for advanced electronics and telecommunications infrastructure grows, this application area may see a modest increase in carbon tetrachloride consumption.
The market for carbon tetrachloride is heavily influenced by regulatory frameworks, particularly those aimed at protecting the ozone layer and reducing greenhouse gas emissions. The Montreal Protocol and subsequent amendments have significantly restricted the production and consumption of carbon tetrachloride, leading to a constrained supply and higher prices for legitimate uses.
Looking ahead, the market for carbon tetrachloride is expected to remain relatively stable, with potential for slight growth in specialized applications. Research into alternative compounds and processes that can replace carbon tetrachloride in its remaining applications continues, which may further impact future demand. The emphasis on sustainable and environmentally friendly industrial processes is likely to drive innovation in this area, potentially leading to the development of new markets for alternative compounds that can match or exceed the performance of carbon tetrachloride in specific applications.
Current Understanding and Challenges
Carbon tetrachloride (CCl4) has been extensively studied due to its historical industrial importance and environmental impact. The current understanding of its structure is well-established, with a tetrahedral molecular geometry where the carbon atom is at the center, bonded to four chlorine atoms. This arrangement results in a highly symmetrical molecule with a non-polar nature.
Recent advancements in spectroscopic techniques have provided more precise measurements of bond lengths and angles within the CCl4 molecule. The C-Cl bond length is approximately 1.77 Å, and the Cl-C-Cl bond angle is close to the ideal tetrahedral angle of 109.5°. These refined measurements have contributed to a more accurate understanding of the molecule's electronic structure and bonding characteristics.
Despite the well-established structural knowledge, researchers continue to face challenges in fully understanding the behavior of CCl4 under various conditions. One significant area of ongoing research is the molecule's interaction with surfaces and other materials. The adsorption and desorption processes of CCl4 on different substrates are complex and not yet fully elucidated, presenting challenges in environmental remediation efforts.
Another challenge lies in understanding the photochemical behavior of CCl4. While it is known that the molecule can undergo photodissociation when exposed to UV light, the exact mechanisms and intermediate species formed during this process are still subjects of investigation. This knowledge gap has implications for atmospheric chemistry and the molecule's role in ozone depletion.
The behavior of CCl4 under extreme conditions, such as high pressure or in supercritical states, is another area where current understanding is limited. Researchers are working to elucidate how the molecular structure and properties change under these conditions, which could have implications for industrial processes and geological studies.
In the field of theoretical chemistry, accurately modeling the electronic structure of CCl4 remains challenging. While density functional theory (DFT) and other computational methods have provided valuable insights, achieving high-accuracy predictions of certain properties, particularly those related to excited states, continues to be difficult.
Lastly, the interaction of CCl4 with biological systems at the molecular level is not fully understood. While its toxicity is well-documented, the specific mechanisms of cellular damage and the potential for long-term effects on genetic material are areas that require further investigation. This knowledge is crucial for developing more effective treatments for CCl4 exposure and understanding its environmental impact on living organisms.
Recent advancements in spectroscopic techniques have provided more precise measurements of bond lengths and angles within the CCl4 molecule. The C-Cl bond length is approximately 1.77 Å, and the Cl-C-Cl bond angle is close to the ideal tetrahedral angle of 109.5°. These refined measurements have contributed to a more accurate understanding of the molecule's electronic structure and bonding characteristics.
Despite the well-established structural knowledge, researchers continue to face challenges in fully understanding the behavior of CCl4 under various conditions. One significant area of ongoing research is the molecule's interaction with surfaces and other materials. The adsorption and desorption processes of CCl4 on different substrates are complex and not yet fully elucidated, presenting challenges in environmental remediation efforts.
Another challenge lies in understanding the photochemical behavior of CCl4. While it is known that the molecule can undergo photodissociation when exposed to UV light, the exact mechanisms and intermediate species formed during this process are still subjects of investigation. This knowledge gap has implications for atmospheric chemistry and the molecule's role in ozone depletion.
The behavior of CCl4 under extreme conditions, such as high pressure or in supercritical states, is another area where current understanding is limited. Researchers are working to elucidate how the molecular structure and properties change under these conditions, which could have implications for industrial processes and geological studies.
In the field of theoretical chemistry, accurately modeling the electronic structure of CCl4 remains challenging. While density functional theory (DFT) and other computational methods have provided valuable insights, achieving high-accuracy predictions of certain properties, particularly those related to excited states, continues to be difficult.
Lastly, the interaction of CCl4 with biological systems at the molecular level is not fully understood. While its toxicity is well-documented, the specific mechanisms of cellular damage and the potential for long-term effects on genetic material are areas that require further investigation. This knowledge is crucial for developing more effective treatments for CCl4 exposure and understanding its environmental impact on living organisms.
Modern Analytical Techniques for CCl4 Structure
01 Molecular structure of carbon tetrachloride
Carbon tetrachloride is a tetrahedral molecule with a central carbon atom bonded to four chlorine atoms. This structure gives it unique chemical and physical properties, including its high stability and non-flammability.- Molecular structure of carbon tetrachloride: Carbon tetrachloride is a tetrahedral molecule with a central carbon atom bonded to four chlorine atoms. This structure gives it unique chemical and physical properties, including its high stability and non-flammability.
- Synthesis methods for carbon tetrachloride: Various methods exist for synthesizing carbon tetrachloride, including chlorination of methane or carbon disulfide, and the reaction of chloroform with chlorine. These processes often involve specific catalysts and reaction conditions to optimize yield and purity.
- Applications of carbon tetrachloride: Carbon tetrachloride has been used in various industrial and commercial applications, such as a solvent for oils and fats, a refrigerant, and a fire extinguishing agent. However, its use has been restricted due to environmental and health concerns.
- Environmental impact and regulations: Due to its ozone-depleting properties and potential health hazards, the production and use of carbon tetrachloride have been heavily regulated. Many countries have phased out its use in consumer products and restricted its industrial applications.
- Detection and analysis methods: Various analytical techniques have been developed to detect and quantify carbon tetrachloride in environmental samples and industrial processes. These methods often involve spectroscopic or chromatographic techniques to identify and measure the compound accurately.
02 Production methods for carbon tetrachloride
Various methods exist for producing carbon tetrachloride, including chlorination of methane or carbon disulfide, and the reaction of chloroform with chlorine. These processes often involve specific catalysts and reaction conditions to optimize yield and purity.Expand Specific Solutions03 Applications of carbon tetrachloride
Carbon tetrachloride has been used in various industrial and commercial applications, such as a solvent for oils and fats, a refrigerant, and a fire extinguishing agent. However, its use has been restricted due to environmental and health concerns.Expand Specific Solutions04 Environmental impact and regulations
Due to its ozone-depleting properties and potential health hazards, the use of carbon tetrachloride has been heavily regulated. Many countries have phased out its production and use in accordance with international agreements such as the Montreal Protocol.Expand Specific Solutions05 Detection and analysis methods
Various analytical techniques have been developed to detect and quantify carbon tetrachloride in environmental samples and industrial processes. These methods often involve spectroscopic or chromatographic techniques, ensuring accurate monitoring of this compound.Expand Specific Solutions
Key Research Institutions and Scientists
The research landscape surrounding carbon tetrachloride's structure is characterized by a mature technology in a well-established field. The market for this research is relatively stable, with ongoing interest from both academic institutions and industrial players. Key competitors include major chemical companies like ExxonMobil, Occidental Chemical, and JSR Corporation, alongside prestigious research institutions such as MIT, Columbia University, and Max Planck Society. The involvement of these diverse stakeholders indicates a continued relevance of carbon tetrachloride research, likely driven by environmental concerns and potential industrial applications. The technology's maturity suggests that innovations may focus on refinements, alternative applications, or addressing environmental impacts rather than fundamental structural discoveries.
Occidental Chemical Corp.
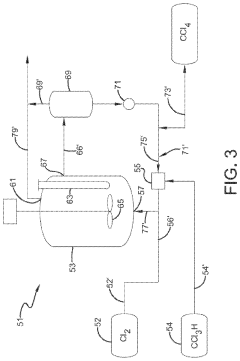
Technical Solution: Occidental Chemical Corp. has developed advanced purification techniques for carbon tetrachloride, focusing on its tetrahedral molecular structure. Their process involves multi-stage distillation and selective adsorption to remove impurities, resulting in ultra-high purity CCl4 (99.99+%) [1]. The company has also implemented in-line spectroscopic analysis to monitor the structural integrity of CCl4 during production, ensuring consistent quality. Additionally, they have explored the use of carbon tetrachloride as a precursor in the synthesis of chlorofluorocarbons (CFCs) alternatives, leveraging its stable tetrahedral configuration [3].
Strengths: High-purity production capabilities, advanced quality control. Weaknesses: Environmental concerns due to CCl4's ozone-depleting potential, limited applications due to regulatory restrictions.
ExxonMobil Technology & Engineering Co.
Technical Solution: ExxonMobil has focused on understanding the reactivity of carbon tetrachloride's structure in various industrial processes. They have developed catalytic systems that can selectively cleave C-Cl bonds in CCl4, allowing for controlled transformation into less harmful compounds [2]. Their research includes computational modeling of CCl4's molecular orbitals to predict its behavior in complex chemical environments. ExxonMobil has also investigated the use of carbon tetrachloride as a model compound for studying chlorinated hydrocarbon remediation techniques, utilizing its well-defined tetrahedral structure to develop more effective cleanup methods for contaminated sites [4].
Strengths: Advanced catalytic technologies, computational modeling capabilities. Weaknesses: Limited commercial applications due to environmental regulations on CCl4 use.
Breakthrough Discoveries in CCl4 Bonding
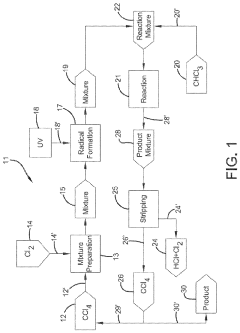
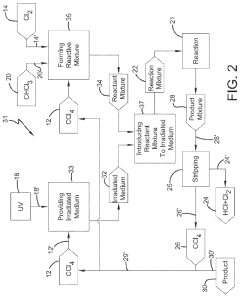
Photochlorination of partially-chlorinated chloromethanes to carbon tetrachloride
PatentActiveUS20240025823A1
Innovation
- A method involving the photochlorination of a chloromethanes stream containing chloroform, methyl chloride, and methylene chloride, combined with chlorine and additional carbon tetrachloride, and subjected to electromagnetic radiation to form carbon tetrachloride, achieving high conversion rates with reduced levels of unwanted chlorinated hydrocarbons.
Method for determining carbohydrates structure
PatentWO2017036545A1
Innovation
- The use of ion mobility-mass spectrometry (IM-MS) in negative ionization mode, combined with fragmentation and a database of collision cross section and mass-to-charge ratio values, allows for the determination of carbohydrate structures by measuring drift time and converting it into collision cross section, enabling the differentiation of isomers and determination of stereochemistry.
Environmental Impact and Regulations
Carbon tetrachloride, a synthetic chemical compound, has been widely used in various industrial applications for decades. However, its environmental impact and associated regulations have become increasingly significant concerns for researchers and policymakers alike.
The release of carbon tetrachloride into the environment can have severe consequences for both ecosystems and human health. When released into the atmosphere, it contributes to the depletion of the ozone layer, which protects Earth from harmful ultraviolet radiation. This ozone-depleting property has led to strict regulations on its production and use under the Montreal Protocol, an international treaty designed to protect the ozone layer.
In aquatic environments, carbon tetrachloride can persist for long periods due to its low biodegradability. It can accumulate in sediments and bioaccumulate in aquatic organisms, potentially entering the food chain. This persistence and bioaccumulation potential have raised concerns about its long-term effects on aquatic ecosystems and the potential for human exposure through contaminated water sources or seafood consumption.
Soil contamination by carbon tetrachloride is another significant environmental issue. The compound can leach into groundwater, potentially contaminating drinking water supplies. Its presence in soil can also inhibit plant growth and affect soil microbial communities, disrupting ecosystem functions.
Due to these environmental risks, many countries have implemented stringent regulations on the production, use, and disposal of carbon tetrachloride. In the United States, for example, the Environmental Protection Agency (EPA) has classified it as a hazardous air pollutant and a priority pollutant under the Clean Air Act and Clean Water Act, respectively. The EPA has also established maximum contaminant levels for carbon tetrachloride in drinking water.
Internationally, the Stockholm Convention on Persistent Organic Pollutants has listed carbon tetrachloride as a substance targeted for global elimination. This treaty requires signatories to take measures to reduce or eliminate its production and use.
Researchers studying carbon tetrachloride's structure must be aware of these environmental impacts and regulatory frameworks. Understanding the compound's behavior in different environmental compartments is crucial for developing effective remediation strategies and assessing potential risks. Moreover, knowledge of current regulations is essential for ensuring compliance in research activities and for informing policy decisions related to the management of this chemical.
As environmental concerns continue to grow, researchers may need to explore alternative compounds or technologies that can replace carbon tetrachloride in its various applications. This shift towards more environmentally friendly alternatives represents an important area for future research and innovation in the field.
The release of carbon tetrachloride into the environment can have severe consequences for both ecosystems and human health. When released into the atmosphere, it contributes to the depletion of the ozone layer, which protects Earth from harmful ultraviolet radiation. This ozone-depleting property has led to strict regulations on its production and use under the Montreal Protocol, an international treaty designed to protect the ozone layer.
In aquatic environments, carbon tetrachloride can persist for long periods due to its low biodegradability. It can accumulate in sediments and bioaccumulate in aquatic organisms, potentially entering the food chain. This persistence and bioaccumulation potential have raised concerns about its long-term effects on aquatic ecosystems and the potential for human exposure through contaminated water sources or seafood consumption.
Soil contamination by carbon tetrachloride is another significant environmental issue. The compound can leach into groundwater, potentially contaminating drinking water supplies. Its presence in soil can also inhibit plant growth and affect soil microbial communities, disrupting ecosystem functions.
Due to these environmental risks, many countries have implemented stringent regulations on the production, use, and disposal of carbon tetrachloride. In the United States, for example, the Environmental Protection Agency (EPA) has classified it as a hazardous air pollutant and a priority pollutant under the Clean Air Act and Clean Water Act, respectively. The EPA has also established maximum contaminant levels for carbon tetrachloride in drinking water.
Internationally, the Stockholm Convention on Persistent Organic Pollutants has listed carbon tetrachloride as a substance targeted for global elimination. This treaty requires signatories to take measures to reduce or eliminate its production and use.
Researchers studying carbon tetrachloride's structure must be aware of these environmental impacts and regulatory frameworks. Understanding the compound's behavior in different environmental compartments is crucial for developing effective remediation strategies and assessing potential risks. Moreover, knowledge of current regulations is essential for ensuring compliance in research activities and for informing policy decisions related to the management of this chemical.
As environmental concerns continue to grow, researchers may need to explore alternative compounds or technologies that can replace carbon tetrachloride in its various applications. This shift towards more environmentally friendly alternatives represents an important area for future research and innovation in the field.
Safety Protocols in CCl4 Research
Carbon tetrachloride (CCl4) is a highly toxic compound that requires stringent safety protocols in research settings. Researchers working with CCl4 must adhere to strict guidelines to protect themselves and others from potential exposure and associated health risks. Personal protective equipment (PPE) is essential when handling CCl4. This includes wearing chemical-resistant gloves, lab coats, and safety goggles or face shields. Respiratory protection may also be necessary, depending on the concentration and duration of exposure.
Proper ventilation is crucial when working with CCl4. All experiments involving this compound should be conducted in a fume hood or other well-ventilated area to minimize inhalation risks. Researchers should ensure that fume hoods are functioning correctly and maintain adequate airflow before beginning any work with CCl4.
Storage and handling of CCl4 require special attention. The compound should be stored in tightly sealed containers in a cool, dry, and well-ventilated area away from sources of heat or ignition. Researchers must use appropriate secondary containment to prevent spills and leaks. When transferring CCl4, use of a safety pipette or closed system is recommended to minimize the risk of accidental release.
Spill response procedures must be in place and clearly communicated to all laboratory personnel. In the event of a CCl4 spill, immediate evacuation of the area may be necessary. Only trained personnel equipped with appropriate PPE should attempt to clean up spills. Absorbent materials specifically designed for chemical spills should be readily available in the laboratory.
Waste disposal is another critical aspect of CCl4 safety protocols. The compound and any materials contaminated with it must be treated as hazardous waste and disposed of according to local, state, and federal regulations. Proper labeling and segregation of CCl4 waste are essential to ensure safe handling and disposal.
Regular safety training and education are vital for all researchers working with CCl4. This should include information on the compound's properties, health hazards, proper handling techniques, and emergency procedures. Researchers should also be familiar with the location and use of safety equipment such as eyewash stations and emergency showers.
Monitoring and health surveillance may be necessary for researchers regularly exposed to CCl4. This can include periodic medical examinations and air quality monitoring in the laboratory. Implementing a system for tracking CCl4 usage and exposure can help identify potential risks and improve safety measures over time.
Proper ventilation is crucial when working with CCl4. All experiments involving this compound should be conducted in a fume hood or other well-ventilated area to minimize inhalation risks. Researchers should ensure that fume hoods are functioning correctly and maintain adequate airflow before beginning any work with CCl4.
Storage and handling of CCl4 require special attention. The compound should be stored in tightly sealed containers in a cool, dry, and well-ventilated area away from sources of heat or ignition. Researchers must use appropriate secondary containment to prevent spills and leaks. When transferring CCl4, use of a safety pipette or closed system is recommended to minimize the risk of accidental release.
Spill response procedures must be in place and clearly communicated to all laboratory personnel. In the event of a CCl4 spill, immediate evacuation of the area may be necessary. Only trained personnel equipped with appropriate PPE should attempt to clean up spills. Absorbent materials specifically designed for chemical spills should be readily available in the laboratory.
Waste disposal is another critical aspect of CCl4 safety protocols. The compound and any materials contaminated with it must be treated as hazardous waste and disposed of according to local, state, and federal regulations. Proper labeling and segregation of CCl4 waste are essential to ensure safe handling and disposal.
Regular safety training and education are vital for all researchers working with CCl4. This should include information on the compound's properties, health hazards, proper handling techniques, and emergency procedures. Researchers should also be familiar with the location and use of safety equipment such as eyewash stations and emergency showers.
Monitoring and health surveillance may be necessary for researchers regularly exposed to CCl4. This can include periodic medical examinations and air quality monitoring in the laboratory. Implementing a system for tracking CCl4 usage and exposure can help identify potential risks and improve safety measures over time.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!