A perfusion culture method for expressing recombinant factor eight
A technology of perfusion culture and factors, which is applied in the field of perfusion culture for high-efficiency expression of eight recombinant factors, to achieve the effects of high retention efficiency, large perfusion efficiency, easy cleaning and sterilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
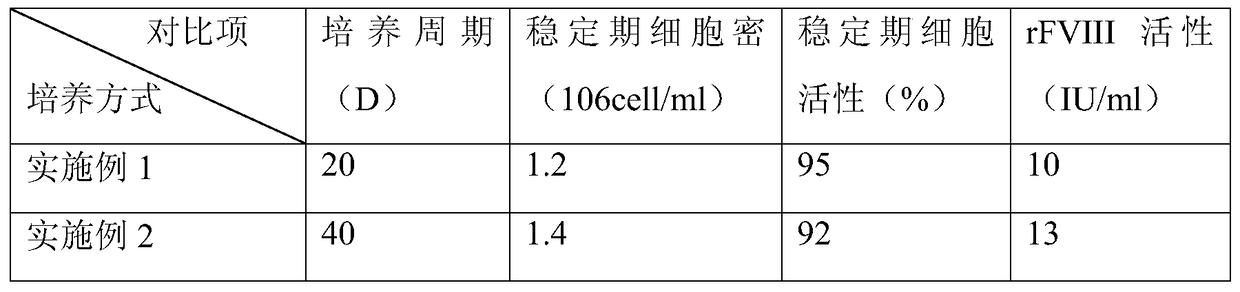
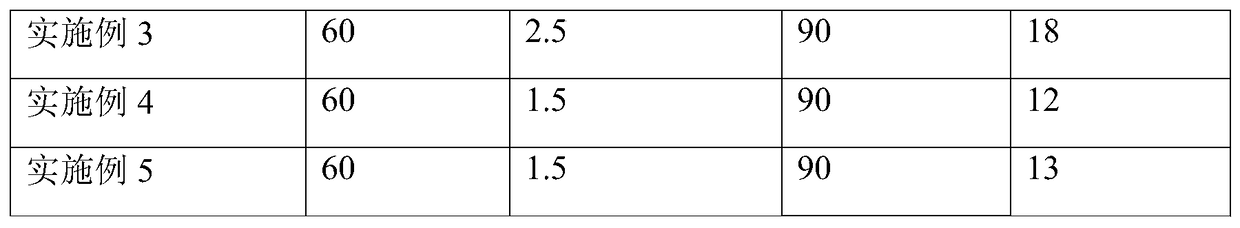
Embodiment 1
[0039] (1) Inoculation of cells
[0040] A cell line expressing recombinant FVIII (preservation number: CCTCC C2015214) was revived in the cell bank, cultured and expanded in a shake flask with serum-free medium, and inoculated into a reactor of a 5L culture system after the number of cells was sufficient. The cell density after inoculation is 0.8*10 6 a / ml;
[0041] (2) Initial growth stage
[0042] After the cells were inserted into the reactor, various culture parameters were set, such as temperature 37°C, pH 6.9, dissolved oxygen (DO) 30-50%, and initial rotation speed 90 rpm. Sampling was aseptically taken every day to detect the content of glucose and FVIII.
[0043] (3) Enter the perfusion culture mode
[0044] When the glucose content is lower than 1g / L, turn on the Spin filter (CelliGen Plus, New Brunswick Scientific) to enter the perfusion culture mode, and the perfusion volume is to keep the glucose content at about 1g / L. Remove part of the cells from the react...
Embodiment 2
[0046] (1) Inoculation of cells
[0047] A cell line expressing recombinant FVIII (same as in Example 1) was revived in the cell bank, cultured and amplified in a shake flask with a serum-free medium, and inoculated into a reactor of a 5L culture system after the number of cells was sufficient. The cell density after inoculation is 0.8*10 6 a / ml;
[0048] (2) Initial growth stage
[0049] After the cells were inserted into the reactor, various culture parameters were set, such as temperature 37°C, pH 6.9, dissolved oxygen (DO) 30-50%, and initial rotation speed 90 rpm. Sampling was aseptically taken every day to detect the content of glucose and FVIII.
[0050] (3) Enter the perfusion culture mode
[0051] When the glucose content is lower than 1g / L, turn on the audio frequency perfusion device (10L Biosep, Applikon) for perfusion culture. The perfusion volume is to keep the glucose content at about 1g / L. Remove part of the cells from the container and keep the cells at 1...
Embodiment 3
[0053] (1) Inoculation of cells
[0054] A cell line expressing recombinant FVIII (same as in Example 1) was revived in the cell bank, cultured and amplified in a shake flask with a serum-free medium, and inoculated into a reactor of a 5L culture system after the number of cells was sufficient. The cell density after inoculation is 0.8*10 6 a / ml;
[0055] (2) Initial growth stage
[0056] After the cells were inserted into the reactor, various culture parameters were set, such as temperature 37°C, pH 6.9, dissolved oxygen (DO) 30-50%, and initial rotation speed 90 rpm. Sampling was aseptically taken every day to detect the content of glucose and FVIII.
[0057] (3) Change the training mode
[0058] When the glucose content is lower than 1g / L, turn on the spin filter first, and carry out perfusion culture. The perfusion volume is to keep the glucose content at about 1g / L, and maintain the cells at 1-2*10 7 pieces / ml.
[0059] (4) Continuous perfusion culture stage
[006...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com