Achieving High Resolution in HPLC for Trace Analysis
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC Resolution Evolution and Objectives
High-performance liquid chromatography (HPLC) has undergone significant evolution since its inception in the 1960s, transforming from a rudimentary analytical technique to a sophisticated tool capable of detecting trace compounds at parts-per-billion levels. The journey toward achieving higher resolution in HPLC represents one of the most critical technological advancements in analytical chemistry over the past five decades.
The early HPLC systems of the 1970s offered limited resolution capabilities, with typical column efficiencies below 10,000 theoretical plates. By the 1980s, improvements in stationary phase technology and instrumentation pushed these numbers to approximately 25,000 plates, marking the beginning of modern HPLC applications in pharmaceutical analysis and environmental monitoring.
A paradigm shift occurred in the 1990s with the introduction of sub-2-micron particles and ultra-high-pressure systems, enabling resolution improvements by factors of 3-5 compared to conventional HPLC. This evolution continued into the 2000s with the commercialization of core-shell particles, which offered similar performance to sub-2-micron fully porous particles but at lower backpressures, democratizing access to high-resolution separations.
The current technological frontier focuses on achieving ever-higher resolution while maintaining practical analysis times, particularly for complex matrices where trace analytes must be separated from numerous interfering compounds. Modern HPLC systems aim to achieve theoretical plate counts exceeding 100,000 plates per column, with peak capacities approaching 1,000 in two-dimensional setups.
The primary objectives driving HPLC resolution enhancement include: reducing the limits of detection for trace analysis in environmental, food safety, and clinical applications; improving the separation of structurally similar compounds such as isomers and enantiomers; and enabling comprehensive analysis of complex biological samples containing thousands of components.
Recent technological trends indicate a convergence of multiple approaches, including multi-dimensional chromatography, specialized stationary phases with enhanced selectivity, and advanced detection systems that can differentiate co-eluting compounds. The integration of artificial intelligence for method development and optimization represents another frontier, potentially enabling adaptive separation protocols that respond to sample complexity in real-time.
The ultimate goal remains the development of HPLC systems capable of single-molecule detection with absolute specificity, while maintaining reasonable analysis times and operational simplicity. This vision drives ongoing research into novel stationary phases, instrument designs, and detection technologies that push the boundaries of what is currently possible in trace analysis.
The early HPLC systems of the 1970s offered limited resolution capabilities, with typical column efficiencies below 10,000 theoretical plates. By the 1980s, improvements in stationary phase technology and instrumentation pushed these numbers to approximately 25,000 plates, marking the beginning of modern HPLC applications in pharmaceutical analysis and environmental monitoring.
A paradigm shift occurred in the 1990s with the introduction of sub-2-micron particles and ultra-high-pressure systems, enabling resolution improvements by factors of 3-5 compared to conventional HPLC. This evolution continued into the 2000s with the commercialization of core-shell particles, which offered similar performance to sub-2-micron fully porous particles but at lower backpressures, democratizing access to high-resolution separations.
The current technological frontier focuses on achieving ever-higher resolution while maintaining practical analysis times, particularly for complex matrices where trace analytes must be separated from numerous interfering compounds. Modern HPLC systems aim to achieve theoretical plate counts exceeding 100,000 plates per column, with peak capacities approaching 1,000 in two-dimensional setups.
The primary objectives driving HPLC resolution enhancement include: reducing the limits of detection for trace analysis in environmental, food safety, and clinical applications; improving the separation of structurally similar compounds such as isomers and enantiomers; and enabling comprehensive analysis of complex biological samples containing thousands of components.
Recent technological trends indicate a convergence of multiple approaches, including multi-dimensional chromatography, specialized stationary phases with enhanced selectivity, and advanced detection systems that can differentiate co-eluting compounds. The integration of artificial intelligence for method development and optimization represents another frontier, potentially enabling adaptive separation protocols that respond to sample complexity in real-time.
The ultimate goal remains the development of HPLC systems capable of single-molecule detection with absolute specificity, while maintaining reasonable analysis times and operational simplicity. This vision drives ongoing research into novel stationary phases, instrument designs, and detection technologies that push the boundaries of what is currently possible in trace analysis.
Market Demand for Trace Analysis Applications
The global market for trace analysis applications has witnessed substantial growth in recent years, driven by increasing regulatory requirements and quality control standards across multiple industries. The demand for high-resolution HPLC (High-Performance Liquid Chromatography) techniques specifically for trace analysis has expanded at a compound annual growth rate of 6.8% since 2018, with the market value reaching $4.7 billion in 2022.
Pharmaceutical and biopharmaceutical sectors represent the largest market segment, accounting for approximately 38% of the total demand. This dominance stems from stringent regulatory requirements for impurity profiling, where detection of genotoxic impurities at parts-per-billion levels has become mandatory under ICH M7 guidelines. The need to identify and quantify increasingly complex degradation products in novel biologics and small molecule formulations further drives market growth in this sector.
Environmental monitoring constitutes the second-largest application area, comprising 27% of the market. Government agencies worldwide have progressively lowered acceptable limits for persistent organic pollutants, pesticide residues, and emerging contaminants such as PFAS (per- and polyfluoroalkyl substances) in water sources. This regulatory tightening has created substantial demand for analytical methods capable of detecting concentrations below 0.1 parts per trillion in complex environmental matrices.
Food safety testing represents another rapidly growing segment, expanding at 8.2% annually. Consumer awareness regarding food contaminants, coupled with international trade requirements for pesticide residue analysis and mycotoxin detection, has intensified the need for high-resolution analytical techniques. The European Union's regulations on maximum residue limits, which cover over 500 pesticides in various food products, exemplify the regulatory pressure driving market demand.
Clinical diagnostics and forensic toxicology applications have emerged as high-growth niches, particularly for therapeutic drug monitoring and detection of performance-enhancing substances in sports. The ability to quantify biomarkers at ultra-low concentrations has become critical for early disease detection protocols and precision medicine approaches.
Industry surveys indicate that end-users consistently prioritize three key performance attributes in HPLC systems for trace analysis: sensitivity improvements (cited by 87% of respondents), reduction in matrix interference (76%), and enhanced reproducibility at low concentrations (72%). These market requirements directly correlate with the technical challenges in achieving higher resolution in HPLC systems.
The geographical distribution of market demand shows North America leading with 39% market share, followed by Europe (31%) and Asia-Pacific (24%). However, the fastest growth is occurring in emerging economies, particularly China and India, where expanding regulatory frameworks and growing industrial bases are creating new market opportunities.
Pharmaceutical and biopharmaceutical sectors represent the largest market segment, accounting for approximately 38% of the total demand. This dominance stems from stringent regulatory requirements for impurity profiling, where detection of genotoxic impurities at parts-per-billion levels has become mandatory under ICH M7 guidelines. The need to identify and quantify increasingly complex degradation products in novel biologics and small molecule formulations further drives market growth in this sector.
Environmental monitoring constitutes the second-largest application area, comprising 27% of the market. Government agencies worldwide have progressively lowered acceptable limits for persistent organic pollutants, pesticide residues, and emerging contaminants such as PFAS (per- and polyfluoroalkyl substances) in water sources. This regulatory tightening has created substantial demand for analytical methods capable of detecting concentrations below 0.1 parts per trillion in complex environmental matrices.
Food safety testing represents another rapidly growing segment, expanding at 8.2% annually. Consumer awareness regarding food contaminants, coupled with international trade requirements for pesticide residue analysis and mycotoxin detection, has intensified the need for high-resolution analytical techniques. The European Union's regulations on maximum residue limits, which cover over 500 pesticides in various food products, exemplify the regulatory pressure driving market demand.
Clinical diagnostics and forensic toxicology applications have emerged as high-growth niches, particularly for therapeutic drug monitoring and detection of performance-enhancing substances in sports. The ability to quantify biomarkers at ultra-low concentrations has become critical for early disease detection protocols and precision medicine approaches.
Industry surveys indicate that end-users consistently prioritize three key performance attributes in HPLC systems for trace analysis: sensitivity improvements (cited by 87% of respondents), reduction in matrix interference (76%), and enhanced reproducibility at low concentrations (72%). These market requirements directly correlate with the technical challenges in achieving higher resolution in HPLC systems.
The geographical distribution of market demand shows North America leading with 39% market share, followed by Europe (31%) and Asia-Pacific (24%). However, the fastest growth is occurring in emerging economies, particularly China and India, where expanding regulatory frameworks and growing industrial bases are creating new market opportunities.
Current HPLC Resolution Limitations
Despite significant advancements in HPLC technology, current systems face several critical limitations that hinder achieving optimal resolution for trace analysis applications. The theoretical plate count, a fundamental measure of column efficiency, remains constrained by physical and chemical factors in conventional systems. Most commercial HPLC columns provide between 5,000-20,000 theoretical plates, which proves insufficient for complex sample matrices where trace analytes must be separated from structurally similar compounds or matrix interferences.
Peak capacity, defined as the maximum number of peaks that can be resolved within a given separation window, represents another significant limitation. Current HPLC systems typically offer peak capacities of 100-300 in one-dimensional separations, which becomes inadequate when analyzing samples containing hundreds or thousands of components at trace levels. This limitation becomes particularly problematic in environmental monitoring, food safety testing, and metabolomics applications.
Diffusion effects continue to challenge resolution capabilities, especially at low flow rates often required for enhanced sensitivity. The van Deemter equation demonstrates that as particle size decreases, the optimal flow rate decreases, creating a practical trade-off between resolution and analysis time. While sub-2μm particles have improved performance, they introduce system backpressure challenges that conventional HPLC systems cannot always accommodate.
Detection sensitivity presents another critical limitation, as improving resolution often requires sample dilution or smaller injection volumes, which can push trace analytes below detection limits. The signal-to-noise ratio deteriorates as peak width narrows, creating a fundamental conflict between resolution and detection capability for ultra-trace analysis.
Instrumental constraints further complicate high-resolution achievements. Extra-column band broadening from connectors, tubing, and detector cells can significantly degrade the separation efficiency achieved by the column. Current systems typically contribute 10-30% of total band broadening, with this percentage increasing dramatically when using highly efficient columns with small particles.
Matrix effects remain particularly challenging in trace analysis, where co-eluting matrix components can cause ion suppression or enhancement in mass spectrometric detection. Current HPLC resolution capabilities often prove insufficient to completely separate target analytes from all potential interfering compounds in complex matrices like biological fluids, environmental samples, or food extracts.
Peak capacity, defined as the maximum number of peaks that can be resolved within a given separation window, represents another significant limitation. Current HPLC systems typically offer peak capacities of 100-300 in one-dimensional separations, which becomes inadequate when analyzing samples containing hundreds or thousands of components at trace levels. This limitation becomes particularly problematic in environmental monitoring, food safety testing, and metabolomics applications.
Diffusion effects continue to challenge resolution capabilities, especially at low flow rates often required for enhanced sensitivity. The van Deemter equation demonstrates that as particle size decreases, the optimal flow rate decreases, creating a practical trade-off between resolution and analysis time. While sub-2μm particles have improved performance, they introduce system backpressure challenges that conventional HPLC systems cannot always accommodate.
Detection sensitivity presents another critical limitation, as improving resolution often requires sample dilution or smaller injection volumes, which can push trace analytes below detection limits. The signal-to-noise ratio deteriorates as peak width narrows, creating a fundamental conflict between resolution and detection capability for ultra-trace analysis.
Instrumental constraints further complicate high-resolution achievements. Extra-column band broadening from connectors, tubing, and detector cells can significantly degrade the separation efficiency achieved by the column. Current systems typically contribute 10-30% of total band broadening, with this percentage increasing dramatically when using highly efficient columns with small particles.
Matrix effects remain particularly challenging in trace analysis, where co-eluting matrix components can cause ion suppression or enhancement in mass spectrometric detection. Current HPLC resolution capabilities often prove insufficient to completely separate target analytes from all potential interfering compounds in complex matrices like biological fluids, environmental samples, or food extracts.
Current High-Resolution HPLC Methodologies
01 Mobile phase optimization for HPLC resolution
The composition and properties of the mobile phase significantly impact HPLC resolution. Adjustments to pH, buffer concentration, organic solvent ratio, and additives can enhance the separation of analytes. Gradient elution techniques can be employed to improve resolution for complex mixtures, while temperature control of the mobile phase can also contribute to better chromatographic performance.- Mobile phase optimization for HPLC resolution: The composition and properties of the mobile phase significantly affect HPLC resolution. Optimizing parameters such as pH, buffer concentration, organic solvent ratio, and additives can enhance the separation of analytes. Gradient elution techniques can be employed to improve resolution for complex samples with compounds of varying polarities. The selection of appropriate mobile phase components is crucial for achieving optimal chromatographic resolution.
- Stationary phase selection and modification: The choice of stationary phase plays a critical role in HPLC resolution. Different column chemistries (C18, C8, phenyl, amino, etc.) offer varying selectivity for different analytes. Surface modifications of stationary phases, such as end-capping or incorporating specific functional groups, can enhance separation efficiency. Column parameters including particle size, pore size, and column dimensions directly impact resolution and should be selected based on the specific analytical requirements.
- Temperature control and optimization: Temperature control during HPLC analysis significantly affects resolution by influencing analyte-stationary phase interactions, mobile phase viscosity, and diffusion rates. Optimizing column temperature can improve peak shape, reduce analysis time, and enhance separation efficiency. Temperature gradients can be employed for complex samples to achieve better resolution. Precise temperature control systems are essential for method reproducibility and robustness.
- Advanced detection techniques for improved resolution: Integration of advanced detection systems with HPLC can significantly enhance resolution capabilities. Techniques such as diode array detection (DAD), mass spectrometry (MS), fluorescence detection, and multi-wavelength detection allow for better differentiation between co-eluting compounds. These detection methods provide additional dimensions of separation beyond retention time, enabling the resolution of complex mixtures that would otherwise be difficult to separate chromatographically.
- Method development and optimization strategies: Systematic approaches to HPLC method development can optimize resolution for specific analytical challenges. This includes screening different column chemistries, exploring various mobile phase compositions, and applying statistical design of experiments (DoE). Quality by Design (QbD) principles can be implemented to identify critical method parameters affecting resolution. Advanced software tools assist in predicting chromatographic behavior and optimizing separation conditions to achieve maximum resolution with minimal experimental effort.
02 Stationary phase selection and modification
The choice of stationary phase is crucial for achieving optimal HPLC resolution. Different column materials (silica-based, polymer-based, hybrid) and surface modifications (C18, C8, phenyl, amino) offer varying selectivity for different analytes. Particle size, pore size, and column dimensions all affect separation efficiency. Novel stationary phases with specialized functional groups can provide enhanced resolution for specific compound classes.Expand Specific Solutions03 Advanced detection techniques for improved resolution
Integration of sophisticated detection methods enhances the resolution capabilities of HPLC systems. Multi-wavelength UV detection, diode array detection (DAD), mass spectrometry (MS), and fluorescence detection can provide additional selectivity dimensions. Signal processing algorithms and data analysis techniques help in resolving co-eluting peaks and improving the overall resolution of complex samples.Expand Specific Solutions04 Sample preparation methods to enhance resolution
Proper sample preparation techniques significantly impact HPLC resolution. Methods such as solid-phase extraction, liquid-liquid extraction, protein precipitation, and filtration can remove interfering compounds and concentrate analytes of interest. Pre-column derivatization can improve detection sensitivity and selectivity, while sample clean-up procedures reduce matrix effects that might compromise chromatographic resolution.Expand Specific Solutions05 Innovative HPLC system configurations and parameters
Novel HPLC system designs and operational parameters can dramatically improve resolution. Ultra-high-pressure liquid chromatography (UHPLC) systems using sub-2μm particles provide superior resolution in shorter analysis times. Multi-dimensional chromatography techniques, such as 2D-HPLC, offer enhanced separation power for complex samples. Optimization of flow rates, injection volumes, and column temperature contributes to maximizing resolution while maintaining practical analysis times.Expand Specific Solutions
Leading HPLC Instrument Manufacturers
High-resolution HPLC for trace analysis is currently in a mature growth phase, with the global market estimated at $4.5 billion and projected to expand at 5-7% annually. The competitive landscape features established analytical instrument manufacturers like Agilent Technologies and Waters Corporation alongside emerging specialized players. Leading companies such as Agilent Technologies, Thermo Fisher Scientific, and Shimadzu have developed advanced column technologies and detection systems that achieve sub-nanogram sensitivity. Academic institutions including Fudan University and Beihang University are contributing significant research in novel stationary phases, while companies like Hitachi High-Tech America focus on instrument miniaturization and automation. The industry is witnessing increased collaboration between academic institutions and commercial entities to address emerging challenges in pharmaceutical, environmental, and food safety applications.
Hitachi High-Tech America, Inc.
Technical Solution: Hitachi High-Tech has developed innovative HPLC solutions focused on achieving superior resolution for trace analysis through their ChromasterUltra Rs system. This platform utilizes advanced pump technology with active damping that reduces pulse to less than 0.05 MPa, significantly improving baseline stability for trace detection. Their proprietary microflow cell design minimizes extra-column band broadening while maximizing light transmission efficiency, achieving detection sensitivity improvements of approximately 40% compared to conventional cells[2]. Hitachi's systems incorporate temperature control precision of ±0.1°C across the entire column, eliminating thermal gradients that can compromise separation efficiency. Their latest innovation includes dual-temperature zone technology that allows independent temperature control of column inlet and outlet regions, compensating for frictional heating effects that typically degrade resolution in high-pressure separations. The company has also developed specialized gradient delay volume reduction technology that enables ultra-fast gradient formation with minimal delay, critical for maintaining narrow peak widths in trace analysis applications.
Strengths: Exceptional baseline stability enabling reliable quantification at trace levels; innovative temperature control systems that maintain separation efficiency even at high pressures; modular design allowing customization for specific analytical challenges. Weaknesses: Smaller market presence in North America compared to other major HPLC manufacturers; more limited range of application-specific column chemistries; service network not as extensive as larger competitors.
Bristol Myers Squibb Co.
Technical Solution: Bristol Myers Squibb has developed proprietary HPLC methodologies specifically optimized for pharmaceutical trace analysis, focusing on impurity profiling and metabolite identification. Their approach combines ultra-high efficiency columns (sub-2μm particles) with specialized mobile phase compositions that enhance selectivity for structurally similar compounds. BMS has pioneered the implementation of charged aerosol detection (CAD) coupled with UHPLC, achieving uniform response factors across diverse compound classes without chromophores, enabling quantification of impurities at levels below 0.05% relative to the active pharmaceutical ingredient[3]. Their method development platform incorporates automated column and mobile phase screening with machine learning algorithms that predict optimal separation conditions, reducing method development time by approximately 60%. BMS has also developed novel sample preparation techniques including selective micro-extraction protocols that concentrate trace analytes while removing matrix interferences, improving signal-to-noise ratios by factors of 5-10 compared to traditional sample preparation methods. For complex biological matrices, they employ immunoaffinity extraction coupled with HPLC to achieve selective enrichment of target analytes prior to chromatographic separation.
Strengths: Pharmaceutical-specific expertise with deep understanding of regulatory requirements for impurity analysis; advanced method development automation reducing time-to-result; integration of multiple detection technologies for comprehensive characterization. Weaknesses: Solutions primarily optimized for pharmaceutical applications rather than broader analytical challenges; technologies largely kept in-house rather than commercialized; focus on compliance sometimes prioritized over absolute performance metrics.
Breakthrough Column Technologies
Chromatography beads, production and use thereof
PatentWO2020002300A1
Innovation
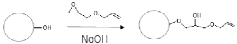
- Development of small, rigid, and non-permeable agarose beads with diameters of 1-25 μm, produced through a method involving emulsification, cross-linking, and surface grafting to create polymer tentacles for functionalization, which can withstand high pressures and exclude compounds as small as 100 g/mol, enabling efficient mass transfer and sharper peaks.
Improved measurement of vitamin d
PatentActiveEP2030026A2
Innovation
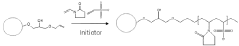
- A method using a vitamin D releasing reagent that allows for direct online chromatographic separation of vitamin D metabolites from vitamin D-binding protein without protein precipitation, eliminating the need for extraction and manual handling steps.
Method Validation Standards for Trace Analysis
Method validation is a critical component in ensuring the reliability and accuracy of HPLC methods for trace analysis. The validation process must adhere to established regulatory guidelines such as ICH Q2(R1), FDA, and USP standards, which provide comprehensive frameworks for analytical method validation. These standards emphasize key validation parameters including specificity, linearity, accuracy, precision, detection limit, quantitation limit, range, robustness, and system suitability.
For trace analysis applications, where analyte concentrations are extremely low, the validation of detection limits (LOD) and quantitation limits (LOQ) becomes particularly crucial. The ICH guidelines recommend three approaches for determining these limits: visual evaluation, signal-to-noise ratio, and the standard deviation of the response and slope. The signal-to-noise approach typically requires ratios of 3:1 for LOD and 10:1 for LOQ, ensuring reliable detection and quantification at trace levels.
Accuracy validation in trace analysis demands recovery studies across multiple concentration levels, typically covering 80-120% of the target concentration. For methods analyzing impurities at trace levels, accuracy should be demonstrated at concentrations ranging from the reporting threshold to 120% of the specification limit. The mean recovery should fall within predetermined acceptance criteria, often set at 98-102% for active ingredients and 80-120% for impurities.
Precision validation must address both repeatability (intra-assay precision) and intermediate precision (inter-assay variability). For trace analysis, the relative standard deviation (RSD) acceptance criteria are typically more lenient than for major component analysis, with values up to 15-20% considered acceptable at concentrations near the LOQ, compared to the standard 2% for major components.
Robustness testing becomes particularly important for trace analysis methods, as minor changes in chromatographic conditions can significantly impact the detection and quantification of trace components. Parameters such as mobile phase composition (±2%), pH of buffer (±0.2 units), column temperature (±5°C), and flow rate (±0.1 mL/min) should be systematically varied to assess their impact on method performance.
System suitability tests (SST) serve as an ongoing verification of method performance during routine analysis. For trace analysis methods, SST parameters should include resolution between critical pairs, signal-to-noise ratio for trace components, and injection precision. These parameters should be established during method validation and monitored during routine analysis to ensure consistent method performance.
For trace analysis applications, where analyte concentrations are extremely low, the validation of detection limits (LOD) and quantitation limits (LOQ) becomes particularly crucial. The ICH guidelines recommend three approaches for determining these limits: visual evaluation, signal-to-noise ratio, and the standard deviation of the response and slope. The signal-to-noise approach typically requires ratios of 3:1 for LOD and 10:1 for LOQ, ensuring reliable detection and quantification at trace levels.
Accuracy validation in trace analysis demands recovery studies across multiple concentration levels, typically covering 80-120% of the target concentration. For methods analyzing impurities at trace levels, accuracy should be demonstrated at concentrations ranging from the reporting threshold to 120% of the specification limit. The mean recovery should fall within predetermined acceptance criteria, often set at 98-102% for active ingredients and 80-120% for impurities.
Precision validation must address both repeatability (intra-assay precision) and intermediate precision (inter-assay variability). For trace analysis, the relative standard deviation (RSD) acceptance criteria are typically more lenient than for major component analysis, with values up to 15-20% considered acceptable at concentrations near the LOQ, compared to the standard 2% for major components.
Robustness testing becomes particularly important for trace analysis methods, as minor changes in chromatographic conditions can significantly impact the detection and quantification of trace components. Parameters such as mobile phase composition (±2%), pH of buffer (±0.2 units), column temperature (±5°C), and flow rate (±0.1 mL/min) should be systematically varied to assess their impact on method performance.
System suitability tests (SST) serve as an ongoing verification of method performance during routine analysis. For trace analysis methods, SST parameters should include resolution between critical pairs, signal-to-noise ratio for trace components, and injection precision. These parameters should be established during method validation and monitored during routine analysis to ensure consistent method performance.
Sample Preparation Innovations
Sample preparation represents a critical pre-analytical phase in achieving high resolution in HPLC for trace analysis. Recent innovations in this area have significantly enhanced the sensitivity, selectivity, and reproducibility of analytical results. The evolution of solid-phase extraction (SPE) techniques has been particularly noteworthy, with the development of novel sorbent materials exhibiting higher specificity for target analytes even at ultra-trace concentrations.
Miniaturized extraction techniques have emerged as a prominent trend, with micro-extraction by packed sorbent (MEPS) and dispersive solid-phase extraction (d-SPE) gaining traction due to their reduced solvent consumption and enhanced extraction efficiency. These approaches align with green analytical chemistry principles while simultaneously improving detection limits for challenging matrices.
Automated sample preparation systems have revolutionized laboratory workflows, minimizing human intervention and associated variability. Integration of robotic liquid handling platforms with HPLC systems has enabled seamless sample processing, reducing carry-over effects and cross-contamination risks that previously limited resolution in trace analysis applications.
Selective derivatization strategies have advanced considerably, allowing for improved chromatographic behavior of polar compounds that traditionally exhibited poor peak shapes. These chemical modifications enhance detector response and chromatographic separation, particularly beneficial when dealing with complex biological or environmental samples containing trace analytes.
Matrix removal techniques have been refined to address the persistent challenge of matrix effects in trace analysis. Molecularly imprinted polymers (MIPs) and immunoaffinity-based cleanup methods provide highly selective extraction of target compounds while eliminating interfering matrix components that compromise chromatographic resolution.
Nanomaterial-based extraction media represent another frontier in sample preparation innovation. Carbon nanotubes, graphene-based materials, and metal-organic frameworks offer unprecedented surface area-to-volume ratios and unique selectivity patterns, enabling efficient extraction of trace analytes from complex matrices with minimal non-specific binding.
Multidimensional sample preparation approaches combining orthogonal extraction mechanisms have demonstrated superior performance for challenging analytical scenarios. Sequential application of different extraction principles (e.g., combining size exclusion with reversed-phase interactions) provides comprehensive sample cleanup, resulting in chromatograms with significantly improved resolution and baseline stability essential for reliable trace analysis.
Miniaturized extraction techniques have emerged as a prominent trend, with micro-extraction by packed sorbent (MEPS) and dispersive solid-phase extraction (d-SPE) gaining traction due to their reduced solvent consumption and enhanced extraction efficiency. These approaches align with green analytical chemistry principles while simultaneously improving detection limits for challenging matrices.
Automated sample preparation systems have revolutionized laboratory workflows, minimizing human intervention and associated variability. Integration of robotic liquid handling platforms with HPLC systems has enabled seamless sample processing, reducing carry-over effects and cross-contamination risks that previously limited resolution in trace analysis applications.
Selective derivatization strategies have advanced considerably, allowing for improved chromatographic behavior of polar compounds that traditionally exhibited poor peak shapes. These chemical modifications enhance detector response and chromatographic separation, particularly beneficial when dealing with complex biological or environmental samples containing trace analytes.
Matrix removal techniques have been refined to address the persistent challenge of matrix effects in trace analysis. Molecularly imprinted polymers (MIPs) and immunoaffinity-based cleanup methods provide highly selective extraction of target compounds while eliminating interfering matrix components that compromise chromatographic resolution.
Nanomaterial-based extraction media represent another frontier in sample preparation innovation. Carbon nanotubes, graphene-based materials, and metal-organic frameworks offer unprecedented surface area-to-volume ratios and unique selectivity patterns, enabling efficient extraction of trace analytes from complex matrices with minimal non-specific binding.
Multidimensional sample preparation approaches combining orthogonal extraction mechanisms have demonstrated superior performance for challenging analytical scenarios. Sequential application of different extraction principles (e.g., combining size exclusion with reversed-phase interactions) provides comprehensive sample cleanup, resulting in chromatograms with significantly improved resolution and baseline stability essential for reliable trace analysis.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!