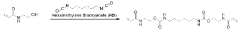
Achieving New Standards in Polyurethane Biocompatibility
JUN 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PU Biocompatibility Evolution and Objectives
Polyurethane (PU) has been a cornerstone material in biomedical applications for decades, owing to its versatility and adaptable properties. The evolution of PU biocompatibility has been marked by significant milestones and continuous improvements, driven by the increasing demands of medical devices and implants.
In the early stages of PU development, the focus was primarily on mechanical properties and durability. However, as the medical field advanced, the need for materials that could seamlessly integrate with biological systems became paramount. This shift in focus led to the emergence of biocompatibility as a critical factor in PU research and development.
The 1970s and 1980s saw the first generation of biocompatible PUs, which were designed to minimize adverse reactions in the body. These early efforts laid the groundwork for future advancements but were limited by the understanding of biomaterial-tissue interactions at the time.
As research progressed, the objectives for PU biocompatibility expanded beyond mere tolerance by the body. The aim shifted towards creating materials that could actively promote positive biological responses, such as enhanced cell adhesion, proliferation, and tissue integration. This paradigm shift marked the beginning of the bioactive PU era in the 1990s and early 2000s.
Recent years have witnessed a surge in nanotechnology and surface modification techniques, opening new avenues for improving PU biocompatibility. The integration of nanoparticles, surface functionalization, and biomimetic approaches have pushed the boundaries of what is possible in terms of material-tissue interactions.
Looking ahead, the objectives for achieving new standards in PU biocompatibility are multifaceted and ambitious. One primary goal is to develop PUs that can dynamically respond to the biological environment, adapting their properties to optimize healing and integration. This includes smart materials that can release bioactive agents on-demand or change their surface characteristics based on physiological cues.
Another critical objective is to enhance the long-term stability and performance of PU-based implants and devices. This involves addressing issues such as oxidative degradation, calcification, and immune response modulation to ensure sustained biocompatibility over extended periods.
Furthermore, there is a growing emphasis on personalized medicine, driving the need for customizable PU formulations that can be tailored to individual patient needs. This objective aligns with the broader trend towards precision healthcare and may involve the development of 3D-printable biocompatible PUs for patient-specific implants and devices.
In the early stages of PU development, the focus was primarily on mechanical properties and durability. However, as the medical field advanced, the need for materials that could seamlessly integrate with biological systems became paramount. This shift in focus led to the emergence of biocompatibility as a critical factor in PU research and development.
The 1970s and 1980s saw the first generation of biocompatible PUs, which were designed to minimize adverse reactions in the body. These early efforts laid the groundwork for future advancements but were limited by the understanding of biomaterial-tissue interactions at the time.
As research progressed, the objectives for PU biocompatibility expanded beyond mere tolerance by the body. The aim shifted towards creating materials that could actively promote positive biological responses, such as enhanced cell adhesion, proliferation, and tissue integration. This paradigm shift marked the beginning of the bioactive PU era in the 1990s and early 2000s.
Recent years have witnessed a surge in nanotechnology and surface modification techniques, opening new avenues for improving PU biocompatibility. The integration of nanoparticles, surface functionalization, and biomimetic approaches have pushed the boundaries of what is possible in terms of material-tissue interactions.
Looking ahead, the objectives for achieving new standards in PU biocompatibility are multifaceted and ambitious. One primary goal is to develop PUs that can dynamically respond to the biological environment, adapting their properties to optimize healing and integration. This includes smart materials that can release bioactive agents on-demand or change their surface characteristics based on physiological cues.
Another critical objective is to enhance the long-term stability and performance of PU-based implants and devices. This involves addressing issues such as oxidative degradation, calcification, and immune response modulation to ensure sustained biocompatibility over extended periods.
Furthermore, there is a growing emphasis on personalized medicine, driving the need for customizable PU formulations that can be tailored to individual patient needs. This objective aligns with the broader trend towards precision healthcare and may involve the development of 3D-printable biocompatible PUs for patient-specific implants and devices.
Market Demand Analysis for Biocompatible PU
The market demand for biocompatible polyurethane (PU) has been experiencing significant growth in recent years, driven by the increasing need for advanced medical devices and implants. The global biocompatible materials market, which includes biocompatible PU, is projected to reach substantial value in the coming years, with a compound annual growth rate outpacing many other sectors in the healthcare industry.
The healthcare sector, particularly in developed countries, is the primary driver of demand for biocompatible PU. As populations age and chronic diseases become more prevalent, there is a growing need for long-term implantable devices and tissue engineering scaffolds. Biocompatible PU is particularly sought after in cardiovascular applications, such as artificial heart valves, vascular grafts, and pacemaker leads, due to its excellent mechanical properties and durability.
Another significant market segment for biocompatible PU is orthopedic implants. With the rise in sports-related injuries and the increasing prevalence of osteoarthritis, the demand for joint replacements and other orthopedic devices is surging. Biocompatible PU's ability to mimic the properties of natural tissue makes it an attractive material for these applications.
The wound care market is also contributing to the demand for biocompatible PU. Advanced wound dressings that incorporate biocompatible PU are gaining popularity due to their ability to promote healing while reducing the risk of infection. This trend is particularly strong in regions with a high incidence of chronic wounds, such as diabetic ulcers.
Emerging applications in drug delivery systems and 3D-printed medical devices are expected to further boost the market for biocompatible PU. The material's versatility allows for controlled release of pharmaceuticals and the creation of patient-specific implants, aligning with the growing trend towards personalized medicine.
Geographically, North America and Europe currently dominate the market for biocompatible PU, owing to their advanced healthcare infrastructure and higher healthcare spending. However, the Asia-Pacific region is anticipated to show the fastest growth in the coming years, driven by improving healthcare access, increasing disposable incomes, and a rapidly aging population in countries like China and Japan.
Despite the positive outlook, challenges remain in the biocompatible PU market. Stringent regulatory requirements and long approval processes for medical devices can slow market growth. Additionally, concerns about the long-term performance and potential degradation of PU in the body continue to drive research and development efforts to improve the material's biocompatibility and stability.
The healthcare sector, particularly in developed countries, is the primary driver of demand for biocompatible PU. As populations age and chronic diseases become more prevalent, there is a growing need for long-term implantable devices and tissue engineering scaffolds. Biocompatible PU is particularly sought after in cardiovascular applications, such as artificial heart valves, vascular grafts, and pacemaker leads, due to its excellent mechanical properties and durability.
Another significant market segment for biocompatible PU is orthopedic implants. With the rise in sports-related injuries and the increasing prevalence of osteoarthritis, the demand for joint replacements and other orthopedic devices is surging. Biocompatible PU's ability to mimic the properties of natural tissue makes it an attractive material for these applications.
The wound care market is also contributing to the demand for biocompatible PU. Advanced wound dressings that incorporate biocompatible PU are gaining popularity due to their ability to promote healing while reducing the risk of infection. This trend is particularly strong in regions with a high incidence of chronic wounds, such as diabetic ulcers.
Emerging applications in drug delivery systems and 3D-printed medical devices are expected to further boost the market for biocompatible PU. The material's versatility allows for controlled release of pharmaceuticals and the creation of patient-specific implants, aligning with the growing trend towards personalized medicine.
Geographically, North America and Europe currently dominate the market for biocompatible PU, owing to their advanced healthcare infrastructure and higher healthcare spending. However, the Asia-Pacific region is anticipated to show the fastest growth in the coming years, driven by improving healthcare access, increasing disposable incomes, and a rapidly aging population in countries like China and Japan.
Despite the positive outlook, challenges remain in the biocompatible PU market. Stringent regulatory requirements and long approval processes for medical devices can slow market growth. Additionally, concerns about the long-term performance and potential degradation of PU in the body continue to drive research and development efforts to improve the material's biocompatibility and stability.
Current Challenges in PU Biocompatibility
Despite significant advancements in polyurethane (PU) biocompatibility, several challenges persist in achieving new standards for medical applications. One of the primary concerns is the potential for adverse biological reactions when PU materials come into contact with living tissues or blood. These reactions can include inflammation, thrombosis, and foreign body responses, which may compromise the safety and efficacy of medical devices.
The degradation of PU materials in biological environments remains a significant challenge. While some degree of biodegradation may be desirable for certain applications, uncontrolled degradation can lead to the release of potentially harmful byproducts and compromise the structural integrity of medical devices. Balancing the stability of PU materials with their biocompatibility is an ongoing area of research and development.
Another critical challenge lies in the surface properties of PU materials. The hydrophobicity of many PU formulations can lead to protein adsorption and subsequent cellular adhesion, potentially triggering unwanted biological responses. Modifying surface characteristics to enhance biocompatibility without compromising the bulk properties of the material is a complex task that requires innovative approaches.
The variability in PU composition and manufacturing processes also presents challenges in achieving consistent biocompatibility across different batches and applications. Slight variations in chemical composition, processing conditions, or sterilization methods can significantly impact the material's interaction with biological systems, making it difficult to establish standardized protocols for biocompatibility testing and evaluation.
Furthermore, the long-term effects of PU implants and devices on the human body are not fully understood. While short-term biocompatibility can be assessed through in vitro and animal studies, predicting the long-term performance and potential complications of PU materials in diverse physiological environments remains challenging. This uncertainty poses risks for patients and creates regulatory hurdles for the approval of new PU-based medical devices.
The development of PU materials that can actively promote tissue integration and healing, rather than merely being inert, represents another frontier in biocompatibility research. Creating bioactive PU surfaces that can guide cell behavior, promote tissue regeneration, and resist bacterial colonization is a complex multidisciplinary challenge that requires advances in material science, biology, and nanotechnology.
Lastly, the increasing demand for personalized medicine and patient-specific devices adds another layer of complexity to PU biocompatibility. Developing PU materials that can be tailored to individual patient needs while maintaining consistent biocompatibility profiles across different formulations and manufacturing processes is a significant challenge that requires innovative approaches to material design and production.
The degradation of PU materials in biological environments remains a significant challenge. While some degree of biodegradation may be desirable for certain applications, uncontrolled degradation can lead to the release of potentially harmful byproducts and compromise the structural integrity of medical devices. Balancing the stability of PU materials with their biocompatibility is an ongoing area of research and development.
Another critical challenge lies in the surface properties of PU materials. The hydrophobicity of many PU formulations can lead to protein adsorption and subsequent cellular adhesion, potentially triggering unwanted biological responses. Modifying surface characteristics to enhance biocompatibility without compromising the bulk properties of the material is a complex task that requires innovative approaches.
The variability in PU composition and manufacturing processes also presents challenges in achieving consistent biocompatibility across different batches and applications. Slight variations in chemical composition, processing conditions, or sterilization methods can significantly impact the material's interaction with biological systems, making it difficult to establish standardized protocols for biocompatibility testing and evaluation.
Furthermore, the long-term effects of PU implants and devices on the human body are not fully understood. While short-term biocompatibility can be assessed through in vitro and animal studies, predicting the long-term performance and potential complications of PU materials in diverse physiological environments remains challenging. This uncertainty poses risks for patients and creates regulatory hurdles for the approval of new PU-based medical devices.
The development of PU materials that can actively promote tissue integration and healing, rather than merely being inert, represents another frontier in biocompatibility research. Creating bioactive PU surfaces that can guide cell behavior, promote tissue regeneration, and resist bacterial colonization is a complex multidisciplinary challenge that requires advances in material science, biology, and nanotechnology.
Lastly, the increasing demand for personalized medicine and patient-specific devices adds another layer of complexity to PU biocompatibility. Developing PU materials that can be tailored to individual patient needs while maintaining consistent biocompatibility profiles across different formulations and manufacturing processes is a significant challenge that requires innovative approaches to material design and production.
Existing PU Biocompatibility Solutions
01 Surface modification of polyurethane for improved biocompatibility
Various surface modification techniques are employed to enhance the biocompatibility of polyurethane materials. These methods include plasma treatment, grafting of bioactive molecules, and coating with biocompatible substances. Such modifications can improve cell adhesion, reduce protein adsorption, and minimize inflammatory responses, making polyurethane more suitable for medical applications.- Surface modification of polyurethane for improved biocompatibility: Various surface modification techniques are employed to enhance the biocompatibility of polyurethane materials. These methods include plasma treatment, chemical grafting, and coating with biocompatible substances. Such modifications can improve cell adhesion, reduce protein adsorption, and minimize inflammatory responses, making the polyurethane more suitable for medical applications.
- Incorporation of bioactive compounds into polyurethane: Bioactive compounds such as growth factors, antimicrobial agents, and antioxidants are incorporated into polyurethane matrices to enhance their biological performance. This approach can promote tissue regeneration, prevent infections, and reduce oxidative stress, leading to improved biocompatibility and functionality of polyurethane-based medical devices.
- Development of biodegradable polyurethanes: Biodegradable polyurethanes are designed to break down into non-toxic components within the body over time. These materials are particularly useful for temporary implants or tissue engineering scaffolds. The degradation rate and mechanical properties can be tailored by adjusting the chemical composition and structure of the polyurethane.
- Polyurethane-based drug delivery systems: Polyurethanes are utilized as carriers for controlled drug release systems. The polymer's structure can be modified to achieve desired drug release kinetics, improving the efficacy of therapeutic agents while minimizing side effects. This application enhances the biocompatibility of polyurethane by combining it with pharmaceutical interventions.
- Nanocomposite polyurethanes for biomedical applications: Nanocomposite polyurethanes incorporate nanomaterials such as nanoparticles, nanofibers, or nanotubes to enhance their mechanical, thermal, and biological properties. These advanced materials can exhibit improved strength, flexibility, and biocompatibility, making them suitable for a wide range of biomedical applications, including implants and tissue engineering scaffolds.
02 Incorporation of bioactive compounds in polyurethane
Bioactive compounds such as growth factors, antimicrobial agents, and antioxidants are incorporated into polyurethane matrices to enhance their biocompatibility and functionality. This approach can create polyurethane materials with improved wound healing properties, reduced infection risks, and enhanced tissue integration for various biomedical applications.Expand Specific Solutions03 Development of biodegradable polyurethanes
Research focuses on creating biodegradable polyurethanes that can be safely absorbed by the body over time. These materials are designed to maintain their mechanical properties during the healing process and then gradually degrade into non-toxic components. This approach is particularly useful for tissue engineering scaffolds and temporary implants.Expand Specific Solutions04 Polyurethane-based drug delivery systems
Polyurethanes are utilized as drug delivery systems due to their versatile chemistry and biocompatibility. These systems can be designed to release drugs in a controlled manner, improving therapeutic efficacy and reducing side effects. The polymer structure can be tailored to achieve desired release profiles for various medical applications.Expand Specific Solutions05 Evaluation and testing of polyurethane biocompatibility
Various in vitro and in vivo methods are developed and standardized to assess the biocompatibility of polyurethane materials. These tests evaluate cytotoxicity, hemocompatibility, inflammatory responses, and long-term tissue interactions. Advanced techniques such as 3D cell culture models and high-throughput screening are employed to improve the accuracy and efficiency of biocompatibility assessments.Expand Specific Solutions
Key Players in Biocompatible PU Industry
The polyurethane biocompatibility market is in a growth phase, driven by increasing demand for advanced medical devices and implants. The global market size is expanding rapidly, with projections indicating significant growth in the coming years. Technologically, the field is advancing, with companies like PolyNovo Biomaterials, DePuy Synthes, and Boston Scientific leading innovation. Academic institutions such as Carnegie Mellon University and Vanderbilt University are contributing to fundamental research. The technology maturity varies, with some established players like Bayer AG offering commercial products, while others like Tensive Srl and RUA Life Sciences are developing novel approaches. Collaboration between industry and academia is accelerating progress, as seen with partnerships involving institutions like Zhejiang University and companies such as Wanhua Chemical Group.
PolyNovo Biomaterials Pty Ltd.
Technical Solution: PolyNovo's NovoSorb BTM (Biodegradable Temporising Matrix) is a revolutionary polyurethane-based technology for biocompatible tissue scaffolds. This synthetic polymer platform is designed to provide a temporary scaffold for tissue regeneration, gradually biodegrading as new tissue forms. The NovoSorb technology features a unique porous structure that promotes cell infiltration and vascularization, crucial for effective tissue integration. Its controlled degradation rate allows for optimal tissue regeneration timing, while its mechanical properties can be tailored to match specific tissue requirements.
Strengths: Highly customizable, excellent biocompatibility, controlled biodegradation. Weaknesses: Potential for variability in degradation rates, higher cost compared to traditional materials.
Poly-Med, Inc.
Technical Solution: Poly-Med specializes in the development of absorbable polymers and fibers for medical applications. Their approach to enhancing polyurethane biocompatibility involves the incorporation of bioactive molecules and surface modifications. They have developed a range of polyurethane-based materials with improved cell adhesion and proliferation properties. Their technology includes the use of bioresorbable polyurethanes that can be engineered to have specific degradation profiles, matching the rate of tissue regeneration. Additionally, they have explored the incorporation of anti-inflammatory agents into the polymer matrix to reduce foreign body responses.
Strengths: Tailored degradation profiles, reduced inflammatory response. Weaknesses: Complexity in manufacturing process, potential for uneven drug release in drug-eluting variants.
Core Innovations in PU Biocompatibility
Biocompatible, Biodegradable Polyurethane Materials With Controlled Hydrophobic to Hydrophilic Ratio
PatentInactiveUS20150231305A1
Innovation
- Development of biocompatible, biodegradable polyurethane and polyurethaneacrylate materials with controlled elasticity, hydrophilicity, and degradation rates, using aliphatic diisocyanates, mixtures of polyols with varying hydrophilicity, and biologically active chain extenders to enhance interaction with tissues and promote tissue healing, along with the ability to form injectable and porous scaffolds.
Medical devices formed from high purity polyurethane
PatentWO2024112802A1
Innovation
- Development of high purity poly(urethane amide) compositions with urethane, amide, and thioether linkages, formed through reactions between hydroxyalkyl acrylamides and diisocyanates, polythiols, or polyamines, using Michael addition reactions to avoid urea formation and ensure biocompatibility and scalability.
Regulatory Framework for Biomedical PU
The regulatory framework for biomedical polyurethanes (PU) is a complex and evolving landscape that plays a crucial role in ensuring the safety and efficacy of medical devices and implants. At the forefront of this framework are regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), which set stringent guidelines for the development, testing, and approval of biomedical PU materials.
These regulatory agencies have established comprehensive protocols for evaluating the biocompatibility of PU materials, including in vitro and in vivo testing methods. The ISO 10993 series of standards, particularly ISO 10993-1, serves as a cornerstone for assessing the biological evaluation of medical devices. This standard outlines a systematic approach to biocompatibility testing, encompassing cytotoxicity, sensitization, irritation, and long-term implantation studies.
For biomedical PU applications, manufacturers must adhere to Good Manufacturing Practices (GMP) and Quality Management Systems (QMS) to ensure consistent production of high-quality materials. The FDA's 21 CFR Part 820 and the EU's Medical Device Regulation (MDR) 2017/745 provide detailed requirements for quality control and risk management throughout the product lifecycle.
Specific to polyurethanes, regulatory bodies have focused on the potential leaching of harmful substances, such as unreacted isocyanates or catalysts. As a result, manufacturers are required to conduct thorough chemical characterization and leachable/extractable studies to demonstrate the safety of their PU formulations. The FDA's guidance on the use of ISO 10993-18 for chemical characterization of materials is particularly relevant in this context.
Environmental considerations have also become increasingly important in the regulatory framework. The EU's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation impacts the use of certain chemicals in PU production, driving the industry towards more sustainable and environmentally friendly formulations.
As the field of biomedical PU advances, regulatory agencies are adapting their frameworks to address emerging technologies. For instance, the development of biodegradable PU materials has led to new considerations in long-term safety assessments and the need for guidelines on degradation products and their potential biological effects.
The global nature of the medical device industry necessitates harmonization efforts between regulatory bodies. Initiatives such as the International Medical Device Regulators Forum (IMDRF) aim to align regulatory requirements across different regions, potentially streamlining the approval process for biomedical PU products in multiple markets.
These regulatory agencies have established comprehensive protocols for evaluating the biocompatibility of PU materials, including in vitro and in vivo testing methods. The ISO 10993 series of standards, particularly ISO 10993-1, serves as a cornerstone for assessing the biological evaluation of medical devices. This standard outlines a systematic approach to biocompatibility testing, encompassing cytotoxicity, sensitization, irritation, and long-term implantation studies.
For biomedical PU applications, manufacturers must adhere to Good Manufacturing Practices (GMP) and Quality Management Systems (QMS) to ensure consistent production of high-quality materials. The FDA's 21 CFR Part 820 and the EU's Medical Device Regulation (MDR) 2017/745 provide detailed requirements for quality control and risk management throughout the product lifecycle.
Specific to polyurethanes, regulatory bodies have focused on the potential leaching of harmful substances, such as unreacted isocyanates or catalysts. As a result, manufacturers are required to conduct thorough chemical characterization and leachable/extractable studies to demonstrate the safety of their PU formulations. The FDA's guidance on the use of ISO 10993-18 for chemical characterization of materials is particularly relevant in this context.
Environmental considerations have also become increasingly important in the regulatory framework. The EU's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation impacts the use of certain chemicals in PU production, driving the industry towards more sustainable and environmentally friendly formulations.
As the field of biomedical PU advances, regulatory agencies are adapting their frameworks to address emerging technologies. For instance, the development of biodegradable PU materials has led to new considerations in long-term safety assessments and the need for guidelines on degradation products and their potential biological effects.
The global nature of the medical device industry necessitates harmonization efforts between regulatory bodies. Initiatives such as the International Medical Device Regulators Forum (IMDRF) aim to align regulatory requirements across different regions, potentially streamlining the approval process for biomedical PU products in multiple markets.
Environmental Impact of Biocompatible PU
The environmental impact of biocompatible polyurethane (PU) is a critical consideration in the development of new standards for PU biocompatibility. As the demand for biocompatible materials in medical applications continues to grow, it is essential to assess the ecological footprint of these materials throughout their lifecycle.
Biocompatible PU offers significant advantages in terms of reduced toxicity and improved patient outcomes. However, the production process of PU still involves the use of petrochemical-based raw materials, which can have negative environmental consequences. The synthesis of polyols and isocyanates, key components in PU production, often requires energy-intensive processes and may generate harmful byproducts.
One of the primary environmental concerns associated with biocompatible PU is its end-of-life disposal. Traditional PU materials are not biodegradable and can persist in the environment for extended periods. This has led to increased research efforts focused on developing biodegradable PU formulations that maintain biocompatibility while reducing long-term environmental impact.
Recent advancements in green chemistry have shown promise in mitigating some of these environmental issues. The use of bio-based polyols derived from renewable resources, such as vegetable oils or lignin, has gained traction as a more sustainable alternative to petroleum-based polyols. These bio-based materials not only reduce reliance on fossil fuels but also often exhibit improved biodegradability.
Another area of focus is the development of non-isocyanate polyurethanes (NIPUs), which eliminate the need for toxic isocyanates in the production process. NIPUs can be synthesized using more environmentally friendly methods, such as the reaction between cyclic carbonates and amines. This approach not only reduces the environmental impact of production but also enhances worker safety.
The manufacturing processes for biocompatible PU are also being optimized to reduce energy consumption and waste generation. Implementation of closed-loop systems, solvent recovery, and more efficient catalysts are some of the strategies being employed to minimize the environmental footprint of production facilities.
As the field progresses, life cycle assessments (LCAs) are becoming increasingly important in evaluating the overall environmental impact of biocompatible PU. These assessments consider factors such as raw material extraction, production processes, use phase, and end-of-life disposal to provide a comprehensive view of the material's ecological impact. LCAs help identify areas for improvement and guide the development of more sustainable biocompatible PU formulations.
Biocompatible PU offers significant advantages in terms of reduced toxicity and improved patient outcomes. However, the production process of PU still involves the use of petrochemical-based raw materials, which can have negative environmental consequences. The synthesis of polyols and isocyanates, key components in PU production, often requires energy-intensive processes and may generate harmful byproducts.
One of the primary environmental concerns associated with biocompatible PU is its end-of-life disposal. Traditional PU materials are not biodegradable and can persist in the environment for extended periods. This has led to increased research efforts focused on developing biodegradable PU formulations that maintain biocompatibility while reducing long-term environmental impact.
Recent advancements in green chemistry have shown promise in mitigating some of these environmental issues. The use of bio-based polyols derived from renewable resources, such as vegetable oils or lignin, has gained traction as a more sustainable alternative to petroleum-based polyols. These bio-based materials not only reduce reliance on fossil fuels but also often exhibit improved biodegradability.
Another area of focus is the development of non-isocyanate polyurethanes (NIPUs), which eliminate the need for toxic isocyanates in the production process. NIPUs can be synthesized using more environmentally friendly methods, such as the reaction between cyclic carbonates and amines. This approach not only reduces the environmental impact of production but also enhances worker safety.
The manufacturing processes for biocompatible PU are also being optimized to reduce energy consumption and waste generation. Implementation of closed-loop systems, solvent recovery, and more efficient catalysts are some of the strategies being employed to minimize the environmental footprint of production facilities.
As the field progresses, life cycle assessments (LCAs) are becoming increasingly important in evaluating the overall environmental impact of biocompatible PU. These assessments consider factors such as raw material extraction, production processes, use phase, and end-of-life disposal to provide a comprehensive view of the material's ecological impact. LCAs help identify areas for improvement and guide the development of more sustainable biocompatible PU formulations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!