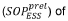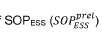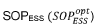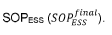Advanced modeling of transport phenomena in lithium-sulfur batteries
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Li-S Battery Transport Phenomena Background & Objectives
Lithium-sulfur (Li-S) batteries have emerged as a promising next-generation energy storage technology due to their theoretical energy density of 2600 Wh/kg, which significantly surpasses the capabilities of conventional lithium-ion batteries. The development trajectory of Li-S battery technology spans several decades, with initial conceptualization in the 1960s, followed by periods of limited research interest until a resurgence in the early 2000s driven by the increasing demand for high-energy density storage solutions.
The evolution of Li-S battery technology has been characterized by incremental improvements in understanding the complex electrochemical processes and transport phenomena that govern battery performance. Early research primarily focused on basic electrochemical reactions, while recent advancements have shifted toward comprehensive modeling of multi-phase, multi-scale transport processes that occur during charge-discharge cycles.
Transport phenomena in Li-S batteries present unique challenges compared to conventional battery systems due to the complex phase transitions of sulfur species. The conversion between solid sulfur (S8) and various polysulfide intermediates (Li2Sx, where 2≤x≤8) during discharge and charge processes creates a dynamic environment where multiple transport mechanisms operate simultaneously across different phases.
Current research trends indicate growing interest in developing advanced computational models that can accurately capture these complex transport phenomena. These models aim to integrate electrochemical kinetics, mass transport in multiple phases, and the spatial distribution of reaction products to predict battery performance under various operating conditions. The integration of multi-physics approaches with experimental validation has become increasingly important in advancing the fundamental understanding of Li-S battery systems.
The primary technical objectives for advanced modeling of transport phenomena in Li-S batteries include: developing comprehensive mathematical frameworks that accurately describe polysulfide shuttle effects; creating multi-scale models that bridge atomic-level interactions with macroscopic battery performance; establishing predictive capabilities for capacity fade mechanisms related to transport limitations; and designing computational tools that can guide the optimization of electrode architectures and electrolyte compositions.
Additionally, there is a growing emphasis on incorporating machine learning and artificial intelligence techniques to enhance model accuracy and computational efficiency. These approaches aim to process large datasets from experimental measurements and simulations to identify patterns and correlations that might not be apparent through traditional modeling approaches.
The ultimate goal of advanced transport phenomena modeling in Li-S batteries is to overcome the persistent challenges of capacity fading, low Coulombic efficiency, and limited cycle life that have hindered commercial viability. By developing a deeper understanding of the fundamental transport processes, researchers aim to design next-generation Li-S batteries with improved performance metrics that can meet the demands of applications ranging from electric vehicles to grid-scale energy storage.
The evolution of Li-S battery technology has been characterized by incremental improvements in understanding the complex electrochemical processes and transport phenomena that govern battery performance. Early research primarily focused on basic electrochemical reactions, while recent advancements have shifted toward comprehensive modeling of multi-phase, multi-scale transport processes that occur during charge-discharge cycles.
Transport phenomena in Li-S batteries present unique challenges compared to conventional battery systems due to the complex phase transitions of sulfur species. The conversion between solid sulfur (S8) and various polysulfide intermediates (Li2Sx, where 2≤x≤8) during discharge and charge processes creates a dynamic environment where multiple transport mechanisms operate simultaneously across different phases.
Current research trends indicate growing interest in developing advanced computational models that can accurately capture these complex transport phenomena. These models aim to integrate electrochemical kinetics, mass transport in multiple phases, and the spatial distribution of reaction products to predict battery performance under various operating conditions. The integration of multi-physics approaches with experimental validation has become increasingly important in advancing the fundamental understanding of Li-S battery systems.
The primary technical objectives for advanced modeling of transport phenomena in Li-S batteries include: developing comprehensive mathematical frameworks that accurately describe polysulfide shuttle effects; creating multi-scale models that bridge atomic-level interactions with macroscopic battery performance; establishing predictive capabilities for capacity fade mechanisms related to transport limitations; and designing computational tools that can guide the optimization of electrode architectures and electrolyte compositions.
Additionally, there is a growing emphasis on incorporating machine learning and artificial intelligence techniques to enhance model accuracy and computational efficiency. These approaches aim to process large datasets from experimental measurements and simulations to identify patterns and correlations that might not be apparent through traditional modeling approaches.
The ultimate goal of advanced transport phenomena modeling in Li-S batteries is to overcome the persistent challenges of capacity fading, low Coulombic efficiency, and limited cycle life that have hindered commercial viability. By developing a deeper understanding of the fundamental transport processes, researchers aim to design next-generation Li-S batteries with improved performance metrics that can meet the demands of applications ranging from electric vehicles to grid-scale energy storage.
Market Analysis for Li-S Battery Technology
The lithium-sulfur (Li-S) battery market is experiencing significant growth potential due to the technology's promising energy density advantages over conventional lithium-ion batteries. Current market projections indicate that the global Li-S battery market could reach $2.1 billion by 2030, with a compound annual growth rate of approximately 35% from 2023 to 2030. This growth is primarily driven by increasing demand for high-energy-density storage solutions in electric vehicles, aerospace applications, and portable electronics.
The market demand for Li-S batteries stems from their theoretical energy density of 2600 Wh/kg, which substantially exceeds the 250-300 Wh/kg limit of current commercial lithium-ion technologies. This significant performance advantage positions Li-S batteries as a potential game-changer for applications where weight and energy capacity are critical factors.
Electric vehicle manufacturers represent the largest potential market segment, with several major automotive companies investing in Li-S research partnerships. The aerospace sector follows closely, with drone manufacturers and satellite producers showing particular interest in the technology's lightweight properties. Consumer electronics manufacturers are also monitoring developments, though they remain more cautious due to current cycle life limitations.
Geographically, North America and Europe lead in Li-S battery research and development investments, while Asian markets, particularly China, Japan, and South Korea, are rapidly expanding their presence in this space through strategic government funding initiatives and corporate research programs.
Market barriers include the current high production costs, estimated at 2-3 times that of conventional lithium-ion batteries, primarily due to specialized materials and manufacturing processes. Additionally, the limited cycle life of current Li-S prototypes (typically 200-500 cycles compared to 1000+ for commercial lithium-ion) represents a significant market adoption challenge.
Customer requirements vary by sector, with automotive applications demanding 1000+ cycles, 10+ year calendar life, and cost targets below $100/kWh. Aerospace applications prioritize energy density and weight reduction, with somewhat more tolerance for higher costs and lower cycle life.
The competitive landscape includes specialized Li-S startups like OXIS Energy and Sion Power, alongside major battery manufacturers such as Samsung SDI and LG Energy Solution who are developing parallel research programs. Several academic-industrial partnerships are also emerging, particularly focused on solving the transport phenomena challenges that currently limit commercial viability.
The market demand for Li-S batteries stems from their theoretical energy density of 2600 Wh/kg, which substantially exceeds the 250-300 Wh/kg limit of current commercial lithium-ion technologies. This significant performance advantage positions Li-S batteries as a potential game-changer for applications where weight and energy capacity are critical factors.
Electric vehicle manufacturers represent the largest potential market segment, with several major automotive companies investing in Li-S research partnerships. The aerospace sector follows closely, with drone manufacturers and satellite producers showing particular interest in the technology's lightweight properties. Consumer electronics manufacturers are also monitoring developments, though they remain more cautious due to current cycle life limitations.
Geographically, North America and Europe lead in Li-S battery research and development investments, while Asian markets, particularly China, Japan, and South Korea, are rapidly expanding their presence in this space through strategic government funding initiatives and corporate research programs.
Market barriers include the current high production costs, estimated at 2-3 times that of conventional lithium-ion batteries, primarily due to specialized materials and manufacturing processes. Additionally, the limited cycle life of current Li-S prototypes (typically 200-500 cycles compared to 1000+ for commercial lithium-ion) represents a significant market adoption challenge.
Customer requirements vary by sector, with automotive applications demanding 1000+ cycles, 10+ year calendar life, and cost targets below $100/kWh. Aerospace applications prioritize energy density and weight reduction, with somewhat more tolerance for higher costs and lower cycle life.
The competitive landscape includes specialized Li-S startups like OXIS Energy and Sion Power, alongside major battery manufacturers such as Samsung SDI and LG Energy Solution who are developing parallel research programs. Several academic-industrial partnerships are also emerging, particularly focused on solving the transport phenomena challenges that currently limit commercial viability.
Current Challenges in Li-S Battery Transport Modeling
Despite significant advancements in lithium-ion battery technology, lithium-sulfur (Li-S) batteries face unique modeling challenges due to their complex electrochemical processes. The multi-phase, multi-scale transport phenomena in Li-S systems create substantial difficulties for accurate simulation and prediction of battery performance. Current models struggle to capture the complete dissolution-precipitation mechanisms of sulfur species and the resulting "shuttle effect" that significantly impacts battery efficiency and cycle life.
A primary challenge lies in modeling the polysulfide shuttle phenomenon, where soluble lithium polysulfides migrate between electrodes, causing active material loss and capacity fade. Existing models often oversimplify this process, failing to account for the concentration-dependent diffusion coefficients and the complex reaction kinetics involved in the transformation between various polysulfide species (Li₂Sₙ, 2≤n≤8).
The precipitation of solid discharge products (Li₂S₂ and Li₂S) presents another significant modeling hurdle. Current approaches inadequately represent nucleation and growth processes, particularly the spatial distribution of precipitates within the cathode microstructure. This limitation prevents accurate prediction of electrode passivation and pore clogging effects that critically influence battery performance.
Electrolyte transport modeling in Li-S batteries remains problematic due to the dramatic changes in electrolyte properties during cycling. As polysulfides dissolve and precipitate, electrolyte viscosity, ionic conductivity, and diffusion coefficients change substantially. Most models employ constant parameters that fail to capture these dynamic variations, leading to significant discrepancies between simulations and experimental results.
The multi-scale nature of transport phenomena in Li-S batteries further complicates modeling efforts. Processes range from molecular-level reactions to macroscopic mass transport across the cell, spanning multiple time and length scales. Current computational approaches struggle to integrate these scales efficiently while maintaining reasonable calculation times.
Additionally, the highly porous carbon structures typically used in Li-S cathodes create tortuous diffusion pathways that are difficult to characterize and model accurately. Most models rely on effective medium approximations that inadequately represent the actual microstructure and its impact on species transport and reaction distribution.
Temperature effects on transport phenomena represent another underexplored area in current Li-S battery models. The strong temperature dependence of polysulfide solubility, diffusion rates, and reaction kinetics is often neglected, limiting model applicability across different operating conditions and thermal management strategies.
A primary challenge lies in modeling the polysulfide shuttle phenomenon, where soluble lithium polysulfides migrate between electrodes, causing active material loss and capacity fade. Existing models often oversimplify this process, failing to account for the concentration-dependent diffusion coefficients and the complex reaction kinetics involved in the transformation between various polysulfide species (Li₂Sₙ, 2≤n≤8).
The precipitation of solid discharge products (Li₂S₂ and Li₂S) presents another significant modeling hurdle. Current approaches inadequately represent nucleation and growth processes, particularly the spatial distribution of precipitates within the cathode microstructure. This limitation prevents accurate prediction of electrode passivation and pore clogging effects that critically influence battery performance.
Electrolyte transport modeling in Li-S batteries remains problematic due to the dramatic changes in electrolyte properties during cycling. As polysulfides dissolve and precipitate, electrolyte viscosity, ionic conductivity, and diffusion coefficients change substantially. Most models employ constant parameters that fail to capture these dynamic variations, leading to significant discrepancies between simulations and experimental results.
The multi-scale nature of transport phenomena in Li-S batteries further complicates modeling efforts. Processes range from molecular-level reactions to macroscopic mass transport across the cell, spanning multiple time and length scales. Current computational approaches struggle to integrate these scales efficiently while maintaining reasonable calculation times.
Additionally, the highly porous carbon structures typically used in Li-S cathodes create tortuous diffusion pathways that are difficult to characterize and model accurately. Most models rely on effective medium approximations that inadequately represent the actual microstructure and its impact on species transport and reaction distribution.
Temperature effects on transport phenomena represent another underexplored area in current Li-S battery models. The strong temperature dependence of polysulfide solubility, diffusion rates, and reaction kinetics is often neglected, limiting model applicability across different operating conditions and thermal management strategies.
State-of-the-Art Transport Modeling Solutions
01 Electrolyte transport mechanisms in Li-S batteries
The transport of lithium ions through the electrolyte is a critical factor affecting the performance of lithium-sulfur batteries. Various electrolyte compositions and additives can enhance ion mobility and reduce the shuttle effect of polysulfides. Research focuses on understanding how electrolyte properties influence ion transport phenomena, including viscosity effects, ion conductivity, and the formation of the solid-electrolyte interphase (SEI) layer that impacts long-term cycling stability.- Electrolyte transport mechanisms in lithium-sulfur batteries: The transport of electrolyte components plays a crucial role in lithium-sulfur battery performance. Various mechanisms affect ion mobility, including solvent-mediated transport, concentration gradients, and interfacial phenomena. Optimizing electrolyte composition and structure can enhance lithium-ion conductivity while minimizing polysulfide shuttling, which is a major cause of capacity fade. Advanced electrolyte systems incorporate additives and modified solvents to control transport phenomena at the electrode-electrolyte interface.
- Sulfur diffusion and polysulfide shuttling control: Controlling the diffusion of sulfur species, particularly soluble polysulfides, is essential for improving lithium-sulfur battery performance. The shuttle effect, where polysulfides migrate between electrodes, causes capacity loss and reduced efficiency. Various approaches to mitigate this include physical barriers, chemical trapping mechanisms, and modified separator designs. Advanced materials that can selectively block polysulfide migration while allowing lithium-ion transport help maintain capacity over extended cycling.
- Electrode structure optimization for mass transport: The porous structure of electrodes significantly influences mass transport in lithium-sulfur batteries. Optimizing pore size distribution, tortuosity, and connectivity enhances the transport of both ions and electrons while accommodating volume changes during cycling. Hierarchical porous structures with tailored macro/meso/micropores facilitate efficient electrolyte penetration and sulfur utilization. Advanced electrode designs incorporate conductive networks that maintain pathways for electron transport throughout battery operation.
- Thermal transport phenomena and temperature effects: Thermal transport phenomena significantly impact lithium-sulfur battery performance and safety. Temperature gradients affect reaction kinetics, electrolyte viscosity, and ion diffusion rates. Managing heat generation and dissipation is crucial for preventing thermal runaway and ensuring uniform reaction distribution. Advanced thermal management systems incorporate phase change materials and optimized cell designs to maintain optimal operating temperatures and enhance battery longevity.
- Interface phenomena and solid-electrolyte interphase formation: Interface phenomena, particularly the formation and evolution of the solid-electrolyte interphase (SEI), critically affect lithium-sulfur battery performance. Transport across these interfaces determines reaction kinetics and overall cell efficiency. The composition and structure of the SEI layer influence lithium-ion transport while potentially blocking polysulfide diffusion. Advanced interface engineering approaches incorporate functional additives and surface modifications to create stable interfaces that facilitate desired transport processes while inhibiting degradation mechanisms.
02 Sulfur cathode structure and polysulfide diffusion control
The structure of sulfur cathodes significantly impacts the transport phenomena in lithium-sulfur batteries. Innovative cathode designs aim to physically confine polysulfides and prevent their diffusion to the anode. These designs include porous carbon hosts, conductive polymers, and various nanostructured materials that can trap polysulfides while maintaining efficient electron and ion transport pathways, thereby improving capacity retention and cycle life.Expand Specific Solutions03 Separator modifications for polysulfide blocking
Modified separators play a crucial role in controlling transport phenomena in lithium-sulfur batteries. Functional separators with selective permeability can block polysulfide migration while allowing lithium ion transport. Various coating materials and structural modifications are employed to create barriers against polysulfide shuttling, including the use of conductive layers, metal oxide coatings, and polymer membranes with tailored pore structures.Expand Specific Solutions04 Anode protection strategies against polysulfide reactions
Protecting the lithium metal anode from detrimental reactions with migrating polysulfides is essential for improving lithium-sulfur battery performance. Various approaches focus on modifying the anode surface or creating protective layers that prevent direct contact with polysulfides while maintaining efficient lithium ion transport. These strategies include artificial SEI formation, lithiophilic coatings, and three-dimensional current collectors that regulate lithium deposition patterns.Expand Specific Solutions05 Modeling and simulation of transport phenomena
Computational modeling and simulation techniques are increasingly used to understand complex transport phenomena in lithium-sulfur batteries. These approaches help predict ion diffusion, polysulfide dissolution and precipitation, and electrochemical reactions under various operating conditions. Advanced models incorporate multiphysics simulations that account for coupled electrochemical, thermal, and mechanical processes, enabling the optimization of battery design and operating parameters for improved performance and safety.Expand Specific Solutions
Key Research Groups and Industry Players
The lithium-sulfur battery transport phenomena modeling market is currently in an early growth phase, characterized by intensive R&D activities across academic and industrial sectors. With a projected market size reaching $2.5-3 billion by 2030, this technology represents a significant advancement in next-generation energy storage. The competitive landscape features established battery manufacturers like LG Energy Solution and automotive companies such as Toyota and GM developing proprietary technologies, alongside specialized players like Lyten and Honeycomb Battery focusing exclusively on lithium-sulfur chemistry. Research institutions including California Institute of Technology, University of California, and Dalian Institute of Chemical Physics are driving fundamental breakthroughs, while industrial giants Shell and Bosch are investing in applications for transportation and energy storage markets. Technical maturity varies significantly, with most solutions still at TRL 4-6, indicating ongoing challenges in modeling complex electrochemical processes.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed advanced multi-scale modeling approaches for lithium-sulfur (Li-S) batteries that integrate molecular dynamics simulations with continuum-level transport models. Their technology focuses on accurately predicting polysulfide shuttle effects - a critical challenge in Li-S batteries. The company employs a comprehensive modeling framework that captures the dissolution, diffusion, and precipitation of sulfur species across multiple length scales[1]. Their models incorporate detailed electrochemical kinetics coupled with species transport phenomena, allowing for precise prediction of concentration gradients and reaction distributions within the cell. LG's approach also accounts for the volume changes and morphological evolution of the sulfur cathode during cycling, which significantly impacts long-term performance. They have integrated these models with machine learning algorithms to accelerate parameter optimization and improve predictive capabilities for various operating conditions[3].
Strengths: Superior integration of multi-scale modeling techniques that bridge molecular and macroscopic phenomena; robust prediction of polysulfide shuttle effects leading to practical design improvements. Weaknesses: Computational intensity requires significant resources; models may still struggle to fully capture the complex phase transformations in real-world operating conditions.
The Regents of the University of California
Technical Solution: The University of California has developed sophisticated multi-physics modeling frameworks for lithium-sulfur batteries that integrate electrochemical, thermal, and mechanical phenomena. Their approach employs advanced numerical methods to solve coupled partial differential equations describing species transport across multiple phases. UC researchers have pioneered the use of phase-field models to capture the precipitation/dissolution dynamics of sulfur species, which is crucial for understanding capacity fade mechanisms[1]. Their models incorporate detailed reaction kinetics at electrode interfaces, accounting for the complex speciation of polysulfides and their spatial distribution throughout the cell architecture. The university's research teams have developed specialized computational techniques that bridge atomistic and continuum scales, allowing for more accurate prediction of electrolyte transport properties and interfacial phenomena[3]. Their modeling framework also addresses the mechanical stresses induced by volume changes during cycling, providing insights into structural degradation mechanisms. UC researchers have validated their models against extensive experimental data, enabling the identification of rate-limiting steps in the overall battery performance[5].
Strengths: Comprehensive multi-physics approach with strong theoretical foundations; excellent integration of experimental validation with model refinement; advanced numerical methods for solving complex coupled equations. Weaknesses: Models can be computationally intensive and challenging to implement in industrial settings; some aspects may prioritize academic rigor over practical application.
Critical Transport Mechanisms and Governing Equations
Improved method for controlling an energy storage system
PatentWO2020128066A1
Innovation
- A predictive method using a dynamic state-space model for load distribution among parallel battery units, considering connection resistances and aging, to calculate optimal load thresholds and balance dynamic interactions, ensuring neither battery unit exceeds its load threshold, thus prolonging battery life and maximizing overall power capability.
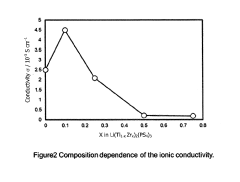
Increasing ionic conductivity of liti2(PS4)3 by zr doping
PatentActiveUS20190229369A1
Innovation
- A Zr-doped compound Li(Ti1-xZrx)2(PS4)3 is synthesized through a method involving mechanical milling or melt-quenching followed by heat treatment at temperatures between 350°C and 500°C, optimizing the crystal structure for enhanced ionic conductivity.
Environmental Impact and Sustainability Assessment
The environmental impact of lithium-sulfur (Li-S) battery technology represents a critical consideration in its development trajectory. Unlike conventional lithium-ion batteries, Li-S batteries utilize sulfur cathodes, which offer significant sustainability advantages due to sulfur's natural abundance, low cost, and reduced environmental toxicity compared to traditional cathode materials like cobalt and nickel. Advanced modeling of transport phenomena in these batteries provides crucial insights into optimizing their environmental performance throughout their lifecycle.
Life cycle assessment (LCA) studies incorporating transport phenomena models reveal that Li-S batteries potentially reduce carbon footprint by 25-30% compared to conventional lithium-ion technologies. This reduction stems primarily from the elimination of energy-intensive mining operations associated with rare earth metals. However, the environmental benefits are partially offset by the complex electrolyte systems required to manage polysulfide shuttling effects, which often contain fluorinated compounds with high global warming potential.
The sustainability profile of Li-S batteries is significantly influenced by the transport mechanisms of lithium ions and polysulfides within the battery structure. Models that accurately predict these transport phenomena enable the design of more efficient electrolyte compositions and separator materials, potentially reducing the need for environmentally harmful additives. Recent research indicates that optimized transport properties could extend cycle life by 40-60%, thereby reducing the environmental impact per unit of energy delivered over the battery lifetime.
Water consumption represents another critical environmental consideration for Li-S battery production. Current manufacturing processes require approximately 65-80 liters of water per kWh of battery capacity. Advanced modeling approaches that incorporate multiphase transport phenomena can identify opportunities to reduce water usage by optimizing electrode preparation techniques and electrolyte formulations, potentially decreasing water requirements by up to 35%.
End-of-life management presents both challenges and opportunities for Li-S technology. The sulfur component is theoretically 100% recyclable, offering a significant advantage over conventional batteries. However, the complex transport phenomena during battery degradation create challenges for efficient material recovery. Models that accurately predict material distribution and transformation during cycling can inform more effective recycling processes, potentially increasing sulfur recovery rates from the current 70-75% to over 90%.
Regional environmental impacts vary significantly based on manufacturing location and energy mix. Transport phenomena models that account for temperature-dependent performance can help optimize battery designs for specific deployment regions, reducing the need for energy-intensive thermal management systems and thereby lowering operational environmental footprints by an estimated 15-20% in extreme climate conditions.
Life cycle assessment (LCA) studies incorporating transport phenomena models reveal that Li-S batteries potentially reduce carbon footprint by 25-30% compared to conventional lithium-ion technologies. This reduction stems primarily from the elimination of energy-intensive mining operations associated with rare earth metals. However, the environmental benefits are partially offset by the complex electrolyte systems required to manage polysulfide shuttling effects, which often contain fluorinated compounds with high global warming potential.
The sustainability profile of Li-S batteries is significantly influenced by the transport mechanisms of lithium ions and polysulfides within the battery structure. Models that accurately predict these transport phenomena enable the design of more efficient electrolyte compositions and separator materials, potentially reducing the need for environmentally harmful additives. Recent research indicates that optimized transport properties could extend cycle life by 40-60%, thereby reducing the environmental impact per unit of energy delivered over the battery lifetime.
Water consumption represents another critical environmental consideration for Li-S battery production. Current manufacturing processes require approximately 65-80 liters of water per kWh of battery capacity. Advanced modeling approaches that incorporate multiphase transport phenomena can identify opportunities to reduce water usage by optimizing electrode preparation techniques and electrolyte formulations, potentially decreasing water requirements by up to 35%.
End-of-life management presents both challenges and opportunities for Li-S technology. The sulfur component is theoretically 100% recyclable, offering a significant advantage over conventional batteries. However, the complex transport phenomena during battery degradation create challenges for efficient material recovery. Models that accurately predict material distribution and transformation during cycling can inform more effective recycling processes, potentially increasing sulfur recovery rates from the current 70-75% to over 90%.
Regional environmental impacts vary significantly based on manufacturing location and energy mix. Transport phenomena models that account for temperature-dependent performance can help optimize battery designs for specific deployment regions, reducing the need for energy-intensive thermal management systems and thereby lowering operational environmental footprints by an estimated 15-20% in extreme climate conditions.
Computational Methods and Simulation Techniques
The computational landscape for lithium-sulfur (Li-S) battery modeling has evolved significantly over the past decade, with increasing sophistication in simulation techniques to capture the complex transport phenomena. Molecular Dynamics (MD) simulations have emerged as a powerful tool for investigating atomic-level interactions between lithium ions, polysulfides, and electrolyte molecules. These simulations provide critical insights into diffusion coefficients and activation energies that govern the transport processes within the battery system.
Density Functional Theory (DFT) calculations complement MD simulations by offering quantum mechanical insights into electronic structures and reaction energetics. The combination of DFT with continuum models has enabled researchers to bridge the gap between atomic-scale phenomena and macroscopic battery behavior, particularly in understanding the dissolution and precipitation of sulfur species during cycling.
Finite Element Method (FEM) and Computational Fluid Dynamics (CFD) approaches have been instrumental in modeling the multiphase flow and species transport within the porous electrode structures. These numerical methods solve the coupled partial differential equations that describe mass, charge, and energy transport across multiple length scales. Advanced meshing techniques have improved the computational efficiency while maintaining accuracy in representing the complex electrode geometries.
Machine Learning (ML) algorithms are increasingly being integrated with physics-based models to create hybrid modeling frameworks. These data-driven approaches can predict battery performance with reduced computational cost compared to full-scale simulations. Neural networks trained on simulation data have shown promise in capturing the non-linear relationships between operating conditions and transport parameters.
Monte Carlo methods provide probabilistic insights into the stochastic nature of nucleation and growth processes of Li2S precipitates. These simulations are particularly valuable for understanding capacity fade mechanisms related to the spatial distribution of insulating precipitates within the cathode structure.
High-Performance Computing (HPC) infrastructures have enabled more realistic 3D simulations of entire cell architectures. Parallel computing techniques and GPU acceleration have reduced simulation times from weeks to hours, making parameter optimization and sensitivity analysis more feasible for industrial applications.
Open-source software packages like COMSOL Multiphysics, OpenFOAM, and Battery Design Studio have democratized access to advanced simulation capabilities. These platforms offer pre-built modules for electrochemical systems while allowing customization for specific Li-S battery phenomena such as the shuttle effect and precipitation dynamics.
Density Functional Theory (DFT) calculations complement MD simulations by offering quantum mechanical insights into electronic structures and reaction energetics. The combination of DFT with continuum models has enabled researchers to bridge the gap between atomic-scale phenomena and macroscopic battery behavior, particularly in understanding the dissolution and precipitation of sulfur species during cycling.
Finite Element Method (FEM) and Computational Fluid Dynamics (CFD) approaches have been instrumental in modeling the multiphase flow and species transport within the porous electrode structures. These numerical methods solve the coupled partial differential equations that describe mass, charge, and energy transport across multiple length scales. Advanced meshing techniques have improved the computational efficiency while maintaining accuracy in representing the complex electrode geometries.
Machine Learning (ML) algorithms are increasingly being integrated with physics-based models to create hybrid modeling frameworks. These data-driven approaches can predict battery performance with reduced computational cost compared to full-scale simulations. Neural networks trained on simulation data have shown promise in capturing the non-linear relationships between operating conditions and transport parameters.
Monte Carlo methods provide probabilistic insights into the stochastic nature of nucleation and growth processes of Li2S precipitates. These simulations are particularly valuable for understanding capacity fade mechanisms related to the spatial distribution of insulating precipitates within the cathode structure.
High-Performance Computing (HPC) infrastructures have enabled more realistic 3D simulations of entire cell architectures. Parallel computing techniques and GPU acceleration have reduced simulation times from weeks to hours, making parameter optimization and sensitivity analysis more feasible for industrial applications.
Open-source software packages like COMSOL Multiphysics, OpenFOAM, and Battery Design Studio have democratized access to advanced simulation capabilities. These platforms offer pre-built modules for electrochemical systems while allowing customization for specific Li-S battery phenomena such as the shuttle effect and precipitation dynamics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!