Sulfur loading optimization for high-energy-density lithium-sulfur batteries
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Li-S Battery Technology Background and Objectives
Lithium-sulfur (Li-S) batteries have emerged as a promising next-generation energy storage technology due to their theoretical energy density of 2600 Wh/kg, which significantly surpasses the capabilities of conventional lithium-ion batteries (typically 250-300 Wh/kg). This remarkable potential stems from sulfur's high theoretical specific capacity of 1675 mAh/g and its natural abundance, making it both technically attractive and economically viable as a cathode material.
The development of Li-S battery technology can be traced back to the 1960s when the first conceptual designs were proposed. However, significant research momentum only began building in the early 2000s as limitations of traditional lithium-ion technologies became apparent and the demand for higher energy density storage solutions increased across multiple sectors including electric vehicles, portable electronics, and grid storage applications.
Despite their theoretical advantages, Li-S batteries have faced persistent challenges that have hindered their commercialization. These include the insulating nature of sulfur, the dissolution of lithium polysulfides leading to the "shuttle effect," and substantial volume changes during cycling. These issues collectively result in rapid capacity fading and limited cycle life, which have been the focus of intensive research efforts globally.
The optimization of sulfur loading represents a critical frontier in Li-S battery development. Traditional approaches typically employed low sulfur loadings (< 2 mg/cm²) to mitigate the aforementioned challenges, but this strategy severely limits the practical energy density of the resulting batteries. Recent research has increasingly focused on achieving high sulfur loadings (> 5 mg/cm²) while maintaining electrochemical performance, which is essential for realizing the technology's full potential.
The primary technical objectives in sulfur loading optimization include: developing novel electrode architectures that can accommodate high sulfur content while maintaining electronic conductivity; creating effective strategies to suppress polysulfide shuttling; designing electrolyte systems compatible with high-loading electrodes; and engineering cell components that can withstand the mechanical stresses associated with volume expansion during cycling.
Current technological trends indicate a shift toward multifunctional electrode designs that integrate conductive networks, polysulfide trapping mechanisms, and catalytic sites to enhance reaction kinetics. Additionally, there is growing interest in the development of solid-state and semi-solid electrolytes as potential solutions to the polysulfide dissolution problem.
The ultimate goal of these research efforts is to develop Li-S batteries with energy densities exceeding 500 Wh/kg at the cell level, with cycle life comparable to commercial lithium-ion batteries (>1000 cycles), while maintaining cost advantages through the use of abundant materials like sulfur.
The development of Li-S battery technology can be traced back to the 1960s when the first conceptual designs were proposed. However, significant research momentum only began building in the early 2000s as limitations of traditional lithium-ion technologies became apparent and the demand for higher energy density storage solutions increased across multiple sectors including electric vehicles, portable electronics, and grid storage applications.
Despite their theoretical advantages, Li-S batteries have faced persistent challenges that have hindered their commercialization. These include the insulating nature of sulfur, the dissolution of lithium polysulfides leading to the "shuttle effect," and substantial volume changes during cycling. These issues collectively result in rapid capacity fading and limited cycle life, which have been the focus of intensive research efforts globally.
The optimization of sulfur loading represents a critical frontier in Li-S battery development. Traditional approaches typically employed low sulfur loadings (< 2 mg/cm²) to mitigate the aforementioned challenges, but this strategy severely limits the practical energy density of the resulting batteries. Recent research has increasingly focused on achieving high sulfur loadings (> 5 mg/cm²) while maintaining electrochemical performance, which is essential for realizing the technology's full potential.
The primary technical objectives in sulfur loading optimization include: developing novel electrode architectures that can accommodate high sulfur content while maintaining electronic conductivity; creating effective strategies to suppress polysulfide shuttling; designing electrolyte systems compatible with high-loading electrodes; and engineering cell components that can withstand the mechanical stresses associated with volume expansion during cycling.
Current technological trends indicate a shift toward multifunctional electrode designs that integrate conductive networks, polysulfide trapping mechanisms, and catalytic sites to enhance reaction kinetics. Additionally, there is growing interest in the development of solid-state and semi-solid electrolytes as potential solutions to the polysulfide dissolution problem.
The ultimate goal of these research efforts is to develop Li-S batteries with energy densities exceeding 500 Wh/kg at the cell level, with cycle life comparable to commercial lithium-ion batteries (>1000 cycles), while maintaining cost advantages through the use of abundant materials like sulfur.
Market Analysis for High-Energy-Density Battery Demand
The global market for high-energy-density batteries is experiencing unprecedented growth, driven primarily by the rapid expansion of electric vehicles (EVs), portable electronics, and renewable energy storage systems. Lithium-sulfur (Li-S) batteries, with their theoretical energy density of 2600 Wh/kg—approximately five times higher than conventional lithium-ion batteries—are positioned as a promising next-generation energy storage solution.
Current market projections indicate that the global high-energy-density battery market will reach significant valuation by 2030, with compound annual growth rates exceeding 12% between 2023 and 2030. The EV sector represents the largest demand segment, accounting for over 60% of the market share, as automotive manufacturers increasingly commit to electrification targets and zero-emission mandates take effect across major markets.
Consumer electronics constitute the second-largest market segment, with demand for longer-lasting portable devices driving innovation in battery technologies. This sector values both energy density and form factor flexibility, making Li-S batteries particularly attractive due to their potential for lightweight, high-capacity applications.
Grid-scale energy storage represents an emerging but rapidly growing market segment, projected to expand at the fastest rate among all applications. The intermittent nature of renewable energy sources necessitates high-capacity, cost-effective storage solutions—a need that optimized Li-S batteries could potentially address.
Regional analysis reveals Asia-Pacific as the dominant market for high-energy-density batteries, with China leading manufacturing capacity and consumption. North America and Europe follow closely, with significant investments in battery research and production facilities to reduce dependency on Asian suppliers.
Market barriers for Li-S battery adoption include cost considerations, with current production estimates ranging significantly higher than established lithium-ion technologies. However, cost projections indicate potential parity by 2028-2030 as manufacturing scales and sulfur utilization improves through loading optimization.
Consumer and industrial demand for longer-lasting, faster-charging batteries continues to outpace technological development, creating a substantial market opportunity for breakthrough technologies like optimized Li-S batteries. Market surveys indicate consumers are willing to pay premium prices for devices with significantly improved battery performance, particularly in high-end electronics and luxury EV segments.
The regulatory landscape further supports market growth, with policies in major economies incentivizing the development and adoption of higher-energy-density, more sustainable battery technologies. These include manufacturing subsidies, research grants, and end-of-life recycling requirements that favor more environmentally compatible chemistries like sulfur-based systems.
Current market projections indicate that the global high-energy-density battery market will reach significant valuation by 2030, with compound annual growth rates exceeding 12% between 2023 and 2030. The EV sector represents the largest demand segment, accounting for over 60% of the market share, as automotive manufacturers increasingly commit to electrification targets and zero-emission mandates take effect across major markets.
Consumer electronics constitute the second-largest market segment, with demand for longer-lasting portable devices driving innovation in battery technologies. This sector values both energy density and form factor flexibility, making Li-S batteries particularly attractive due to their potential for lightweight, high-capacity applications.
Grid-scale energy storage represents an emerging but rapidly growing market segment, projected to expand at the fastest rate among all applications. The intermittent nature of renewable energy sources necessitates high-capacity, cost-effective storage solutions—a need that optimized Li-S batteries could potentially address.
Regional analysis reveals Asia-Pacific as the dominant market for high-energy-density batteries, with China leading manufacturing capacity and consumption. North America and Europe follow closely, with significant investments in battery research and production facilities to reduce dependency on Asian suppliers.
Market barriers for Li-S battery adoption include cost considerations, with current production estimates ranging significantly higher than established lithium-ion technologies. However, cost projections indicate potential parity by 2028-2030 as manufacturing scales and sulfur utilization improves through loading optimization.
Consumer and industrial demand for longer-lasting, faster-charging batteries continues to outpace technological development, creating a substantial market opportunity for breakthrough technologies like optimized Li-S batteries. Market surveys indicate consumers are willing to pay premium prices for devices with significantly improved battery performance, particularly in high-end electronics and luxury EV segments.
The regulatory landscape further supports market growth, with policies in major economies incentivizing the development and adoption of higher-energy-density, more sustainable battery technologies. These include manufacturing subsidies, research grants, and end-of-life recycling requirements that favor more environmentally compatible chemistries like sulfur-based systems.
Current Sulfur Loading Challenges and Limitations
Despite the promising theoretical energy density of lithium-sulfur (Li-S) batteries (2600 Wh/kg), practical applications remain limited due to several critical challenges related to sulfur loading. Current commercial cathodes typically achieve only 1-2 mg/cm² sulfur loading, significantly below the threshold required for competitive energy density compared to conventional lithium-ion batteries. This limitation directly impacts the volumetric and gravimetric energy density of the entire battery system.
The primary technical challenge stems from sulfur's inherent properties. With a density of 2.07 g/cm³, sulfur is relatively light compared to transition metal oxides used in conventional cathodes. This necessitates thicker electrodes to accommodate sufficient active material, creating issues with electron transport and ion diffusion pathways. Additionally, sulfur's poor electrical conductivity (5×10⁻³⁰ S/cm) requires substantial conductive additives, further diluting the energy density.
Polysulfide dissolution and shuttling represent another major obstacle. During discharge, long-chain polysulfides (Li₂Sₙ, 4≤n≤8) dissolve into the electrolyte and migrate to the anode, causing capacity fading and reduced coulombic efficiency. This issue becomes more pronounced at higher sulfur loadings, as the concentration gradient of polysulfides increases, accelerating the shuttling effect and resulting in rapid capacity decay.
Mechanical integrity presents significant challenges for high-loading electrodes. The substantial volume expansion (approximately 80%) during the sulfur to Li₂S conversion creates mechanical stress that can fracture the electrode structure. This problem intensifies with increased sulfur loading, leading to electrical contact loss between active materials and current collectors, ultimately resulting in capacity degradation over cycling.
Electrolyte management emerges as a critical limitation for high-sulfur-loading cathodes. The conventional electrolyte-to-sulfur (E/S) ratio of 10-15 μL/mg is unsustainable for practical applications, as it significantly reduces the energy density of the entire cell. However, reducing this ratio while maintaining high sulfur loading creates issues with electrolyte infiltration, ion transport, and reaction kinetics, particularly in thick electrodes.
Mass transport limitations become increasingly problematic as sulfur loading increases. The diffusion of lithium ions through thick electrodes creates concentration gradients that lead to uneven utilization of active material. This results in lower sulfur utilization rates, typically below 70% for high-loading electrodes, compared to the near-complete utilization possible with thin electrodes under optimal conditions.
The primary technical challenge stems from sulfur's inherent properties. With a density of 2.07 g/cm³, sulfur is relatively light compared to transition metal oxides used in conventional cathodes. This necessitates thicker electrodes to accommodate sufficient active material, creating issues with electron transport and ion diffusion pathways. Additionally, sulfur's poor electrical conductivity (5×10⁻³⁰ S/cm) requires substantial conductive additives, further diluting the energy density.
Polysulfide dissolution and shuttling represent another major obstacle. During discharge, long-chain polysulfides (Li₂Sₙ, 4≤n≤8) dissolve into the electrolyte and migrate to the anode, causing capacity fading and reduced coulombic efficiency. This issue becomes more pronounced at higher sulfur loadings, as the concentration gradient of polysulfides increases, accelerating the shuttling effect and resulting in rapid capacity decay.
Mechanical integrity presents significant challenges for high-loading electrodes. The substantial volume expansion (approximately 80%) during the sulfur to Li₂S conversion creates mechanical stress that can fracture the electrode structure. This problem intensifies with increased sulfur loading, leading to electrical contact loss between active materials and current collectors, ultimately resulting in capacity degradation over cycling.
Electrolyte management emerges as a critical limitation for high-sulfur-loading cathodes. The conventional electrolyte-to-sulfur (E/S) ratio of 10-15 μL/mg is unsustainable for practical applications, as it significantly reduces the energy density of the entire cell. However, reducing this ratio while maintaining high sulfur loading creates issues with electrolyte infiltration, ion transport, and reaction kinetics, particularly in thick electrodes.
Mass transport limitations become increasingly problematic as sulfur loading increases. The diffusion of lithium ions through thick electrodes creates concentration gradients that lead to uneven utilization of active material. This results in lower sulfur utilization rates, typically below 70% for high-loading electrodes, compared to the near-complete utilization possible with thin electrodes under optimal conditions.
Current Sulfur Loading Optimization Approaches
01 High sulfur loading cathode structures
Lithium-sulfur batteries with high sulfur loading cathodes can achieve improved energy density and cycle life. These cathodes typically incorporate porous carbon structures or conductive frameworks that can accommodate large amounts of sulfur while maintaining electrical connectivity. The high loading designs often require careful engineering of the electrode architecture to manage volume changes during cycling and prevent capacity fade.- High sulfur loading cathode structures: Advanced cathode structures have been developed to accommodate high sulfur loading in lithium-sulfur batteries. These structures typically involve porous carbon frameworks, conductive matrices, or hierarchical designs that can hold larger amounts of sulfur while maintaining electrical conductivity. High sulfur loading is critical for achieving higher energy density in lithium-sulfur batteries, with some designs capable of supporting sulfur content exceeding 70% by weight.
- Sulfur-carbon composite materials: Sulfur-carbon composite materials represent a significant advancement in lithium-sulfur battery technology. These composites typically combine sulfur with various carbon materials such as graphene, carbon nanotubes, or mesoporous carbon to enhance conductivity and contain polysulfides. The carbon component provides structural stability and electrical pathways, while also helping to mitigate the volume expansion during cycling, resulting in improved cycle life and capacity retention.
- Polymer binders and electrolyte modifications: Specialized polymer binders and electrolyte modifications have been developed to address challenges associated with high sulfur loading. These include functional polymers that can chemically interact with polysulfides, preventing their dissolution, and electrolyte additives that form protective interfaces. These approaches help maintain the integrity of the cathode structure during cycling, especially important when high amounts of sulfur are present, and contribute to improved capacity retention and cycle life.
- Interlayers and separator modifications: Interlayers and modified separators are employed to trap polysulfides and prevent their migration to the anode in high-sulfur-loading batteries. These components are typically placed between the cathode and separator or integrated into the separator itself. They can be made from carbon materials, polymers, or inorganic compounds with strong polysulfide adsorption capabilities, effectively creating a physical or chemical barrier that improves the utilization of active sulfur material and battery performance.
- Nanostructured sulfur hosts: Nanostructured materials designed specifically to host sulfur have emerged as a promising approach for high-loading lithium-sulfur batteries. These include hollow carbon spheres, yolk-shell structures, and metal-organic frameworks that can encapsulate sulfur while providing void space for expansion. The nanoscale architecture helps to confine polysulfides within the cathode region while maintaining efficient ion transport pathways, addressing key challenges in sulfur utilization and capacity retention.
02 Sulfur-carbon composite materials
Sulfur-carbon composites are widely used in lithium-sulfur batteries to improve sulfur utilization and retention. These composites typically involve sulfur impregnated into various carbon structures such as graphene, carbon nanotubes, or mesoporous carbon. The carbon host provides electrical conductivity while physically constraining the sulfur and polysulfides, leading to better electrochemical performance and higher practical sulfur loading.Expand Specific Solutions03 Binder systems for high sulfur loading
Specialized binder systems are crucial for maintaining the integrity of high-sulfur-loading electrodes during cycling. Advanced polymer binders can accommodate the volume changes associated with sulfur conversion reactions while maintaining good adhesion between active materials and current collectors. These binder systems often incorporate functional groups that can interact with polysulfides, further enhancing cycle stability at high sulfur loadings.Expand Specific Solutions04 Electrolyte modifications for high sulfur loading
Tailored electrolyte formulations are essential for lithium-sulfur batteries with high sulfur loading. These electrolytes often contain additives that can form protective interfaces, suppress the shuttle effect, or enhance ionic conductivity. Optimized electrolyte-to-sulfur ratios and salt concentrations are particularly important when dealing with high sulfur loading to ensure complete utilization of the active material and prevent premature cell failure.Expand Specific Solutions05 Interlayers and separators for polysulfide retention
Functional interlayers and modified separators play a critical role in managing polysulfide migration in high-sulfur-loading lithium-sulfur batteries. These components are designed to physically block or chemically bind polysulfides that dissolve during battery operation. By preventing polysulfide shuttling, these interlayers enable higher sulfur utilization and improved cycling stability, which is particularly important for electrodes with high sulfur content.Expand Specific Solutions
Key Industry Players in Li-S Battery Development
The lithium-sulfur battery market is currently in an early growth phase, characterized by intensive R&D efforts to overcome key technical challenges in sulfur loading optimization. The global market size is projected to reach $1.5 billion by 2028, with a CAGR exceeding 30%. Major industrial players like LG Energy Solution, Samsung SDI, and CATL (through Guangdong Bangpu) are advancing commercial viability, while academic institutions including MIT, Central South University, and City University of Hong Kong are making significant research contributions. Technical maturity varies across approaches, with companies like Asahi Kasei and DuPont developing proprietary sulfur host materials, while LG Chem and Robert Bosch focus on electrolyte formulations to mitigate polysulfide shuttling effects. The technology remains pre-commercial with ongoing efforts to balance high sulfur loading with cycle stability.
LG Chem Ltd.
Technical Solution: LG Chem has developed an innovative approach to sulfur loading optimization through their proprietary "dual-confinement" strategy. This technology utilizes a carbon-sulfur composite structure where sulfur is first infiltrated into mesoporous carbon particles (2-50 nm pore size) at loadings of 6-8 mg/cm², then encapsulated with a microporous carbon shell. This architecture effectively addresses the volume expansion (up to 80%) during lithiation and prevents polysulfide dissolution. LG Chem's approach also incorporates functional interlayers between the cathode and separator, consisting of graphene oxide sheets with oxygen-containing groups that chemically bind polysulfides. Their recent advancements include the development of a gradient-distribution sulfur loading technique, where sulfur concentration varies through the electrode thickness to optimize both energy density and ion transport. This method has demonstrated energy densities exceeding 400 Wh/kg at the cell level while maintaining 80% capacity retention after 200 cycles, a significant improvement over conventional uniform loading approaches.
Strengths: LG Chem's dual-confinement strategy effectively addresses both the volume expansion and polysulfide dissolution issues simultaneously, enabling high practical energy densities. Their gradient loading approach represents a sophisticated engineering solution to the trade-off between loading and performance. Weaknesses: The multi-step manufacturing process increases production complexity and potentially costs, while the long-term stability of their functional interlayers in commercial-scale cells remains to be proven.
Central South University
Technical Solution: Central South University has developed an innovative "multi-functional sulfur host" technology for optimizing sulfur loading in Li-S batteries. Their approach utilizes a composite structure combining graphene aerogels with metal-organic frameworks (MOFs) that can accommodate sulfur loadings of 5-7 mg/cm² while providing multiple functional benefits. The graphene aerogel provides a highly conductive network, while the MOF components offer abundant micropores for sulfur confinement and metal sites for chemical binding of polysulfides. CSU researchers have further enhanced this system by incorporating transition metal sulfides (particularly cobalt and nickel sulfides) as catalytic sites that accelerate the conversion of polysulfides during cycling, addressing the kinetic limitations typically associated with high sulfur loading. Their recent advancement includes a "spatial regulation" strategy where the electrode structure is engineered with varying pore sizes and functional groups across its thickness, optimizing both sulfur utilization and ion transport. This approach has demonstrated impressive performance metrics, including specific capacities exceeding 1200 mAh/g at moderate rates and capacity retention above 75% after 300 cycles, even with sulfur loadings above 6 mg/cm². Additionally, they've developed specialized electrolyte formulations containing fluorinated additives that form protective films on the lithium anode, addressing another critical failure mode in high-energy-density Li-S systems.
Strengths: CSU's multi-functional approach simultaneously addresses multiple failure mechanisms in Li-S batteries, from polysulfide dissolution to conversion kinetics. Their integration of catalytic components represents a distinctive advantage for high-loading electrodes where reaction rates often become limiting. Weaknesses: The complex composite structure involving MOFs and multiple functional components may present challenges for cost-effective manufacturing at scale, and the long-term stability of the catalytic sites remains a potential concern.
Critical Patents and Research on Sulfur Loading
Lithium secondary battery having high energy density
PatentPendingEP4475205A1
Innovation
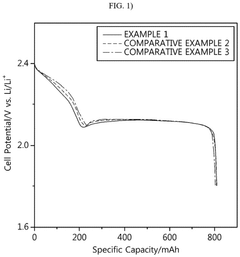
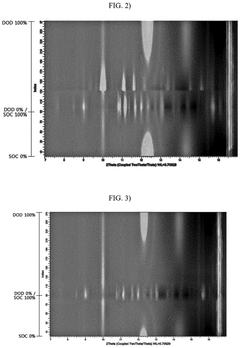
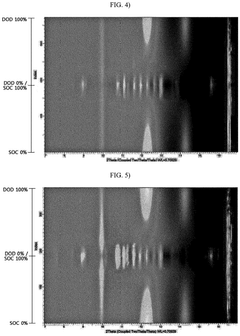
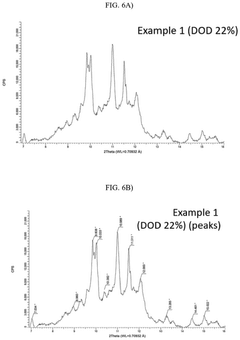
- A lithium-sulfur battery design incorporating a sulfur-carbon composite positive electrode with specific X-ray diffraction peaks and a sulfur loading amount optimized to achieve a high sulfur loading per unit area, along with a controlled electrolyte-to-sulfur ratio, to enhance energy density.
Lithium-sulfur battery with improved energy density and output
PatentWO2023096182A1
Innovation
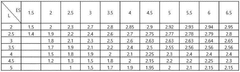
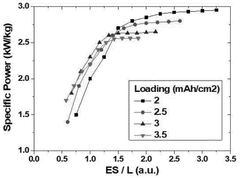
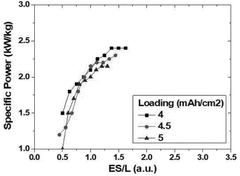
- The lithium-sulfur battery design is optimized by setting the mass ratio of sulfur in the positive electrode to the electrolyte and sulfur loading value within a specific range (1 ≤ ES/L ≤ 1.4), ensuring energy density per cell weight of 300 Wh/kg or more and maximum output of over 2 kW/kg, utilizing a sulfur-carbon composite positive electrode, a lithium metal negative electrode, and a tailored electrolyte with a heterocyclic compound to form a protective film and enhance ionic conductivity.
Material Supply Chain Considerations
The sulfur supply chain presents unique challenges and opportunities for lithium-sulfur battery commercialization. As an abundant by-product of petroleum refining, sulfur offers significant cost advantages compared to traditional cathode materials like cobalt and nickel. Global sulfur production exceeds 70 million tons annually, with major sources concentrated in oil-producing regions such as the Middle East, North America, and Russia. This widespread availability theoretically supports large-scale lithium-sulfur battery production without supply constraints.
However, the quality requirements for battery-grade sulfur differ substantially from industrial-grade sulfur. Battery applications demand high-purity sulfur (>99.9%) with minimal impurities to prevent side reactions and capacity degradation. The purification processes add significant costs to the raw material, potentially offsetting some of the inherent economic advantages.
Transportation and storage of sulfur present additional challenges in the supply chain. While elemental sulfur is relatively stable, prolonged exposure to moisture and air can lead to oxidation and contamination. Specialized handling protocols and controlled environments are necessary throughout the supply chain to maintain material integrity for high-performance battery applications.
The carbon materials required for sulfur loading optimization represent another critical supply chain consideration. High-surface-area carbon hosts, such as carbon nanotubes, graphene, and mesoporous carbons, are essential components for achieving optimal sulfur loading but remain relatively expensive and production-limited. The scalability of these advanced carbon materials presents a potential bottleneck for mass production of high-energy-density lithium-sulfur batteries.
Electrolyte components, particularly lithium salts and specialized additives that suppress polysulfide shuttling, face similar supply chain constraints. Many of these materials are produced in limited quantities for research purposes and lack established industrial-scale production pathways.
Regional disparities in material processing capabilities also impact the lithium-sulfur battery supply chain. While raw sulfur is globally available, the expertise and facilities for battery-grade material processing are concentrated in a few countries, primarily in East Asia. This geographic concentration creates potential vulnerabilities in the supply chain, particularly as demand scales up.
Developing robust recycling processes for lithium-sulfur batteries could significantly improve supply chain sustainability. Unlike lithium-ion batteries, where valuable metals drive recycling economics, sulfur's low intrinsic value presents different recovery incentives. Research into efficient sulfur reclamation methods from spent batteries could create closed-loop material systems that reduce dependence on primary sulfur sources.
However, the quality requirements for battery-grade sulfur differ substantially from industrial-grade sulfur. Battery applications demand high-purity sulfur (>99.9%) with minimal impurities to prevent side reactions and capacity degradation. The purification processes add significant costs to the raw material, potentially offsetting some of the inherent economic advantages.
Transportation and storage of sulfur present additional challenges in the supply chain. While elemental sulfur is relatively stable, prolonged exposure to moisture and air can lead to oxidation and contamination. Specialized handling protocols and controlled environments are necessary throughout the supply chain to maintain material integrity for high-performance battery applications.
The carbon materials required for sulfur loading optimization represent another critical supply chain consideration. High-surface-area carbon hosts, such as carbon nanotubes, graphene, and mesoporous carbons, are essential components for achieving optimal sulfur loading but remain relatively expensive and production-limited. The scalability of these advanced carbon materials presents a potential bottleneck for mass production of high-energy-density lithium-sulfur batteries.
Electrolyte components, particularly lithium salts and specialized additives that suppress polysulfide shuttling, face similar supply chain constraints. Many of these materials are produced in limited quantities for research purposes and lack established industrial-scale production pathways.
Regional disparities in material processing capabilities also impact the lithium-sulfur battery supply chain. While raw sulfur is globally available, the expertise and facilities for battery-grade material processing are concentrated in a few countries, primarily in East Asia. This geographic concentration creates potential vulnerabilities in the supply chain, particularly as demand scales up.
Developing robust recycling processes for lithium-sulfur batteries could significantly improve supply chain sustainability. Unlike lithium-ion batteries, where valuable metals drive recycling economics, sulfur's low intrinsic value presents different recovery incentives. Research into efficient sulfur reclamation methods from spent batteries could create closed-loop material systems that reduce dependence on primary sulfur sources.
Environmental Impact and Sustainability Assessment
The environmental implications of lithium-sulfur (Li-S) battery development, particularly regarding sulfur loading optimization, present both challenges and opportunities for sustainable energy storage solutions. The utilization of sulfur as a cathode material offers significant environmental advantages compared to conventional lithium-ion batteries that rely on cobalt and nickel, which are associated with resource scarcity and environmentally damaging extraction processes.
Sulfur represents an abundant industrial byproduct from petroleum refining processes, making it an environmentally attractive option for large-scale battery production. The repurposing of this waste material for energy storage applications demonstrates circular economy principles and reduces the environmental burden of waste management in petrochemical industries. However, the environmental benefits of high sulfur loading must be evaluated against potential drawbacks throughout the battery lifecycle.
Manufacturing processes for high-sulfur-loaded cathodes typically require less energy-intensive production methods compared to traditional lithium-ion battery materials. This translates to reduced carbon footprints during manufacturing, particularly when renewable energy sources power production facilities. Nevertheless, certain advanced sulfur host materials and conductive additives used to optimize sulfur loading may introduce additional environmental considerations regarding material sourcing and synthesis.
The operational phase of Li-S batteries with optimized sulfur loading demonstrates enhanced sustainability through improved energy density, potentially reducing the total material requirements for equivalent energy storage capacity. This efficiency translates to resource conservation across battery production supply chains. Additionally, the absence of toxic heavy metals in Li-S chemistry significantly reduces environmental hazards associated with battery leakage or improper disposal.
End-of-life management presents both challenges and opportunities for Li-S batteries. The recyclability of sulfur from spent batteries offers a promising pathway for closed-loop material recovery, though current recycling infrastructure requires adaptation to efficiently process Li-S chemistry. Research indicates that sulfur recovery from these batteries could be more straightforward than extracting critical materials from conventional lithium-ion batteries, potentially reducing recycling energy requirements and associated emissions.
Life cycle assessment (LCA) studies comparing optimized Li-S batteries with conventional technologies demonstrate favorable environmental profiles, particularly regarding global warming potential and resource depletion metrics. However, these assessments highlight the importance of developing efficient recycling pathways to fully realize the sustainability benefits of high-sulfur-loaded batteries. Water usage and potential sulfur compound emissions during manufacturing remain areas requiring further optimization to maximize the environmental advantages of this promising technology.
Sulfur represents an abundant industrial byproduct from petroleum refining processes, making it an environmentally attractive option for large-scale battery production. The repurposing of this waste material for energy storage applications demonstrates circular economy principles and reduces the environmental burden of waste management in petrochemical industries. However, the environmental benefits of high sulfur loading must be evaluated against potential drawbacks throughout the battery lifecycle.
Manufacturing processes for high-sulfur-loaded cathodes typically require less energy-intensive production methods compared to traditional lithium-ion battery materials. This translates to reduced carbon footprints during manufacturing, particularly when renewable energy sources power production facilities. Nevertheless, certain advanced sulfur host materials and conductive additives used to optimize sulfur loading may introduce additional environmental considerations regarding material sourcing and synthesis.
The operational phase of Li-S batteries with optimized sulfur loading demonstrates enhanced sustainability through improved energy density, potentially reducing the total material requirements for equivalent energy storage capacity. This efficiency translates to resource conservation across battery production supply chains. Additionally, the absence of toxic heavy metals in Li-S chemistry significantly reduces environmental hazards associated with battery leakage or improper disposal.
End-of-life management presents both challenges and opportunities for Li-S batteries. The recyclability of sulfur from spent batteries offers a promising pathway for closed-loop material recovery, though current recycling infrastructure requires adaptation to efficiently process Li-S chemistry. Research indicates that sulfur recovery from these batteries could be more straightforward than extracting critical materials from conventional lithium-ion batteries, potentially reducing recycling energy requirements and associated emissions.
Life cycle assessment (LCA) studies comparing optimized Li-S batteries with conventional technologies demonstrate favorable environmental profiles, particularly regarding global warming potential and resource depletion metrics. However, these assessments highlight the importance of developing efficient recycling pathways to fully realize the sustainability benefits of high-sulfur-loaded batteries. Water usage and potential sulfur compound emissions during manufacturing remain areas requiring further optimization to maximize the environmental advantages of this promising technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!