Electrode architecture optimization for long-life lithium-sulfur batteries
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Li-S Battery Evolution and Objectives
Lithium-sulfur (Li-S) batteries have emerged as promising candidates for next-generation energy storage systems due to their theoretical energy density of 2600 Wh/kg, which is significantly higher than conventional lithium-ion batteries (typically 100-265 Wh/kg). The evolution of Li-S battery technology can be traced back to the 1960s when the first conceptual designs were proposed. However, meaningful progress was limited until the early 2000s when advances in materials science and nanotechnology enabled researchers to address fundamental challenges.
The development trajectory of Li-S batteries has been characterized by several distinct phases. The initial discovery phase (1960s-1990s) established basic principles but faced insurmountable challenges related to sulfur's insulating nature and the shuttle effect. The renaissance period (2000-2010) saw the introduction of carbon-based materials as conductive hosts for sulfur, marking the first significant breakthrough in making Li-S batteries practically viable.
From 2010 to 2015, research focused on understanding and mitigating the polysulfide shuttle effect through various containment strategies, including physical barriers and chemical bonding approaches. The current phase (2015-present) has shifted toward electrode architecture optimization, recognizing that the spatial arrangement and structural design of electrode components are critical for achieving both high energy density and long cycle life.
The primary technical objectives for Li-S battery development center around overcoming four key challenges: the insulating nature of sulfur and Li2S, volume expansion during cycling (up to 80%), the polysulfide shuttle effect, and lithium metal anode degradation. Electrode architecture optimization has become a focal point as it simultaneously addresses multiple challenges through rational design of the sulfur host structure, electrolyte interaction, and interface engineering.
Specific objectives include developing electrode architectures that: maintain electronic connectivity throughout cycling despite volume changes; provide sufficient space to accommodate sulfur expansion while maintaining high sulfur loading (>5 mg/cm²); create physical and/or chemical barriers to polysulfide migration; facilitate fast ion transport to improve rate capability; and enable stable solid-electrolyte interphase formation on the lithium anode.
The ultimate goal is to achieve Li-S batteries with energy densities exceeding 500 Wh/kg at the cell level, cycle life of more than 1000 cycles with less than 20% capacity fade, and rate capabilities supporting fast charging within 30 minutes. These targets would position Li-S batteries as viable alternatives to lithium-ion batteries for applications ranging from electric vehicles to grid-scale energy storage, particularly in scenarios where weight considerations are paramount.
The development trajectory of Li-S batteries has been characterized by several distinct phases. The initial discovery phase (1960s-1990s) established basic principles but faced insurmountable challenges related to sulfur's insulating nature and the shuttle effect. The renaissance period (2000-2010) saw the introduction of carbon-based materials as conductive hosts for sulfur, marking the first significant breakthrough in making Li-S batteries practically viable.
From 2010 to 2015, research focused on understanding and mitigating the polysulfide shuttle effect through various containment strategies, including physical barriers and chemical bonding approaches. The current phase (2015-present) has shifted toward electrode architecture optimization, recognizing that the spatial arrangement and structural design of electrode components are critical for achieving both high energy density and long cycle life.
The primary technical objectives for Li-S battery development center around overcoming four key challenges: the insulating nature of sulfur and Li2S, volume expansion during cycling (up to 80%), the polysulfide shuttle effect, and lithium metal anode degradation. Electrode architecture optimization has become a focal point as it simultaneously addresses multiple challenges through rational design of the sulfur host structure, electrolyte interaction, and interface engineering.
Specific objectives include developing electrode architectures that: maintain electronic connectivity throughout cycling despite volume changes; provide sufficient space to accommodate sulfur expansion while maintaining high sulfur loading (>5 mg/cm²); create physical and/or chemical barriers to polysulfide migration; facilitate fast ion transport to improve rate capability; and enable stable solid-electrolyte interphase formation on the lithium anode.
The ultimate goal is to achieve Li-S batteries with energy densities exceeding 500 Wh/kg at the cell level, cycle life of more than 1000 cycles with less than 20% capacity fade, and rate capabilities supporting fast charging within 30 minutes. These targets would position Li-S batteries as viable alternatives to lithium-ion batteries for applications ranging from electric vehicles to grid-scale energy storage, particularly in scenarios where weight considerations are paramount.
Market Analysis for Next-Gen Energy Storage
The global energy storage market is experiencing unprecedented growth, driven by the increasing adoption of renewable energy sources and the electrification of transportation. Within this landscape, lithium-sulfur (Li-S) batteries represent a promising next-generation technology with theoretical energy densities up to five times higher than conventional lithium-ion batteries. Market projections indicate that the advanced battery storage market could reach $90 billion by 2030, with Li-S batteries potentially capturing a significant portion of this growth.
Current market demand for high-energy-density batteries is primarily fueled by electric vehicles (EVs), aerospace applications, and grid-scale energy storage systems. The EV market alone is expected to grow at a CAGR of 21% through 2028, creating substantial demand for batteries with higher energy density and longer cycle life. Aerospace and defense sectors are also seeking lightweight, high-performance energy storage solutions where Li-S technology offers compelling advantages.
Despite their theoretical promise, Li-S batteries face significant commercialization challenges related to electrode architecture optimization. The market currently values cycle stability and operational longevity over maximum theoretical capacity, creating a specific demand for research focused on electrode architecture that enhances cycle life rather than just energy density.
Consumer electronics represents another potential market segment, with manufacturers increasingly seeking batteries that offer longer runtime in smaller form factors. This market segment values energy density but demands reliability and safety, areas where electrode architecture optimization is critical for Li-S adoption.
Regional analysis reveals that Asia-Pacific dominates battery manufacturing, with China controlling approximately 75% of global lithium-ion battery production capacity. However, significant investments in North America and Europe aim to establish regional battery supply chains, creating new market opportunities for advanced technologies like optimized Li-S batteries.
Market barriers include the entrenched position of lithium-ion technology, with established manufacturing infrastructure and continuous incremental improvements. For Li-S batteries to penetrate this market, electrode architecture optimization must deliver not only performance improvements but also compatibility with existing manufacturing processes to minimize capital investment requirements.
Price sensitivity varies by application segment, with grid storage being highly cost-conscious (targeting below $100/kWh), while aerospace applications can tolerate higher costs for performance advantages. This market segmentation suggests a staged commercialization approach for electrode-optimized Li-S batteries, targeting premium applications first while scaling to achieve cost reductions for mass markets.
Current market demand for high-energy-density batteries is primarily fueled by electric vehicles (EVs), aerospace applications, and grid-scale energy storage systems. The EV market alone is expected to grow at a CAGR of 21% through 2028, creating substantial demand for batteries with higher energy density and longer cycle life. Aerospace and defense sectors are also seeking lightweight, high-performance energy storage solutions where Li-S technology offers compelling advantages.
Despite their theoretical promise, Li-S batteries face significant commercialization challenges related to electrode architecture optimization. The market currently values cycle stability and operational longevity over maximum theoretical capacity, creating a specific demand for research focused on electrode architecture that enhances cycle life rather than just energy density.
Consumer electronics represents another potential market segment, with manufacturers increasingly seeking batteries that offer longer runtime in smaller form factors. This market segment values energy density but demands reliability and safety, areas where electrode architecture optimization is critical for Li-S adoption.
Regional analysis reveals that Asia-Pacific dominates battery manufacturing, with China controlling approximately 75% of global lithium-ion battery production capacity. However, significant investments in North America and Europe aim to establish regional battery supply chains, creating new market opportunities for advanced technologies like optimized Li-S batteries.
Market barriers include the entrenched position of lithium-ion technology, with established manufacturing infrastructure and continuous incremental improvements. For Li-S batteries to penetrate this market, electrode architecture optimization must deliver not only performance improvements but also compatibility with existing manufacturing processes to minimize capital investment requirements.
Price sensitivity varies by application segment, with grid storage being highly cost-conscious (targeting below $100/kWh), while aerospace applications can tolerate higher costs for performance advantages. This market segmentation suggests a staged commercialization approach for electrode-optimized Li-S batteries, targeting premium applications first while scaling to achieve cost reductions for mass markets.
Current Electrode Challenges in Li-S Technology
Despite significant advancements in lithium-sulfur (Li-S) battery technology, several critical challenges related to electrode architecture continue to impede their widespread commercialization. The cathode structure faces substantial issues due to the insulating nature of sulfur and lithium sulfide, resulting in poor electronic conductivity that limits active material utilization and rate capability. This necessitates the incorporation of conductive additives, which unfortunately reduces the overall energy density of the battery system.
The volume expansion problem presents another major obstacle, as sulfur undergoes approximately 80% volume expansion during the lithium-sulfur conversion reaction. This expansion causes mechanical stress within the electrode structure, leading to pulverization, delamination from current collectors, and ultimately capacity fading over extended cycling. Traditional electrode designs struggle to accommodate this significant volumetric change while maintaining structural integrity.
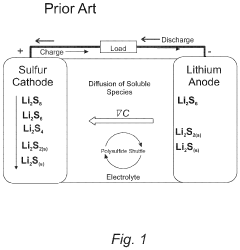
Polysulfide shuttling remains perhaps the most notorious challenge in Li-S technology. The dissolution of intermediate lithium polysulfides into the electrolyte creates a parasitic redox shuttle between electrodes, resulting in active material loss, self-discharge, and rapid capacity decay. Current electrode architectures often lack effective mechanisms to physically or chemically confine these soluble species within the cathode region.
The anode side faces equally critical issues, particularly lithium dendrite formation during repeated cycling. These dendrites can penetrate the separator, causing internal short circuits and safety hazards. Additionally, the continuous reaction between lithium metal and polysulfides forms an unstable solid electrolyte interphase (SEI), consuming both active lithium and sulfur while increasing cell impedance.
Interface engineering between electrode components presents significant challenges. Poor adhesion between active materials, conductive additives, and binders leads to electrical contact loss during cycling. The conventional slurry-casting approach often creates random, non-optimized pore structures that fail to facilitate efficient ion transport while containing polysulfides.
Mass loading limitations further restrict practical applications, as high-loading electrodes (>5 mg/cm²) typically suffer from severe transport limitations and accelerated degradation. Achieving the theoretical energy density of Li-S batteries requires significantly higher sulfur loadings than currently feasible with conventional electrode designs.
Addressing these interconnected challenges requires innovative electrode architectures that simultaneously enhance conductivity, accommodate volume changes, suppress polysulfide shuttling, and enable high mass loading while maintaining mechanical stability throughout extended cycling.
The volume expansion problem presents another major obstacle, as sulfur undergoes approximately 80% volume expansion during the lithium-sulfur conversion reaction. This expansion causes mechanical stress within the electrode structure, leading to pulverization, delamination from current collectors, and ultimately capacity fading over extended cycling. Traditional electrode designs struggle to accommodate this significant volumetric change while maintaining structural integrity.
Polysulfide shuttling remains perhaps the most notorious challenge in Li-S technology. The dissolution of intermediate lithium polysulfides into the electrolyte creates a parasitic redox shuttle between electrodes, resulting in active material loss, self-discharge, and rapid capacity decay. Current electrode architectures often lack effective mechanisms to physically or chemically confine these soluble species within the cathode region.
The anode side faces equally critical issues, particularly lithium dendrite formation during repeated cycling. These dendrites can penetrate the separator, causing internal short circuits and safety hazards. Additionally, the continuous reaction between lithium metal and polysulfides forms an unstable solid electrolyte interphase (SEI), consuming both active lithium and sulfur while increasing cell impedance.
Interface engineering between electrode components presents significant challenges. Poor adhesion between active materials, conductive additives, and binders leads to electrical contact loss during cycling. The conventional slurry-casting approach often creates random, non-optimized pore structures that fail to facilitate efficient ion transport while containing polysulfides.
Mass loading limitations further restrict practical applications, as high-loading electrodes (>5 mg/cm²) typically suffer from severe transport limitations and accelerated degradation. Achieving the theoretical energy density of Li-S batteries requires significantly higher sulfur loadings than currently feasible with conventional electrode designs.
Addressing these interconnected challenges requires innovative electrode architectures that simultaneously enhance conductivity, accommodate volume changes, suppress polysulfide shuttling, and enable high mass loading while maintaining mechanical stability throughout extended cycling.
State-of-Art Electrode Architectures
01 Cathode architecture with carbon-sulfur composites
Carbon-sulfur composites are used in cathode architectures to improve the performance of lithium-sulfur batteries. These composites help to contain sulfur within the electrode structure, preventing dissolution into the electrolyte and improving cycle life. Various carbon materials such as porous carbon, carbon nanotubes, and graphene can be used as hosts for sulfur, providing conductive pathways and physical confinement for the active material.- Porous carbon structures for sulfur cathodes: Porous carbon materials are used as hosts for sulfur in lithium-sulfur battery cathodes. These structures provide high surface area and conductive pathways while accommodating sulfur's volume changes during cycling. Various forms include carbon nanotubes, graphene, and hierarchical porous carbons that trap polysulfides and enhance electrochemical performance. The porous architecture allows for better electrolyte penetration and facilitates faster ion transport.
- Sulfur-carbon composite electrodes: Sulfur-carbon composites are designed to improve the conductivity and cycling stability of lithium-sulfur batteries. These composites integrate sulfur with carbon materials through various methods including melt diffusion, chemical deposition, and mechanical mixing. The carbon component provides electronic conductivity while the composite structure helps contain sulfur and its discharge products, preventing polysulfide dissolution and shuttle effect, thereby enhancing capacity retention.
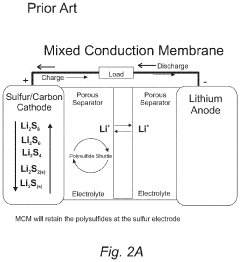
- Protective layers and interlayers for electrodes: Protective layers and interlayers are incorporated into lithium-sulfur battery electrode architectures to mitigate polysulfide shuttling. These layers can be applied on cathodes, anodes, or as separators between electrodes. Materials used include polymers, metal oxides, and functionalized carbon. These barriers physically block polysulfides while allowing lithium ion transport, significantly improving cycling stability and coulombic efficiency of the batteries.
- 3D electrode architectures: Three-dimensional electrode architectures are developed to address the challenges of lithium-sulfur batteries. These structures feature high surface area, interconnected pores, and conductive networks that accommodate sulfur loading while maintaining electronic contact. 3D architectures include foam-like structures, vertically aligned arrays, and hierarchical designs that provide multiple benefits: higher sulfur utilization, better mechanical stability during cycling, and enhanced electrolyte accessibility.
- Binder systems and electrode formulations: Advanced binder systems and electrode formulations are crucial for lithium-sulfur battery performance. Water-soluble binders like PVP and PAA, as well as functional binders with polar groups, help maintain electrode integrity during cycling. Optimized formulations balance sulfur content, conductive additives, and binder ratios to achieve high capacity while maintaining mechanical stability. These formulations also consider electrolyte compatibility and processing conditions to create uniform, crack-free electrodes with strong adhesion to current collectors.
02 Interlayer and separator modifications
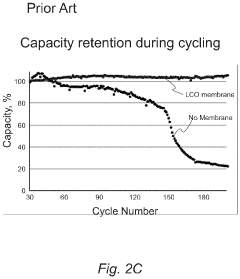
Specialized interlayers and modified separators are incorporated into lithium-sulfur battery electrode architectures to mitigate polysulfide shuttling. These components act as physical barriers or chemical traps that prevent soluble polysulfides from migrating between electrodes. Functionalized separators with coatings of carbon materials, metal oxides, or polymers can selectively block polysulfides while allowing lithium ions to pass through, significantly improving battery cycling stability and capacity retention.Expand Specific Solutions03 Nanostructured sulfur host materials
Nanostructured materials are designed as hosts for sulfur in lithium-sulfur battery electrodes to address volume expansion and polysulfide dissolution issues. These architectures include hollow carbon spheres, mesoporous structures, and hierarchical porous frameworks that accommodate sulfur's volume changes during cycling. The nanostructured hosts provide large surface areas for sulfur deposition, short diffusion paths for lithium ions, and physical confinement to trap polysulfides, resulting in improved electrochemical performance.Expand Specific Solutions04 Binder systems and electrode formulation
Advanced binder systems and electrode formulations are crucial for lithium-sulfur battery performance. Water-soluble binders like polyethylene oxide and carboxymethyl cellulose, as well as functional polymeric binders with strong affinity to polysulfides, help maintain electrode integrity during cycling. The electrode formulation, including the ratio of active material to conductive additives and binders, significantly impacts the electrochemical performance, mechanical stability, and cycle life of lithium-sulfur batteries.Expand Specific Solutions05 Metal-based additives and catalysts
Metal-based additives and catalysts are incorporated into lithium-sulfur battery electrode architectures to enhance reaction kinetics and trap polysulfides. Transition metal oxides, sulfides, nitrides, and metal-organic frameworks serve as chemical anchors for polysulfides through polar-polar interactions. These materials also catalyze the conversion reactions between sulfur and lithium sulfides, reducing overpotential and improving rate capability. The strategic placement of these additives within the electrode structure is critical for maximizing their effectiveness.Expand Specific Solutions
Leading Companies in Li-S Battery Development
Lithium-sulfur battery electrode architecture optimization is currently in the early growth phase, with the market expected to expand significantly due to increasing demand for high-energy density storage solutions. The global market size is projected to grow substantially as this technology offers theoretical energy densities up to five times that of conventional lithium-ion batteries. Technologically, the field remains in development with key challenges including polysulfide shuttling and electrode degradation. Leading players demonstrate varying levels of technological maturity: LG Energy Solution and LG Chem are advancing commercial applications, while Samsung SDI and CALB Technology focus on scalable manufacturing processes. Academic institutions like Tsinghua University and Northwestern University contribute fundamental research, while specialized companies such as Gelion Technologies and Nanotek Instruments develop proprietary electrode architectures to overcome cycle life limitations.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed a multi-layered electrode architecture for lithium-sulfur batteries that addresses the polysulfide shuttle effect. Their approach incorporates a carbon-sulfur composite cathode with hierarchical pore structures to physically confine sulfur and its discharge products. The architecture features a functional interlayer between the cathode and separator, composed of carbon materials doped with nitrogen and other heteroatoms that chemically bind polysulfides. Additionally, they've implemented a lithium metal anode protection strategy using artificial SEI layers formed through in-situ or ex-situ methods. Their electrode design optimizes sulfur loading (>5 mg/cm²) while maintaining electronic conductivity through 3D conductive networks, enabling high areal capacity exceeding 5 mAh/cm².
Strengths: Superior polysulfide confinement through both physical and chemical mechanisms, resulting in significantly reduced capacity fading. The hierarchical electrode structure allows for high sulfur loading while maintaining good ionic and electronic transport. Weaknesses: The complex multi-layered architecture increases manufacturing complexity and potentially raises production costs. The additional functional layers may reduce the overall energy density of the battery system.
SAMSUNG SDI CO LTD
Technical Solution: Samsung SDI has pioneered an electrode architecture optimization approach for lithium-sulfur batteries centered on their "dual-confinement" strategy. This involves a hierarchically structured carbon host material that provides both physical confinement of sulfur and strong chemical binding sites for polysulfides. Their cathode design incorporates mesoporous carbon spheres with nitrogen-doped surfaces, creating a 3D interconnected network that facilitates electron transport while accommodating volume expansion during cycling. Samsung has also developed a proprietary functional interlayer between the cathode and separator, consisting of graphene oxide modified with polar metal oxides (such as TiO2 or MnO2) that act as polysulfide traps. This architecture is complemented by a specialized electrolyte formulation containing lithium nitrate and organic additives that stabilize the lithium metal anode interface, effectively extending cycle life beyond 500 cycles with capacity retention above 80%.
Strengths: The dual-confinement strategy effectively addresses both physical and chemical aspects of polysulfide shuttling, significantly improving cycle life. Their integrated approach optimizes both cathode and anode interfaces simultaneously. Weaknesses: The complex electrode architecture and specialized materials may increase manufacturing costs and present scaling challenges for mass production. The high-performance carbon materials used may reduce the overall energy density compared to theoretical values.
Key Patents in Sulfur Electrode Design
Long-life lithium-sulfur battery using a novel flexible bi-layer solid state electrolyte
PatentPendingUS20220247039A1
Innovation
- A flexible bi-layer solid-state electrolyte separator is introduced, comprising a mixed conduction membrane layer and a lithium ion-conducting polymer electrolyte layer, which blocks polysulfide transport and prevents lithium dendrite growth, eliminating the need for liquid electrolyte and allowing for improved ion transport without increasing resistance.
Improved Organic-Inorganic Composite Electrolyte to Enhance Performance of Lithium-Sulfur Batteries
PatentInactiveKR1020160056423A
Innovation
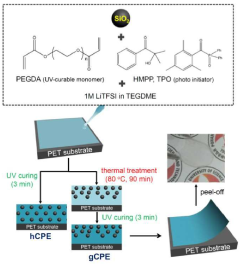
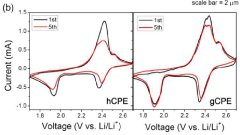
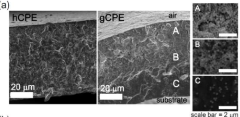
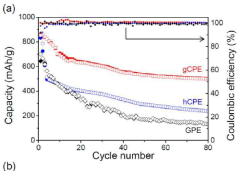
- Development of a unique structured complex gel polyelectrolyte (gCPE) that strategically positions at the anode surface vicinity to suppress polysulfide dissolution.
- Utilization of negatively charged silica nanoparticles within the polyelectrolyte to create effective charge-charge repulsion that reduces polysulfide dissolution during charge/discharge cycles.
- Achievement of high discharge capacity retention from 1140 mAh/g in the first cycle to 970 mAh/g after 100 cycles, demonstrating significant improvement in long-term stability.
Material Supply Chain Considerations
The supply chain for lithium-sulfur (Li-S) battery electrode materials presents unique challenges and opportunities that significantly impact electrode architecture optimization efforts. Sulfur, the primary cathode material, offers advantages of high natural abundance and low cost, with global reserves estimated at over 5 billion tons. This abundance theoretically positions Li-S batteries favorably compared to traditional lithium-ion batteries that rely on scarce cobalt and nickel resources. However, the practical implementation of sulfur in battery manufacturing requires consideration of purification processes, as raw sulfur often contains impurities that can negatively affect electrochemical performance.
Carbon materials, essential components in Li-S electrode architectures, face varying supply chain dynamics. High-surface-area carbons such as activated carbon and carbon black are readily available from established industrial processes, while more specialized carbon nanostructures like carbon nanotubes and graphene remain relatively expensive with limited production scales. The cost disparity between basic and advanced carbon materials can exceed two orders of magnitude, creating significant economic trade-offs in electrode design decisions.
Lithium metal, the conventional anode material for Li-S batteries, presents perhaps the most concerning supply chain vulnerability. Global lithium production is geographically concentrated, with over 80% of reserves located in Australia, Chile, and Argentina. Market volatility has seen lithium prices fluctuate dramatically in recent years, with peaks exceeding $80,000 per ton in 2022. This volatility directly impacts the economic viability of Li-S battery technologies and necessitates consideration of lithium-efficient electrode architectures.
Binder materials and electrolyte components introduce additional supply chain considerations. Fluorinated binders like PVDF rely on fluorine production chains that have environmental implications, while specialized electrolyte additives for Li-S systems often require complex synthesis routes with limited commercial availability. The lithium salt LiTFSI, commonly used in Li-S electrolytes, typically costs 5-10 times more than the LiPF6 used in conventional lithium-ion batteries.
Manufacturing scalability represents another critical dimension of the supply chain landscape. Current electrode architecture innovations often rely on laboratory-scale processes that may face significant barriers to industrial implementation. Techniques such as electrospinning, chemical vapor deposition, and template-based synthesis deliver excellent performance metrics but present challenges for mass production compatibility and cost-effectiveness at commercial scales.
Carbon materials, essential components in Li-S electrode architectures, face varying supply chain dynamics. High-surface-area carbons such as activated carbon and carbon black are readily available from established industrial processes, while more specialized carbon nanostructures like carbon nanotubes and graphene remain relatively expensive with limited production scales. The cost disparity between basic and advanced carbon materials can exceed two orders of magnitude, creating significant economic trade-offs in electrode design decisions.
Lithium metal, the conventional anode material for Li-S batteries, presents perhaps the most concerning supply chain vulnerability. Global lithium production is geographically concentrated, with over 80% of reserves located in Australia, Chile, and Argentina. Market volatility has seen lithium prices fluctuate dramatically in recent years, with peaks exceeding $80,000 per ton in 2022. This volatility directly impacts the economic viability of Li-S battery technologies and necessitates consideration of lithium-efficient electrode architectures.
Binder materials and electrolyte components introduce additional supply chain considerations. Fluorinated binders like PVDF rely on fluorine production chains that have environmental implications, while specialized electrolyte additives for Li-S systems often require complex synthesis routes with limited commercial availability. The lithium salt LiTFSI, commonly used in Li-S electrolytes, typically costs 5-10 times more than the LiPF6 used in conventional lithium-ion batteries.
Manufacturing scalability represents another critical dimension of the supply chain landscape. Current electrode architecture innovations often rely on laboratory-scale processes that may face significant barriers to industrial implementation. Techniques such as electrospinning, chemical vapor deposition, and template-based synthesis deliver excellent performance metrics but present challenges for mass production compatibility and cost-effectiveness at commercial scales.
Sustainability Impact Assessment
The optimization of electrode architectures for lithium-sulfur batteries carries profound implications for environmental sustainability across multiple dimensions. These batteries offer a theoretical energy density up to five times higher than conventional lithium-ion batteries, potentially reducing material consumption per unit of energy storage and extending the operational lifespan of energy storage systems.
From a resource perspective, sulfur represents an abundant, low-cost byproduct of petroleum refining processes, offering a sustainable alternative to cobalt and nickel used in conventional lithium-ion batteries. The utilization of sulfur not only reduces dependency on critical minerals with concentrated supply chains but also provides an avenue for industrial waste valorization, creating a circular economy opportunity.
Life cycle assessment studies indicate that optimized electrode architectures can significantly reduce the carbon footprint of battery production. Advanced carbon host materials and rational design of sulfur-carbon composites minimize energy-intensive processing steps while enhancing cycle life, thereby amortizing manufacturing emissions over longer operational periods.
The extended cycle life achieved through electrode architecture optimization directly addresses one of the most pressing sustainability challenges in battery technology: premature disposal and replacement. By mitigating polysulfide shuttling and volume expansion issues, these architectural innovations can potentially double or triple battery lifespan, substantially reducing electronic waste generation and resource consumption associated with battery replacement.
Water consumption represents another critical sustainability metric improved through electrode optimization. Traditional battery manufacturing processes require significant water resources for material processing and cooling. Advanced electrode architectures that employ dry processing techniques and water-efficient synthesis methods can reduce the water footprint by up to 40% compared to conventional manufacturing approaches.
End-of-life considerations also benefit from optimized electrode designs. The simplified material composition of lithium-sulfur batteries, particularly those with rationally designed carbon-sulfur interfaces, facilitates more efficient recycling processes. The separation of sulfur from carbon hosts can be achieved through relatively low-energy thermal processes, enabling material recovery rates exceeding 80% in laboratory settings.
The sustainability advantages extend to operational safety as well. Optimized electrode architectures with enhanced mechanical stability reduce the risk of internal short circuits and thermal runaway events, minimizing the environmental impact of battery failures and associated hazardous material releases.
From a resource perspective, sulfur represents an abundant, low-cost byproduct of petroleum refining processes, offering a sustainable alternative to cobalt and nickel used in conventional lithium-ion batteries. The utilization of sulfur not only reduces dependency on critical minerals with concentrated supply chains but also provides an avenue for industrial waste valorization, creating a circular economy opportunity.
Life cycle assessment studies indicate that optimized electrode architectures can significantly reduce the carbon footprint of battery production. Advanced carbon host materials and rational design of sulfur-carbon composites minimize energy-intensive processing steps while enhancing cycle life, thereby amortizing manufacturing emissions over longer operational periods.
The extended cycle life achieved through electrode architecture optimization directly addresses one of the most pressing sustainability challenges in battery technology: premature disposal and replacement. By mitigating polysulfide shuttling and volume expansion issues, these architectural innovations can potentially double or triple battery lifespan, substantially reducing electronic waste generation and resource consumption associated with battery replacement.
Water consumption represents another critical sustainability metric improved through electrode optimization. Traditional battery manufacturing processes require significant water resources for material processing and cooling. Advanced electrode architectures that employ dry processing techniques and water-efficient synthesis methods can reduce the water footprint by up to 40% compared to conventional manufacturing approaches.
End-of-life considerations also benefit from optimized electrode designs. The simplified material composition of lithium-sulfur batteries, particularly those with rationally designed carbon-sulfur interfaces, facilitates more efficient recycling processes. The separation of sulfur from carbon hosts can be achieved through relatively low-energy thermal processes, enabling material recovery rates exceeding 80% in laboratory settings.
The sustainability advantages extend to operational safety as well. Optimized electrode architectures with enhanced mechanical stability reduce the risk of internal short circuits and thermal runaway events, minimizing the environmental impact of battery failures and associated hazardous material releases.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!