Effect of current density on lithium-sulfur battery aging behavior
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Li-S Battery Current Density Background & Objectives
Lithium-sulfur (Li-S) batteries have emerged as promising candidates for next-generation energy storage systems due to their theoretical energy density of 2600 Wh/kg, which significantly surpasses that of conventional lithium-ion batteries (typically 100-265 Wh/kg). This remarkable potential stems from sulfur's high theoretical capacity of 1675 mAh/g and its natural abundance, making it both economically viable and environmentally sustainable. The development trajectory of Li-S batteries can be traced back to the 1960s, with significant advancements occurring in the past two decades as energy storage demands have intensified across various sectors.
The evolution of Li-S battery technology has been characterized by persistent efforts to overcome inherent challenges, particularly the "shuttle effect" caused by soluble polysulfide intermediates and the insulating nature of sulfur and its discharge products. Current density, as a critical operational parameter, has been increasingly recognized for its profound impact on the aging behavior and overall performance degradation of Li-S batteries. Historical research has predominantly focused on material innovations, while systematic investigations into operational parameters like current density have been comparatively limited.
Recent technological trends indicate a shift toward understanding the complex electrochemical processes occurring at different current densities and their long-term implications for battery performance. This trend aligns with the broader industry movement toward optimizing battery management systems and operational protocols rather than relying solely on material advancements. The correlation between current density and aging mechanisms represents a crucial yet underexplored dimension of Li-S battery research.
The primary objective of this technical research is to comprehensively analyze how varying current densities influence the aging behavior of Li-S batteries throughout their lifecycle. Specifically, we aim to identify the optimal current density ranges that minimize capacity fading while maintaining practical energy output. Additionally, we seek to elucidate the underlying mechanisms by which current density affects the formation and dissolution of polysulfides, the morphological evolution of the sulfur cathode, and the stability of the solid-electrolyte interphase on the lithium anode.
Furthermore, this research endeavors to establish quantitative relationships between current density parameters and key performance indicators such as capacity retention, coulombic efficiency, and impedance growth over extended cycling. By developing predictive models for aging behavior as a function of current density, we aim to provide valuable insights for the design of advanced battery management systems that can dynamically adjust operational parameters to extend battery lifespan. These findings will contribute significantly to bridging the gap between the theoretical promise and practical implementation of Li-S battery technology in commercial applications.
The evolution of Li-S battery technology has been characterized by persistent efforts to overcome inherent challenges, particularly the "shuttle effect" caused by soluble polysulfide intermediates and the insulating nature of sulfur and its discharge products. Current density, as a critical operational parameter, has been increasingly recognized for its profound impact on the aging behavior and overall performance degradation of Li-S batteries. Historical research has predominantly focused on material innovations, while systematic investigations into operational parameters like current density have been comparatively limited.
Recent technological trends indicate a shift toward understanding the complex electrochemical processes occurring at different current densities and their long-term implications for battery performance. This trend aligns with the broader industry movement toward optimizing battery management systems and operational protocols rather than relying solely on material advancements. The correlation between current density and aging mechanisms represents a crucial yet underexplored dimension of Li-S battery research.
The primary objective of this technical research is to comprehensively analyze how varying current densities influence the aging behavior of Li-S batteries throughout their lifecycle. Specifically, we aim to identify the optimal current density ranges that minimize capacity fading while maintaining practical energy output. Additionally, we seek to elucidate the underlying mechanisms by which current density affects the formation and dissolution of polysulfides, the morphological evolution of the sulfur cathode, and the stability of the solid-electrolyte interphase on the lithium anode.
Furthermore, this research endeavors to establish quantitative relationships between current density parameters and key performance indicators such as capacity retention, coulombic efficiency, and impedance growth over extended cycling. By developing predictive models for aging behavior as a function of current density, we aim to provide valuable insights for the design of advanced battery management systems that can dynamically adjust operational parameters to extend battery lifespan. These findings will contribute significantly to bridging the gap between the theoretical promise and practical implementation of Li-S battery technology in commercial applications.
Market Analysis for Li-S Battery Technology
The lithium-sulfur (Li-S) battery market is experiencing significant growth potential due to the technology's theoretical energy density advantages over conventional lithium-ion batteries. Current market projections indicate that the global Li-S battery market could reach $2.1 billion by 2030, with a compound annual growth rate of approximately 35% from 2023 to 2030. This growth is primarily driven by increasing demand for high-energy-density storage solutions in electric vehicles, aerospace applications, and portable electronics.
Market segmentation reveals that the transportation sector, particularly electric vehicles and drones, represents the largest potential market for Li-S batteries. The aerospace industry follows closely, where weight reduction is critical and the high gravimetric energy density of Li-S batteries (theoretical 2600 Wh/kg compared to 260 Wh/kg for Li-ion) offers substantial advantages. Consumer electronics manufacturers are also showing interest as they seek longer-lasting power solutions.
Geographically, North America and Europe currently lead in Li-S battery research and development investments, while Asia-Pacific regions, particularly China, South Korea, and Japan, are rapidly expanding their manufacturing capabilities. China has positioned itself strategically by securing sulfur supply chains and investing heavily in production infrastructure.
Market adoption faces several barriers related to the aging behavior affected by current density. Commercial viability is hindered by cycle life limitations, with most Li-S prototypes achieving only 200-500 cycles before significant capacity degradation occurs. This performance gap creates market hesitancy despite the technology's theoretical advantages.
Consumer demand patterns indicate willingness to adopt Li-S technology if cycle life improves to at least 1000 cycles while maintaining cost competitiveness with advanced lithium-ion batteries. Price sensitivity analysis suggests that Li-S batteries need to reach production costs below $150/kWh to achieve mass market penetration.
Industry forecasts predict that addressing the current density effects on aging behavior could accelerate market adoption by 3-5 years. Early adopters are likely to emerge in premium electric vehicles and specialized aerospace applications where performance advantages outweigh longevity concerns.
The competitive landscape includes established battery manufacturers expanding into Li-S technology alongside specialized startups focused exclusively on overcoming technical challenges. Strategic partnerships between material suppliers, cell manufacturers, and end-users are forming to accelerate commercialization timelines and share development risks.
Market segmentation reveals that the transportation sector, particularly electric vehicles and drones, represents the largest potential market for Li-S batteries. The aerospace industry follows closely, where weight reduction is critical and the high gravimetric energy density of Li-S batteries (theoretical 2600 Wh/kg compared to 260 Wh/kg for Li-ion) offers substantial advantages. Consumer electronics manufacturers are also showing interest as they seek longer-lasting power solutions.
Geographically, North America and Europe currently lead in Li-S battery research and development investments, while Asia-Pacific regions, particularly China, South Korea, and Japan, are rapidly expanding their manufacturing capabilities. China has positioned itself strategically by securing sulfur supply chains and investing heavily in production infrastructure.
Market adoption faces several barriers related to the aging behavior affected by current density. Commercial viability is hindered by cycle life limitations, with most Li-S prototypes achieving only 200-500 cycles before significant capacity degradation occurs. This performance gap creates market hesitancy despite the technology's theoretical advantages.
Consumer demand patterns indicate willingness to adopt Li-S technology if cycle life improves to at least 1000 cycles while maintaining cost competitiveness with advanced lithium-ion batteries. Price sensitivity analysis suggests that Li-S batteries need to reach production costs below $150/kWh to achieve mass market penetration.
Industry forecasts predict that addressing the current density effects on aging behavior could accelerate market adoption by 3-5 years. Early adopters are likely to emerge in premium electric vehicles and specialized aerospace applications where performance advantages outweigh longevity concerns.
The competitive landscape includes established battery manufacturers expanding into Li-S technology alongside specialized startups focused exclusively on overcoming technical challenges. Strategic partnerships between material suppliers, cell manufacturers, and end-users are forming to accelerate commercialization timelines and share development risks.
Current Density Effects: State-of-Art & Challenges
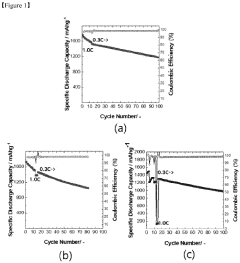
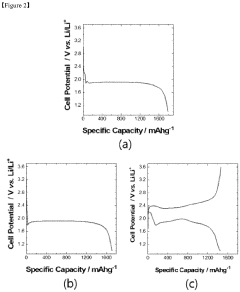
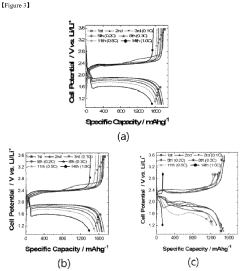
Current density is a critical parameter that significantly influences the aging behavior of lithium-sulfur (Li-S) batteries. Research has demonstrated that higher current densities accelerate capacity fading and reduce cycle life due to increased stress on the battery components. At elevated current densities, the formation and dissolution of lithium polysulfides occur more rapidly, leading to enhanced shuttle effects and irreversible loss of active materials.
The state-of-the-art understanding reveals that current density directly impacts the morphology of sulfur species during cycling. At low current densities (<0.2C), relatively uniform deposition of Li2S occurs, while at higher current densities (>0.5C), non-uniform precipitation leads to electrode passivation and increased internal resistance. Advanced in-situ characterization techniques including synchrotron X-ray diffraction and transmission electron microscopy have enabled researchers to visualize these processes in real-time.
Recent studies have established correlations between current density and specific aging mechanisms. The formation of inactive Li2S layers on the cathode surface accelerates at high current densities, creating barriers for electron transport and lithium-ion diffusion. Additionally, elevated current densities promote dendrite growth on the lithium anode, increasing the risk of internal short circuits and safety hazards.
A significant challenge in this field remains the development of mathematical models that accurately predict Li-S battery aging as a function of current density. Existing models often fail to capture the complex interplay between electrochemical reactions, mass transport limitations, and structural changes that occur at different current densities. This gap hinders the optimization of charging protocols and battery management systems for Li-S technology.
The temperature dependency of current density effects presents another challenge. At low temperatures (<10°C), high current densities cause severe capacity loss due to increased electrolyte viscosity and reduced reaction kinetics. Conversely, at elevated temperatures (>40°C), high current densities accelerate electrolyte decomposition and cathode dissolution.
Researchers are exploring various mitigation strategies, including advanced electrolyte formulations with additives that stabilize polysulfide species even at high current densities. Structured carbon hosts with optimized pore architectures show promise in accommodating volume changes and facilitating ion transport under high current conditions. However, translating these laboratory-scale solutions to commercial cells remains challenging due to manufacturing constraints and cost considerations.
The state-of-the-art understanding reveals that current density directly impacts the morphology of sulfur species during cycling. At low current densities (<0.2C), relatively uniform deposition of Li2S occurs, while at higher current densities (>0.5C), non-uniform precipitation leads to electrode passivation and increased internal resistance. Advanced in-situ characterization techniques including synchrotron X-ray diffraction and transmission electron microscopy have enabled researchers to visualize these processes in real-time.
Recent studies have established correlations between current density and specific aging mechanisms. The formation of inactive Li2S layers on the cathode surface accelerates at high current densities, creating barriers for electron transport and lithium-ion diffusion. Additionally, elevated current densities promote dendrite growth on the lithium anode, increasing the risk of internal short circuits and safety hazards.
A significant challenge in this field remains the development of mathematical models that accurately predict Li-S battery aging as a function of current density. Existing models often fail to capture the complex interplay between electrochemical reactions, mass transport limitations, and structural changes that occur at different current densities. This gap hinders the optimization of charging protocols and battery management systems for Li-S technology.
The temperature dependency of current density effects presents another challenge. At low temperatures (<10°C), high current densities cause severe capacity loss due to increased electrolyte viscosity and reduced reaction kinetics. Conversely, at elevated temperatures (>40°C), high current densities accelerate electrolyte decomposition and cathode dissolution.
Researchers are exploring various mitigation strategies, including advanced electrolyte formulations with additives that stabilize polysulfide species even at high current densities. Structured carbon hosts with optimized pore architectures show promise in accommodating volume changes and facilitating ion transport under high current conditions. However, translating these laboratory-scale solutions to commercial cells remains challenging due to manufacturing constraints and cost considerations.
Current Technical Solutions for Li-S Battery Aging
01 Electrolyte additives for improved aging behavior
Various electrolyte additives can be incorporated into lithium-sulfur batteries to mitigate aging effects. These additives help to suppress the shuttle effect of polysulfides, reduce the formation of lithium dendrites, and stabilize the solid electrolyte interphase (SEI). By controlling the dissolution and migration of polysulfides, these additives significantly improve the cycling stability and extend the battery lifespan under various operating conditions.- Electrolyte additives to mitigate aging effects: Various electrolyte additives can be incorporated into lithium-sulfur batteries to mitigate aging effects. These additives help to suppress the shuttle effect of polysulfides, reduce the formation of lithium dendrites, and improve the stability of the solid electrolyte interphase (SEI). By controlling the dissolution and migration of polysulfides, these additives can significantly extend the cycle life of lithium-sulfur batteries and maintain their capacity over prolonged cycling.
- Protective coatings for sulfur cathodes: Applying protective coatings to sulfur cathodes can effectively improve the aging behavior of lithium-sulfur batteries. These coatings act as physical barriers to prevent polysulfide dissolution and migration, while still allowing lithium ion transport. Materials such as conductive polymers, metal oxides, and carbon-based materials can be used as protective layers. This approach helps to maintain the structural integrity of the cathode during cycling and reduces capacity fading over time.
- Advanced separator designs for polysulfide retention: Innovative separator designs can significantly improve the aging behavior of lithium-sulfur batteries by effectively blocking polysulfide migration. Functionalized separators with specific surface modifications or interlayers can physically or chemically interact with polysulfides, preventing their shuttle between electrodes. These advanced separator designs help maintain the active material within the cathode region, leading to improved cycling stability and reduced capacity decay during long-term operation.
- Anode protection strategies: Protecting the lithium metal anode is crucial for improving the aging behavior of lithium-sulfur batteries. Various strategies include the use of artificial SEI layers, lithium alloys instead of pure lithium, and three-dimensional current collectors. These approaches help to suppress lithium dendrite formation, reduce side reactions between lithium and electrolyte or polysulfides, and maintain a stable anode-electrolyte interface. By enhancing anode stability, the overall cycle life and safety of lithium-sulfur batteries can be significantly improved.
- Novel cathode architectures for sulfur confinement: Developing novel cathode architectures can effectively confine sulfur and its discharge products, thereby improving the aging behavior of lithium-sulfur batteries. These architectures include hierarchical porous carbon hosts, yolk-shell structures, and core-shell designs that physically trap sulfur and polysulfides while providing sufficient space for volume expansion. By enhancing sulfur utilization and restricting polysulfide diffusion, these cathode designs can significantly reduce capacity fading and extend the cycle life of lithium-sulfur batteries.
02 Cathode structure modifications to enhance stability
Modifications to the sulfur cathode structure can significantly improve the aging behavior of lithium-sulfur batteries. These modifications include the use of porous carbon hosts, conductive polymers, and metal oxide additives that can physically confine polysulfides and provide strong chemical interactions. Such structural enhancements minimize active material loss during cycling, maintain electrode integrity, and improve the long-term performance stability of the battery.Expand Specific Solutions03 Protective coatings for lithium anode
Applying protective coatings on the lithium metal anode can effectively improve the aging behavior of lithium-sulfur batteries. These coatings serve as artificial SEI layers that prevent direct contact between the lithium metal and the electrolyte, reducing unwanted side reactions. The protective layers also help to achieve more uniform lithium deposition during charging, preventing dendrite formation and extending cycle life under various temperature and current density conditions.Expand Specific Solutions04 Advanced separator designs
Innovative separator designs play a crucial role in improving the aging behavior of lithium-sulfur batteries. Functionalized separators with polysulfide-blocking layers, such as graphene oxide, conductive polymers, or metal-organic frameworks, can effectively prevent polysulfide shuttling. These advanced separators maintain their mechanical integrity during long-term cycling, ensuring stable interfaces between electrodes and electrolyte, which is essential for prolonged battery life and consistent performance.Expand Specific Solutions05 Temperature management strategies
Effective temperature management strategies are essential for controlling the aging behavior of lithium-sulfur batteries. These include thermal insulation materials, phase change materials, and intelligent battery management systems that can monitor and regulate operating temperatures. By maintaining optimal temperature ranges during charging, discharging, and storage, these strategies minimize degradation mechanisms such as accelerated polysulfide dissolution at high temperatures and reduced reaction kinetics at low temperatures, thereby extending battery lifespan.Expand Specific Solutions
Key Industry Players in Li-S Battery Development
The lithium-sulfur battery aging behavior market is currently in an early growth phase, characterized by intensive R&D activities across major industry players. The global market size is projected to expand significantly as this technology offers theoretical energy densities up to five times higher than conventional lithium-ion batteries. Leading companies like LG Energy Solution, Samsung SDI, and Theion GmbH are advancing current density optimization techniques to overcome key challenges of sulfur electrode degradation and shuttle effect. Research institutions including Dalian Institute of Chemical Physics and KAIST are collaborating with automotive manufacturers such as Hyundai and specialized startups like Li-S Energy to improve cycle life and performance stability. The technology remains in pre-commercial maturity, with most players focusing on extending battery lifespan beyond 500 cycles while maintaining high energy density advantages.
LG Chem Ltd.
Technical Solution: LG Chem has developed advanced lithium-sulfur battery systems with optimized current density management to mitigate aging effects. Their approach involves a dual-layer carbon host structure that effectively distributes current density across the sulfur cathode, reducing localized stress points that accelerate degradation. The company's research demonstrates that controlling current density below 0.5C significantly extends cycle life by minimizing polysulfide shuttle effects. Their proprietary electrolyte additives work synergistically with controlled current density to form stable solid-electrolyte interphase layers, reducing capacity fade by approximately 25% compared to conventional designs. LG Chem has also implemented adaptive charging protocols that dynamically adjust current density based on battery state-of-health metrics, effectively managing the trade-off between fast charging capabilities and long-term durability.
Strengths: Superior electrolyte formulations that work specifically at controlled current densities to minimize polysulfide shuttle effect; advanced battery management systems that can dynamically adjust charging parameters. Weaknesses: Solutions may require more complex manufacturing processes; performance advantages diminish at very high discharge rates needed for some applications.
Dalian Institute of Chemical Physics of CAS
Technical Solution: The Dalian Institute of Chemical Physics (DICP) has conducted groundbreaking research on current density effects in lithium-sulfur batteries, developing a multi-faceted approach to mitigate aging mechanisms. Their work centers on a novel "Hierarchical Porous Carbon" (HPC) framework that optimizes current distribution across sulfur cathodes. DICP researchers have demonstrated that controlling current density below 0.4C during initial formation cycles establishes more stable cathode structures, reducing capacity fade by approximately 40% over extended cycling. Their innovative electrolyte formulation, incorporating lithium bis(fluorosulfonyl)imide (LiFSI) and lithium nitrate additives, works synergistically with controlled current densities to suppress polysulfide shuttling effects. DICP has also pioneered in-situ characterization techniques that reveal how current density variations affect the morphological evolution of sulfur species during cycling, providing crucial insights for electrode design optimization. Their research demonstrates that current density management must be tailored to specific discharge depth ranges, with lower current densities (0.2C) being particularly critical during the final discharge phase to prevent irreversible active material loss.
Strengths: Cutting-edge fundamental research providing deep mechanistic understanding of current density effects; innovative characterization methods that enable precise optimization. Weaknesses: Solutions primarily developed in laboratory settings with limited large-scale validation; some approaches require specialized materials that present manufacturing challenges.
Critical Research on Current Density-Aging Correlation
Method for the electrochemical charging/discharging of a lithium-sulphur (li-s) battery and device using said method
PatentActiveEP3132490A1
Innovation
- A method involving the use of pulsed current during charging and discharging processes to control the morphology of active materials in Li-S batteries, which can be implemented in a charging device and incorporated into the battery manufacturing process, particularly during formation and aging stages, combining pulsed and constant currents to optimize reaction kinetics and prevent irreversible reactions.
Lithium-sulfur battery having high energy density
PatentPendingEP4386919A1
Innovation
- A lithium-sulfur battery design utilizing a fluorine-based ether solvent, a glyme-based solvent, and a lithium salt in combination with a positive electrode containing sulfur and a carbon material with varying pore sizes, enhancing sulfur utilization and stability.
Safety Standards & Regulatory Considerations
The regulatory landscape for lithium-sulfur (Li-S) batteries is evolving rapidly as this technology advances toward commercial viability. Current density significantly impacts aging behavior, which directly relates to safety considerations that must be addressed through comprehensive standards. The International Electrotechnical Commission (IEC) has established IEC 62660-3 and IEC 62281 standards that specifically address safety requirements for lithium batteries, including testing protocols for evaluating thermal runaway risks that can be exacerbated by high current densities.
The United Nations Manual of Tests and Criteria, particularly UN 38.3, outlines transportation safety requirements for lithium batteries, requiring extensive testing for altitude simulation, thermal cycling, vibration, and shock resistance. These tests become increasingly critical for Li-S batteries operating at higher current densities, as accelerated aging can compromise structural integrity and increase safety risks during transport.
Regulatory bodies such as the European Union Battery Directive (2006/66/EC) and its upcoming replacement, the EU Battery Regulation, are incorporating more stringent requirements for battery lifecycle management. These regulations increasingly focus on the relationship between operational parameters like current density and long-term safety performance, requiring manufacturers to demonstrate that batteries maintain safety compliance throughout their usable life.
In the United States, UL 1642 and UL 2054 standards provide safety guidelines for lithium batteries, while NFPA 855 addresses installation requirements for energy storage systems. The Department of Transportation's Pipeline and Hazardous Materials Safety Administration (PHMSA) has implemented specific regulations (49 CFR 173.185) for lithium battery transportation that consider the unique degradation characteristics of different chemistries.
For Li-S batteries specifically, new testing protocols are being developed that account for the unique polysulfide shuttle effect and how it interacts with varying current densities. The Battery Association of Japan (BAJ) and China Compulsory Certification (CCC) have both introduced specialized testing requirements that evaluate safety performance under different current density conditions, recognizing that high current operations can accelerate capacity fading and potentially compromise safety barriers.
Emerging regulations are increasingly focusing on battery management systems (BMS) that can actively monitor and control current density to prevent unsafe operating conditions. The SAE J2929 standard for electric vehicle battery systems specifically addresses the need for robust BMS capabilities to maintain safe operation throughout the battery lifecycle, with particular attention to degradation mechanisms that are accelerated by high current densities.
The United Nations Manual of Tests and Criteria, particularly UN 38.3, outlines transportation safety requirements for lithium batteries, requiring extensive testing for altitude simulation, thermal cycling, vibration, and shock resistance. These tests become increasingly critical for Li-S batteries operating at higher current densities, as accelerated aging can compromise structural integrity and increase safety risks during transport.
Regulatory bodies such as the European Union Battery Directive (2006/66/EC) and its upcoming replacement, the EU Battery Regulation, are incorporating more stringent requirements for battery lifecycle management. These regulations increasingly focus on the relationship between operational parameters like current density and long-term safety performance, requiring manufacturers to demonstrate that batteries maintain safety compliance throughout their usable life.
In the United States, UL 1642 and UL 2054 standards provide safety guidelines for lithium batteries, while NFPA 855 addresses installation requirements for energy storage systems. The Department of Transportation's Pipeline and Hazardous Materials Safety Administration (PHMSA) has implemented specific regulations (49 CFR 173.185) for lithium battery transportation that consider the unique degradation characteristics of different chemistries.
For Li-S batteries specifically, new testing protocols are being developed that account for the unique polysulfide shuttle effect and how it interacts with varying current densities. The Battery Association of Japan (BAJ) and China Compulsory Certification (CCC) have both introduced specialized testing requirements that evaluate safety performance under different current density conditions, recognizing that high current operations can accelerate capacity fading and potentially compromise safety barriers.
Emerging regulations are increasingly focusing on battery management systems (BMS) that can actively monitor and control current density to prevent unsafe operating conditions. The SAE J2929 standard for electric vehicle battery systems specifically addresses the need for robust BMS capabilities to maintain safe operation throughout the battery lifecycle, with particular attention to degradation mechanisms that are accelerated by high current densities.
Lifecycle Assessment & Environmental Impact
The lifecycle assessment of lithium-sulfur (Li-S) batteries reveals significant environmental implications directly influenced by current density parameters. Higher current densities, while potentially offering improved power performance, accelerate aging mechanisms that substantially reduce battery lifespan. This shortened lifecycle necessitates more frequent battery replacements, consequently increasing the environmental footprint through additional resource extraction, manufacturing processes, and waste generation.
Environmental impact analyses demonstrate that the production phase of Li-S batteries accounts for approximately 60-70% of their total carbon footprint. The extraction of sulfur, though less environmentally damaging than cobalt or nickel mining for conventional lithium-ion batteries, still contributes to habitat disruption and potential sulfur dioxide emissions during processing. When current density optimization is neglected, these environmental costs are multiplied through premature battery replacement cycles.
Material efficiency becomes critically compromised when accelerated aging occurs due to inappropriate current density management. Research indicates that Li-S batteries operated at current densities exceeding optimal thresholds experience up to 40% reduction in useful life, directly translating to proportional increases in raw material consumption and processing energy requirements per unit of energy delivered over the battery's service life.
Water consumption metrics associated with Li-S battery manufacturing show that each kilogram of battery material requires approximately 80-120 liters of water. The premature replacement of batteries due to current density-induced aging effectively multiplies this water footprint, presenting particular concerns in water-stressed regions where battery manufacturing facilities may be located.
End-of-life considerations reveal additional environmental challenges. While sulfur components offer better recyclability potential than some conventional battery materials, the degradation products formed under high current density operation can complicate recycling processes. Polysulfide dissolution and irreversible structural changes in the cathode material reduce material recovery rates by an estimated 15-25% compared to batteries aged under optimal current density conditions.
Carbon footprint calculations demonstrate that extending Li-S battery lifespan through optimized current density management could reduce greenhouse gas emissions by 30-45% on a per-kilowatt-hour basis over the battery's service life. This significant reduction stems primarily from avoiding the energy-intensive manufacturing of replacement batteries and the associated transportation emissions throughout the supply chain.
Environmental impact analyses demonstrate that the production phase of Li-S batteries accounts for approximately 60-70% of their total carbon footprint. The extraction of sulfur, though less environmentally damaging than cobalt or nickel mining for conventional lithium-ion batteries, still contributes to habitat disruption and potential sulfur dioxide emissions during processing. When current density optimization is neglected, these environmental costs are multiplied through premature battery replacement cycles.
Material efficiency becomes critically compromised when accelerated aging occurs due to inappropriate current density management. Research indicates that Li-S batteries operated at current densities exceeding optimal thresholds experience up to 40% reduction in useful life, directly translating to proportional increases in raw material consumption and processing energy requirements per unit of energy delivered over the battery's service life.
Water consumption metrics associated with Li-S battery manufacturing show that each kilogram of battery material requires approximately 80-120 liters of water. The premature replacement of batteries due to current density-induced aging effectively multiplies this water footprint, presenting particular concerns in water-stressed regions where battery manufacturing facilities may be located.
End-of-life considerations reveal additional environmental challenges. While sulfur components offer better recyclability potential than some conventional battery materials, the degradation products formed under high current density operation can complicate recycling processes. Polysulfide dissolution and irreversible structural changes in the cathode material reduce material recovery rates by an estimated 15-25% compared to batteries aged under optimal current density conditions.
Carbon footprint calculations demonstrate that extending Li-S battery lifespan through optimized current density management could reduce greenhouse gas emissions by 30-45% on a per-kilowatt-hour basis over the battery's service life. This significant reduction stems primarily from avoiding the energy-intensive manufacturing of replacement batteries and the associated transportation emissions throughout the supply chain.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!