Optimization of electrolyte-to-sulfur ratio for energy density improvement
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrolyte-Sulfur Ratio Background and Objectives
Lithium-sulfur (Li-S) batteries have emerged as promising candidates for next-generation energy storage systems due to their theoretical energy density of 2600 Wh/kg, which far exceeds that of conventional lithium-ion batteries. The development of Li-S battery technology has progressed significantly over the past two decades, with major breakthroughs in addressing the inherent challenges of sulfur cathodes, including the shuttle effect, volume expansion, and poor conductivity.
The electrolyte-to-sulfur (E/S) ratio represents a critical parameter in Li-S battery design that has received increasing attention in recent years. Historically, Li-S battery research has employed high E/S ratios (typically 10-15 μL/mg) to facilitate fundamental studies of reaction mechanisms and material properties. However, this approach has created a significant gap between laboratory research and practical applications, as commercial viability requires much lower E/S ratios to achieve competitive energy densities.
The technical evolution in this field shows a clear trend toward reducing the E/S ratio while maintaining electrochemical performance. Early research (2009-2015) focused primarily on understanding sulfur electrochemistry with minimal consideration for electrolyte optimization. The period from 2016 to 2020 saw increased recognition of the E/S ratio's importance, with researchers beginning to report performance at moderate ratios (5-10 μL/mg). Since 2021, there has been a paradigm shift toward lean electrolyte conditions (<5 μL/mg) as the field matures toward practical applications.
The primary objective of optimizing the E/S ratio is to maximize the energy density of Li-S batteries while maintaining acceptable electrochemical performance. This involves finding the delicate balance between providing sufficient electrolyte for efficient electrochemical reactions and minimizing excess electrolyte that contributes to battery weight and volume without enhancing performance. Specifically, the technical goals include achieving stable cycling at E/S ratios below 3 μL/mg, understanding the fundamental limitations of ultra-low E/S systems, and developing novel electrolyte formulations and cell designs tailored for lean electrolyte conditions.
Additionally, this optimization aims to address the challenges of electrolyte depletion during cycling, which becomes particularly pronounced under lean electrolyte conditions. The formation and dissolution of lithium polysulfides, as well as the irreversible consumption of electrolyte through side reactions with lithium metal anodes, present significant hurdles that must be overcome to realize the full potential of Li-S technology.
The ultimate goal is to enable Li-S batteries with practical energy densities exceeding 400 Wh/kg at the cell level, representing a substantial improvement over state-of-the-art lithium-ion technology and opening new possibilities for applications in electric vehicles, aerospace, and portable electronics.
The electrolyte-to-sulfur (E/S) ratio represents a critical parameter in Li-S battery design that has received increasing attention in recent years. Historically, Li-S battery research has employed high E/S ratios (typically 10-15 μL/mg) to facilitate fundamental studies of reaction mechanisms and material properties. However, this approach has created a significant gap between laboratory research and practical applications, as commercial viability requires much lower E/S ratios to achieve competitive energy densities.
The technical evolution in this field shows a clear trend toward reducing the E/S ratio while maintaining electrochemical performance. Early research (2009-2015) focused primarily on understanding sulfur electrochemistry with minimal consideration for electrolyte optimization. The period from 2016 to 2020 saw increased recognition of the E/S ratio's importance, with researchers beginning to report performance at moderate ratios (5-10 μL/mg). Since 2021, there has been a paradigm shift toward lean electrolyte conditions (<5 μL/mg) as the field matures toward practical applications.
The primary objective of optimizing the E/S ratio is to maximize the energy density of Li-S batteries while maintaining acceptable electrochemical performance. This involves finding the delicate balance between providing sufficient electrolyte for efficient electrochemical reactions and minimizing excess electrolyte that contributes to battery weight and volume without enhancing performance. Specifically, the technical goals include achieving stable cycling at E/S ratios below 3 μL/mg, understanding the fundamental limitations of ultra-low E/S systems, and developing novel electrolyte formulations and cell designs tailored for lean electrolyte conditions.
Additionally, this optimization aims to address the challenges of electrolyte depletion during cycling, which becomes particularly pronounced under lean electrolyte conditions. The formation and dissolution of lithium polysulfides, as well as the irreversible consumption of electrolyte through side reactions with lithium metal anodes, present significant hurdles that must be overcome to realize the full potential of Li-S technology.
The ultimate goal is to enable Li-S batteries with practical energy densities exceeding 400 Wh/kg at the cell level, representing a substantial improvement over state-of-the-art lithium-ion technology and opening new possibilities for applications in electric vehicles, aerospace, and portable electronics.
Market Analysis for High Energy Density Batteries
The high energy density battery market is experiencing unprecedented growth, driven by the expanding electric vehicle (EV) sector, portable electronics, and renewable energy storage systems. The global market for high energy density batteries was valued at approximately $41 billion in 2022 and is projected to reach $97 billion by 2030, representing a compound annual growth rate (CAGR) of 11.4%. Lithium-sulfur (Li-S) batteries, with their theoretical energy density of 2600 Wh/kg, significantly outperform conventional lithium-ion batteries (250-400 Wh/kg), positioning them as a promising next-generation energy storage solution.
The EV segment dominates the demand for high energy density batteries, accounting for 63% of the market share in 2022. This is primarily due to automotive manufacturers' push to extend driving ranges while reducing battery weight and volume. Consumer electronics represents the second-largest market segment at 21%, followed by grid storage applications at 9%.
Regionally, Asia-Pacific leads the market with 47% share, driven by China's aggressive EV adoption policies and substantial manufacturing capacity. North America and Europe follow with 28% and 19% respectively, with both regions investing heavily in battery technology development through initiatives like the European Battery Alliance and the U.S. Battery Materials Processing and Battery Manufacturing program.
The optimization of electrolyte-to-sulfur ratio directly addresses a critical market need: improving energy density while maintaining cycle life. Market research indicates that commercial viability for Li-S batteries requires achieving practical energy densities above 500 Wh/kg with cycle life exceeding 1000 cycles. Current commercial offerings typically deliver 350-400 Wh/kg with limited cycle life, creating a significant market gap.
Key market drivers include increasingly stringent vehicle emission regulations, declining battery costs (projected to fall below $100/kWh by 2025), and growing consumer demand for longer-lasting portable devices. The sustainability aspect of sulfur as an abundant, low-cost material also aligns with market trends toward environmentally friendly technologies.
Market barriers include competition from established lithium-ion technologies, which benefit from mature manufacturing infrastructure and continuous incremental improvements. Additionally, industrial stakeholders report concerns regarding the scalability of electrolyte optimization techniques and the availability of specialized electrolyte components at commercial volumes.
Customer surveys indicate that OEMs would consider adopting Li-S technology if energy density improvements of at least 30% could be achieved without compromising safety or increasing costs by more than 15% compared to current lithium-ion solutions.
The EV segment dominates the demand for high energy density batteries, accounting for 63% of the market share in 2022. This is primarily due to automotive manufacturers' push to extend driving ranges while reducing battery weight and volume. Consumer electronics represents the second-largest market segment at 21%, followed by grid storage applications at 9%.
Regionally, Asia-Pacific leads the market with 47% share, driven by China's aggressive EV adoption policies and substantial manufacturing capacity. North America and Europe follow with 28% and 19% respectively, with both regions investing heavily in battery technology development through initiatives like the European Battery Alliance and the U.S. Battery Materials Processing and Battery Manufacturing program.
The optimization of electrolyte-to-sulfur ratio directly addresses a critical market need: improving energy density while maintaining cycle life. Market research indicates that commercial viability for Li-S batteries requires achieving practical energy densities above 500 Wh/kg with cycle life exceeding 1000 cycles. Current commercial offerings typically deliver 350-400 Wh/kg with limited cycle life, creating a significant market gap.
Key market drivers include increasingly stringent vehicle emission regulations, declining battery costs (projected to fall below $100/kWh by 2025), and growing consumer demand for longer-lasting portable devices. The sustainability aspect of sulfur as an abundant, low-cost material also aligns with market trends toward environmentally friendly technologies.
Market barriers include competition from established lithium-ion technologies, which benefit from mature manufacturing infrastructure and continuous incremental improvements. Additionally, industrial stakeholders report concerns regarding the scalability of electrolyte optimization techniques and the availability of specialized electrolyte components at commercial volumes.
Customer surveys indicate that OEMs would consider adopting Li-S technology if energy density improvements of at least 30% could be achieved without compromising safety or increasing costs by more than 15% compared to current lithium-ion solutions.
Current Limitations in Lithium-Sulfur Battery Technology
Despite significant advancements in lithium-ion battery technology, lithium-sulfur (Li-S) batteries face persistent challenges that hinder their widespread commercial adoption. One of the most critical limitations is the low practical energy density compared to theoretical values. While Li-S batteries boast a theoretical energy density of 2600 Wh/kg, practical implementations typically achieve only 200-300 Wh/kg, representing less than 15% of the theoretical potential.
The shuttle effect remains a fundamental obstacle, where soluble lithium polysulfides migrate between electrodes during cycling, causing rapid capacity fading and shortened battery lifespan. This phenomenon not only reduces energy efficiency but also contributes to self-discharge issues that compromise long-term storage capabilities.
Volume expansion presents another significant challenge, as sulfur undergoes substantial volumetric changes (up to 80%) during charge-discharge cycles. This expansion leads to mechanical stress on the electrode structure, resulting in pulverization and electrical contact loss between active materials and current collectors, ultimately degrading performance over multiple cycles.
Poor sulfur utilization efficiency further limits practical energy density, with typical utilization rates below 70% in many systems. This inefficiency stems from sulfur's insulating nature and the formation of Li2S/Li2S2 discharge products that are electronically and ionically insulating, creating barriers to complete electrochemical reactions.
The electrolyte-to-sulfur (E/S) ratio represents a critical parameter that directly impacts energy density. Current Li-S batteries typically require high E/S ratios (often >10 μL/mg) to facilitate proper electrochemical reactions and accommodate dissolved polysulfides. However, this high electrolyte content significantly increases battery weight and volume, dramatically reducing gravimetric and volumetric energy densities at the cell level.
Low Coulombic efficiency, typically 95-98% compared to >99.5% for commercial lithium-ion batteries, indicates energy losses during cycling and contributes to capacity degradation. This inefficiency stems primarily from the irreversible loss of active materials through side reactions and the incomplete conversion of discharge products.
Temperature sensitivity further complicates Li-S battery implementation, with performance deteriorating significantly at low temperatures due to reduced reaction kinetics and increased electrolyte viscosity. At elevated temperatures, accelerated side reactions and enhanced shuttle effects exacerbate capacity fading.
These interconnected limitations create a complex optimization challenge, where addressing one issue often exacerbates others, necessitating a holistic approach to Li-S battery development with particular focus on optimizing the electrolyte-to-sulfur ratio to improve practical energy density.
The shuttle effect remains a fundamental obstacle, where soluble lithium polysulfides migrate between electrodes during cycling, causing rapid capacity fading and shortened battery lifespan. This phenomenon not only reduces energy efficiency but also contributes to self-discharge issues that compromise long-term storage capabilities.
Volume expansion presents another significant challenge, as sulfur undergoes substantial volumetric changes (up to 80%) during charge-discharge cycles. This expansion leads to mechanical stress on the electrode structure, resulting in pulverization and electrical contact loss between active materials and current collectors, ultimately degrading performance over multiple cycles.
Poor sulfur utilization efficiency further limits practical energy density, with typical utilization rates below 70% in many systems. This inefficiency stems from sulfur's insulating nature and the formation of Li2S/Li2S2 discharge products that are electronically and ionically insulating, creating barriers to complete electrochemical reactions.
The electrolyte-to-sulfur (E/S) ratio represents a critical parameter that directly impacts energy density. Current Li-S batteries typically require high E/S ratios (often >10 μL/mg) to facilitate proper electrochemical reactions and accommodate dissolved polysulfides. However, this high electrolyte content significantly increases battery weight and volume, dramatically reducing gravimetric and volumetric energy densities at the cell level.
Low Coulombic efficiency, typically 95-98% compared to >99.5% for commercial lithium-ion batteries, indicates energy losses during cycling and contributes to capacity degradation. This inefficiency stems primarily from the irreversible loss of active materials through side reactions and the incomplete conversion of discharge products.
Temperature sensitivity further complicates Li-S battery implementation, with performance deteriorating significantly at low temperatures due to reduced reaction kinetics and increased electrolyte viscosity. At elevated temperatures, accelerated side reactions and enhanced shuttle effects exacerbate capacity fading.
These interconnected limitations create a complex optimization challenge, where addressing one issue often exacerbates others, necessitating a holistic approach to Li-S battery development with particular focus on optimizing the electrolyte-to-sulfur ratio to improve practical energy density.
Current Approaches to Electrolyte-Sulfur Ratio Management
01 High energy density cathode materials for lithium-sulfur batteries
Lithium-sulfur batteries utilize sulfur-based cathode materials that offer theoretical energy densities of up to 2600 Wh/kg, significantly higher than conventional lithium-ion batteries. Various cathode material compositions and structures have been developed to maximize the utilization of sulfur and improve energy density, including carbon-sulfur composites and sulfur-polymer composites. These materials aim to address the challenges of sulfur's low conductivity while maintaining its high theoretical capacity.- High energy density cathode materials for lithium-sulfur batteries: Lithium-sulfur batteries utilize sulfur-based cathode materials that offer theoretical energy densities of up to 2600 Wh/kg, significantly higher than conventional lithium-ion batteries. Various cathode material compositions and structures have been developed to maximize the utilization of sulfur's high theoretical capacity. These include sulfur-carbon composites, conductive polymers with sulfur, and nanostructured sulfur materials that help overcome the inherent challenges of sulfur electrodes while maintaining high energy density.
- Electrolyte innovations for improved energy density: Advanced electrolyte formulations play a crucial role in enhancing the energy density of lithium-sulfur batteries. Novel electrolytes help address the polysulfide shuttle effect, which typically reduces energy density and cycle life. These include solid-state electrolytes, ionic liquid electrolytes, and electrolyte additives that suppress polysulfide dissolution. By effectively containing sulfur species within the cathode structure, these electrolyte innovations help lithium-sulfur batteries approach their theoretical energy density limits.
- Anode protection strategies for energy density enhancement: Protecting the lithium metal anode is essential for achieving high energy density in lithium-sulfur batteries. Various approaches include developing protective layers on lithium anodes, using lithium alloys instead of pure lithium, and creating structured anodes that minimize dendrite formation. These strategies prevent capacity loss from side reactions between lithium and polysulfides, allowing the battery to maintain higher energy density over multiple cycles while improving safety characteristics.
- Nanostructured carbon hosts for sulfur cathodes: Nanostructured carbon materials serve as effective hosts for sulfur in lithium-sulfur battery cathodes, significantly improving energy density. These include carbon nanotubes, graphene, mesoporous carbon, and hollow carbon spheres that provide conductive pathways while physically constraining sulfur and its discharge products. The high surface area and pore structure of these carbon hosts enable better sulfur utilization and polysulfide confinement, resulting in batteries with energy densities approaching theoretical limits while maintaining good cycle stability.
- Cell design and engineering for practical energy density: Innovative cell designs and engineering approaches help translate the theoretical high energy density of lithium-sulfur chemistry into practical battery systems. These include optimized electrode thickness ratios, reduced inactive component weight, advanced current collectors, and novel cell architectures. Special attention to electrolyte-to-sulfur ratios and packaging materials helps maximize gravimetric and volumetric energy density at the cell and pack level, bringing lithium-sulfur batteries closer to commercial viability for high-energy applications.
02 Electrolyte innovations for enhanced energy density
Advanced electrolyte formulations play a crucial role in improving the energy density of lithium-sulfur batteries. Novel electrolytes help mitigate the polysulfide shuttle effect, which causes capacity fading and reduces energy density over cycling. Electrolyte additives, ionic liquids, and solid-state electrolytes have been developed to enhance lithium-sulfur battery performance, enabling higher sulfur loading and utilization while maintaining stable cycling, thus increasing practical energy density.Expand Specific Solutions03 Anode protection strategies for lithium-sulfur batteries
Protecting the lithium metal anode is essential for achieving high energy density in lithium-sulfur batteries. Various approaches include developing protective layers, structured anodes, and lithium alloys that prevent dendrite formation and unwanted side reactions with polysulfides. These protection strategies enable the use of thinner lithium anodes and higher sulfur loading, directly contributing to increased energy density while maintaining safety and cycle life of the battery system.Expand Specific Solutions04 Novel cell architectures for maximizing energy density
Innovative cell designs and architectures have been developed to maximize the energy density of lithium-sulfur batteries. These include 3D electrode structures, pouch cell configurations, and bipolar designs that optimize the spatial arrangement of active materials. Advanced manufacturing techniques enable higher sulfur loading and reduced inactive component weight, resulting in lithium-sulfur batteries with practical energy densities exceeding 400-500 Wh/kg at the cell level.Expand Specific Solutions05 Interlayer and separator technologies for energy density improvement
Specialized interlayers and separator technologies have been developed to enhance the energy density of lithium-sulfur batteries. These components trap polysulfides, prevent their migration, and facilitate ion transport while maintaining minimal thickness and weight. Functional separators with selective permeability and interlayers with polysulfide adsorption capabilities allow for higher sulfur utilization and loading, directly contributing to improved energy density while maintaining good cycle performance.Expand Specific Solutions
Leading Companies in Li-S Battery Development
The lithium-sulfur battery market is currently in an early growth phase, with optimization of electrolyte-to-sulfur ratio emerging as a critical factor for improving energy density. The global market is projected to reach $2.5 billion by 2030, driven by demand for higher energy density solutions. Leading companies like LG Energy Solution, Sion Power, and CATL are advancing this technology, with Sion Power's Licerion® technology demonstrating significant progress through sophisticated lithium-metal battery innovations. Research institutions including Beijing Institute of Technology and Huazhong University collaborate with industry players to overcome key challenges of sulfur utilization and electrolyte consumption. The technology is approaching commercial viability, with companies like Guangdong Bangpu and PolyPlus Battery developing scalable manufacturing processes to optimize the electrolyte-sulfur relationship for next-generation energy storage applications.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed an advanced electrolyte management system for lithium-sulfur batteries that optimizes the electrolyte-to-sulfur (E/S) ratio through a multi-layer electrode design. Their approach incorporates a gradient distribution of electrolyte across the cathode structure, with higher concentration near the separator interface and gradually decreasing toward the current collector. This design enables efficient ion transport while minimizing excess electrolyte volume. The company has implemented nano-engineered carbon matrices that can retain electrolyte in precise locations within the sulfur cathode, allowing for E/S ratios as low as 3:1 compared to traditional ratios of 10-15:1. Their proprietary electrolyte formulation includes lithium salt additives and solvating agents specifically designed to enhance sulfur utilization at lower electrolyte volumes, resulting in energy densities exceeding 400 Wh/kg at the cell level.
Strengths: Achieves significantly higher energy density through precise electrolyte management; maintains good cycle life even at reduced E/S ratios; scalable manufacturing process compatible with existing production lines. Weaknesses: Performance degradation at extremely low temperatures; requires specialized electrolyte additives that may increase production costs; still faces challenges with long-term stability beyond 500 cycles.
Sion Power Corp.
Technical Solution: Sion Power has pioneered the Licerion® technology platform specifically addressing the electrolyte-to-sulfur ratio challenge through their protected lithium anode approach. Their system employs a dual-phase electrolyte strategy where a concentrated electrolyte gel layer is maintained at the sulfur cathode interface while a minimal liquid electrolyte volume fills the remaining cell space. This approach has enabled them to reduce E/S ratios to approximately 2-3 mL/g while maintaining high sulfur utilization. The company's proprietary electrolyte formulation includes fluorinated solvents and lithium salts that form stable interfaces with both the protected lithium anode and the sulfur cathode. Sion's electrode architecture incorporates hierarchical porous carbon structures that efficiently trap polysulfide intermediates within the cathode region, preventing their diffusion and allowing for reduced electrolyte volumes. Their latest cells demonstrate energy densities of 450-500 Wh/kg with E/S ratios below 3:1, representing a significant advancement over conventional lithium-sulfur battery designs.
Strengths: Industry-leading energy density achieved through optimized E/S ratio; protected lithium anode technology prevents dendrite formation; demonstrated performance in actual flight tests with aerospace partners. Weaknesses: Higher manufacturing complexity due to specialized anode protection layers; premium pricing compared to conventional lithium-ion batteries; technology still primarily targeted at specialty markets rather than mass consumer applications.
Key Patents in Electrolyte Formulation for Sulfur Cathodes
Lithium secondary battery having high energy density
PatentPendingEP4475205A1
Innovation
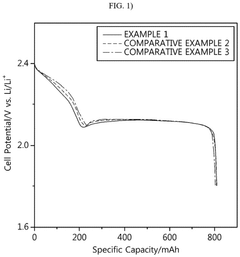
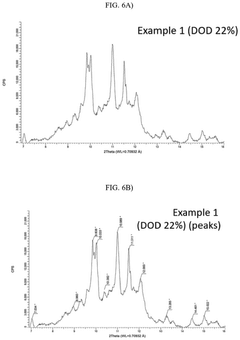
- A lithium-sulfur battery design incorporating a sulfur-carbon composite positive electrode with specific X-ray diffraction peaks and a sulfur loading amount optimized to achieve a high sulfur loading per unit area, along with a controlled electrolyte-to-sulfur ratio, to enhance energy density.
Lithium-sulfur battery with improved energy density and output
PatentWO2023096182A1
Innovation
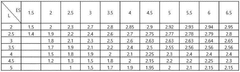
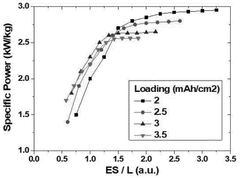
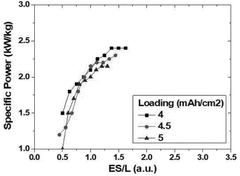
- The lithium-sulfur battery design is optimized by setting the mass ratio of sulfur in the positive electrode to the electrolyte and sulfur loading value within a specific range (1 ≤ ES/L ≤ 1.4), ensuring energy density per cell weight of 300 Wh/kg or more and maximum output of over 2 kW/kg, utilizing a sulfur-carbon composite positive electrode, a lithium metal negative electrode, and a tailored electrolyte with a heterocyclic compound to form a protective film and enhance ionic conductivity.
Environmental Impact and Sustainability Considerations
The optimization of electrolyte-to-sulfur ratio in lithium-sulfur batteries presents significant environmental and sustainability implications that warrant careful consideration. The manufacturing processes for these battery components involve extraction of raw materials, particularly lithium and sulfur, which have varying environmental footprints. Sulfur, often sourced as a byproduct from petroleum refining, represents a sustainable approach to waste utilization, while lithium extraction can be resource-intensive and environmentally disruptive depending on the sourcing method.
Reducing the electrolyte-to-sulfur ratio directly contributes to sustainability by minimizing the amount of electrolyte required per unit of energy storage capacity. This optimization decreases the consumption of fluorinated solvents and lithium salts, many of which have high global warming potential and present end-of-life disposal challenges. Studies indicate that conventional lithium-sulfur batteries with high electrolyte ratios (>10 μL/mg) have substantially higher carbon footprints compared to optimized systems with ratios below 5 μL/mg.
Life cycle assessment (LCA) data demonstrates that electrolyte components typically account for 25-40% of a lithium-sulfur battery's total environmental impact. By optimizing the E/S ratio, manufacturers can achieve up to a 30% reduction in greenhouse gas emissions associated with battery production. Additionally, lower electrolyte volumes reduce the risk of leakage and contamination during operation and disposal phases, mitigating potential ecological damage.
The sustainability benefits extend to resource efficiency and circular economy principles. Optimized E/S ratios enable more efficient use of critical materials, particularly lithium salts, which face supply constraints and geopolitical challenges. This efficiency becomes increasingly important as battery production scales to meet growing global demand for energy storage solutions.
Water consumption represents another critical environmental consideration. Traditional electrolyte production processes are water-intensive, with estimates suggesting that each kilogram of electrolyte requires between 40-100 liters of water. Reducing electrolyte requirements through ratio optimization proportionally decreases this water footprint, contributing to more sustainable manufacturing practices.
From a regulatory perspective, optimized lithium-sulfur batteries with lower electrolyte content may more easily comply with emerging environmental regulations, including the European Union's Battery Directive and similar frameworks being developed globally. These regulations increasingly emphasize reduced toxicity, improved recyclability, and lower carbon footprints—all objectives supported by E/S ratio optimization.
Reducing the electrolyte-to-sulfur ratio directly contributes to sustainability by minimizing the amount of electrolyte required per unit of energy storage capacity. This optimization decreases the consumption of fluorinated solvents and lithium salts, many of which have high global warming potential and present end-of-life disposal challenges. Studies indicate that conventional lithium-sulfur batteries with high electrolyte ratios (>10 μL/mg) have substantially higher carbon footprints compared to optimized systems with ratios below 5 μL/mg.
Life cycle assessment (LCA) data demonstrates that electrolyte components typically account for 25-40% of a lithium-sulfur battery's total environmental impact. By optimizing the E/S ratio, manufacturers can achieve up to a 30% reduction in greenhouse gas emissions associated with battery production. Additionally, lower electrolyte volumes reduce the risk of leakage and contamination during operation and disposal phases, mitigating potential ecological damage.
The sustainability benefits extend to resource efficiency and circular economy principles. Optimized E/S ratios enable more efficient use of critical materials, particularly lithium salts, which face supply constraints and geopolitical challenges. This efficiency becomes increasingly important as battery production scales to meet growing global demand for energy storage solutions.
Water consumption represents another critical environmental consideration. Traditional electrolyte production processes are water-intensive, with estimates suggesting that each kilogram of electrolyte requires between 40-100 liters of water. Reducing electrolyte requirements through ratio optimization proportionally decreases this water footprint, contributing to more sustainable manufacturing practices.
From a regulatory perspective, optimized lithium-sulfur batteries with lower electrolyte content may more easily comply with emerging environmental regulations, including the European Union's Battery Directive and similar frameworks being developed globally. These regulations increasingly emphasize reduced toxicity, improved recyclability, and lower carbon footprints—all objectives supported by E/S ratio optimization.
Scale-up Challenges and Manufacturing Feasibility
The transition from laboratory-scale lithium-sulfur battery prototypes to commercial production presents significant manufacturing challenges, particularly regarding the optimization of electrolyte-to-sulfur ratio. Current laboratory demonstrations typically employ high electrolyte-to-sulfur ratios (E/S) of 10-15 μL/mg, which enables impressive performance but severely limits practical energy density in scaled systems.
Manufacturing feasibility is critically dependent on reducing this ratio to below 3 μL/mg while maintaining electrochemical performance. This reduction represents a fundamental engineering challenge that impacts multiple production processes. The viscosity and wetting properties of electrolytes at lower ratios create difficulties in electrode impregnation during high-speed roll-to-roll manufacturing, potentially leading to incomplete sulfur utilization and accelerated capacity fade.
Material handling complexities also emerge at scale. The highly reactive nature of lithium metal anodes and the sensitivity of polysulfide intermediates to moisture necessitate stringent environmental controls throughout the manufacturing process. These requirements substantially increase production costs and complexity compared to conventional lithium-ion battery manufacturing lines.
Equipment modification represents another significant hurdle. Existing battery production equipment must be adapted to handle the unique rheological properties of low-E/S electrolyte systems. This includes redesigning coating and filling equipment to ensure uniform electrolyte distribution despite reduced volumes and potentially higher viscosities. The capital expenditure for such specialized equipment modifications presents a barrier to market entry for many manufacturers.
Quality control processes must also evolve to accommodate these new parameters. Traditional testing methods may not adequately detect issues specific to low-E/S systems, such as localized electrolyte depletion or non-uniform sulfur utilization. New in-line monitoring techniques will need development to ensure consistent product quality at commercial scales.
Economic viability remains uncertain despite potential energy density improvements. The cost-benefit analysis must account for increased manufacturing complexity against performance gains. Initial production runs will likely face higher unit costs until processes are optimized and economies of scale achieved, potentially delaying market adoption despite technical feasibility.
Addressing these scale-up challenges requires collaborative efforts between materials scientists, chemical engineers, and manufacturing specialists to develop integrated solutions that balance electrochemical performance with production practicality. Success in this domain could significantly accelerate the commercial deployment of high-energy-density lithium-sulfur batteries across multiple applications.
Manufacturing feasibility is critically dependent on reducing this ratio to below 3 μL/mg while maintaining electrochemical performance. This reduction represents a fundamental engineering challenge that impacts multiple production processes. The viscosity and wetting properties of electrolytes at lower ratios create difficulties in electrode impregnation during high-speed roll-to-roll manufacturing, potentially leading to incomplete sulfur utilization and accelerated capacity fade.
Material handling complexities also emerge at scale. The highly reactive nature of lithium metal anodes and the sensitivity of polysulfide intermediates to moisture necessitate stringent environmental controls throughout the manufacturing process. These requirements substantially increase production costs and complexity compared to conventional lithium-ion battery manufacturing lines.
Equipment modification represents another significant hurdle. Existing battery production equipment must be adapted to handle the unique rheological properties of low-E/S electrolyte systems. This includes redesigning coating and filling equipment to ensure uniform electrolyte distribution despite reduced volumes and potentially higher viscosities. The capital expenditure for such specialized equipment modifications presents a barrier to market entry for many manufacturers.
Quality control processes must also evolve to accommodate these new parameters. Traditional testing methods may not adequately detect issues specific to low-E/S systems, such as localized electrolyte depletion or non-uniform sulfur utilization. New in-line monitoring techniques will need development to ensure consistent product quality at commercial scales.
Economic viability remains uncertain despite potential energy density improvements. The cost-benefit analysis must account for increased manufacturing complexity against performance gains. Initial production runs will likely face higher unit costs until processes are optimized and economies of scale achieved, potentially delaying market adoption despite technical feasibility.
Addressing these scale-up challenges requires collaborative efforts between materials scientists, chemical engineers, and manufacturing specialists to develop integrated solutions that balance electrochemical performance with production practicality. Success in this domain could significantly accelerate the commercial deployment of high-energy-density lithium-sulfur batteries across multiple applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!