Design of hybrid electrolytes for lithium-sulfur batteries
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hybrid Electrolyte Technology Background and Objectives
Lithium-sulfur (Li-S) batteries have emerged as a promising next-generation energy storage technology due to their theoretical energy density of 2600 Wh/kg, which far exceeds that of conventional lithium-ion batteries (typically 250-300 Wh/kg). The development of Li-S battery technology can be traced back to the 1960s, but significant research momentum has only built up in the past two decades as the limitations of traditional lithium-ion batteries became increasingly apparent for applications demanding higher energy density.
The evolution of Li-S battery technology has been marked by several key challenges, particularly the "shuttle effect" of polysulfides, poor sulfur utilization, and lithium dendrite growth. These issues have historically limited the practical application of Li-S batteries despite their theoretical advantages. Electrolyte design has emerged as a critical factor in addressing these challenges, with hybrid electrolytes representing one of the most promising approaches in recent years.
Hybrid electrolytes combine different types of electrolyte systems—such as liquid-solid, liquid-gel, or multiple liquid phases—to leverage the advantages of each component while mitigating their individual drawbacks. The concept of hybrid electrolytes for Li-S batteries began gaining traction around 2010, with significant advancements occurring from 2015 onward as researchers recognized the limitations of conventional single-phase electrolytes.
The technological trajectory shows a clear shift from traditional liquid organic electrolytes toward more complex hybrid systems incorporating ionic liquids, solid-state components, and functional additives. This evolution reflects the growing understanding that no single electrolyte type can simultaneously address all the challenges inherent to Li-S chemistry.
The primary objectives of hybrid electrolyte development for Li-S batteries include: suppressing the polysulfide shuttle effect to improve coulombic efficiency; enhancing the ionic conductivity while maintaining mechanical stability; preventing lithium dendrite formation to improve safety and cycle life; and enabling high sulfur loading and utilization to maximize practical energy density.
Recent technological trends indicate increasing interest in localized high-concentration electrolytes, dual-phase liquid systems, and quasi-solid-state hybrid electrolytes. These approaches aim to create spatially controlled chemical environments that can simultaneously address multiple failure mechanisms in Li-S cells.
The ultimate goal of this technological pursuit is to develop hybrid electrolyte systems that enable Li-S batteries with practical energy densities exceeding 500 Wh/kg, cycle life of over 1000 cycles, and safety characteristics suitable for commercial applications ranging from electric vehicles to grid-scale energy storage.
The evolution of Li-S battery technology has been marked by several key challenges, particularly the "shuttle effect" of polysulfides, poor sulfur utilization, and lithium dendrite growth. These issues have historically limited the practical application of Li-S batteries despite their theoretical advantages. Electrolyte design has emerged as a critical factor in addressing these challenges, with hybrid electrolytes representing one of the most promising approaches in recent years.
Hybrid electrolytes combine different types of electrolyte systems—such as liquid-solid, liquid-gel, or multiple liquid phases—to leverage the advantages of each component while mitigating their individual drawbacks. The concept of hybrid electrolytes for Li-S batteries began gaining traction around 2010, with significant advancements occurring from 2015 onward as researchers recognized the limitations of conventional single-phase electrolytes.
The technological trajectory shows a clear shift from traditional liquid organic electrolytes toward more complex hybrid systems incorporating ionic liquids, solid-state components, and functional additives. This evolution reflects the growing understanding that no single electrolyte type can simultaneously address all the challenges inherent to Li-S chemistry.
The primary objectives of hybrid electrolyte development for Li-S batteries include: suppressing the polysulfide shuttle effect to improve coulombic efficiency; enhancing the ionic conductivity while maintaining mechanical stability; preventing lithium dendrite formation to improve safety and cycle life; and enabling high sulfur loading and utilization to maximize practical energy density.
Recent technological trends indicate increasing interest in localized high-concentration electrolytes, dual-phase liquid systems, and quasi-solid-state hybrid electrolytes. These approaches aim to create spatially controlled chemical environments that can simultaneously address multiple failure mechanisms in Li-S cells.
The ultimate goal of this technological pursuit is to develop hybrid electrolyte systems that enable Li-S batteries with practical energy densities exceeding 500 Wh/kg, cycle life of over 1000 cycles, and safety characteristics suitable for commercial applications ranging from electric vehicles to grid-scale energy storage.
Market Analysis for Li-S Battery Electrolytes
The global lithium-sulfur (Li-S) battery electrolyte market is experiencing significant growth, driven by the increasing demand for high-energy-density batteries in various applications. Current market valuations indicate that the Li-S battery market is projected to grow at a compound annual growth rate of 35% between 2023 and 2030, with electrolytes representing approximately 15-20% of the total battery cost structure.
The primary market segments for Li-S battery electrolytes include electric vehicles, aerospace applications, portable electronics, and grid-scale energy storage. Among these, electric vehicles represent the largest potential market, with automotive manufacturers actively seeking alternatives to conventional lithium-ion batteries that offer higher energy density and lower material costs.
Market demand for hybrid electrolytes specifically is being driven by several factors. First, the performance limitations of traditional single-solvent electrolytes have created a clear need for innovative solutions that address the polysulfide shuttle effect and lithium dendrite formation. Second, regulatory pressures toward more environmentally friendly battery technologies favor Li-S systems due to their use of abundant sulfur rather than critical minerals like cobalt.
Regional analysis shows that Asia-Pacific currently dominates the Li-S electrolyte research and development landscape, with China, South Korea, and Japan leading in patent applications. However, significant research activities are also underway in North America and Europe, particularly in Germany and the United Kingdom, where government funding for next-generation battery technologies has increased substantially.
Consumer electronics manufacturers represent an important early-adoption market segment, as they seek batteries with higher energy density for portable devices. This segment values the theoretical energy density of Li-S batteries (2600 Wh/kg) compared to conventional lithium-ion batteries (250-300 Wh/kg).
Market barriers for hybrid electrolytes include high production costs, scalability challenges, and competition from solid-state battery technologies. Current production costs for specialized hybrid electrolytes remain 3-5 times higher than conventional electrolytes, primarily due to complex synthesis processes and limited economies of scale.
Industry analysts predict that as manufacturing processes mature and production volumes increase, the cost differential between hybrid and conventional electrolytes will narrow significantly by 2027-2028, potentially accelerating market adoption. The market is also seeing increased partnership activity between electrolyte manufacturers, battery producers, and end-users, indicating growing commercial interest in bringing Li-S technology to market.
The primary market segments for Li-S battery electrolytes include electric vehicles, aerospace applications, portable electronics, and grid-scale energy storage. Among these, electric vehicles represent the largest potential market, with automotive manufacturers actively seeking alternatives to conventional lithium-ion batteries that offer higher energy density and lower material costs.
Market demand for hybrid electrolytes specifically is being driven by several factors. First, the performance limitations of traditional single-solvent electrolytes have created a clear need for innovative solutions that address the polysulfide shuttle effect and lithium dendrite formation. Second, regulatory pressures toward more environmentally friendly battery technologies favor Li-S systems due to their use of abundant sulfur rather than critical minerals like cobalt.
Regional analysis shows that Asia-Pacific currently dominates the Li-S electrolyte research and development landscape, with China, South Korea, and Japan leading in patent applications. However, significant research activities are also underway in North America and Europe, particularly in Germany and the United Kingdom, where government funding for next-generation battery technologies has increased substantially.
Consumer electronics manufacturers represent an important early-adoption market segment, as they seek batteries with higher energy density for portable devices. This segment values the theoretical energy density of Li-S batteries (2600 Wh/kg) compared to conventional lithium-ion batteries (250-300 Wh/kg).
Market barriers for hybrid electrolytes include high production costs, scalability challenges, and competition from solid-state battery technologies. Current production costs for specialized hybrid electrolytes remain 3-5 times higher than conventional electrolytes, primarily due to complex synthesis processes and limited economies of scale.
Industry analysts predict that as manufacturing processes mature and production volumes increase, the cost differential between hybrid and conventional electrolytes will narrow significantly by 2027-2028, potentially accelerating market adoption. The market is also seeing increased partnership activity between electrolyte manufacturers, battery producers, and end-users, indicating growing commercial interest in bringing Li-S technology to market.
Current Challenges in Hybrid Electrolyte Development
Despite significant advancements in lithium-sulfur (Li-S) battery technology, the development of effective hybrid electrolytes continues to face substantial challenges. The polysulfide shuttle effect remains one of the most critical issues, where soluble lithium polysulfides migrate between electrodes during cycling, causing rapid capacity fading and shortened battery life. Current hybrid electrolyte systems struggle to completely suppress this phenomenon while maintaining adequate ionic conductivity.
The inherent chemical instability at the lithium metal anode interface presents another major obstacle. Hybrid electrolytes must simultaneously protect the highly reactive lithium metal from parasitic reactions while facilitating uniform lithium deposition to prevent dendrite formation. This dual requirement creates a complex design challenge that few current formulations can adequately address.
Viscosity management represents a significant engineering hurdle in hybrid electrolyte development. Many polymer components that effectively suppress polysulfide shuttling also increase system viscosity, negatively impacting ion transport kinetics and rate capability. This trade-off between polysulfide suppression and electrochemical performance continues to limit practical applications.
Temperature sensitivity further complicates hybrid electrolyte design. Many promising systems exhibit dramatic performance variations across different operating temperatures, with particular challenges in low-temperature environments where ion mobility decreases substantially. This temperature dependence restricts the potential application scenarios for Li-S batteries equipped with current hybrid electrolytes.
Long-term stability issues persist across most hybrid electrolyte formulations. The gradual degradation of electrolyte components through reactions with polysulfides, exposure to high voltages, or mechanical stress during cycling leads to diminishing performance over time. This stability challenge directly impacts the commercial viability of Li-S technology.
Manufacturing scalability presents additional complications. Many laboratory-demonstrated hybrid electrolytes utilize complex synthesis procedures or expensive materials that prove challenging to scale for mass production. The transition from promising research results to commercially viable manufacturing processes remains a significant barrier to widespread adoption.
Compatibility with existing battery manufacturing infrastructure represents another practical challenge. Novel hybrid electrolyte systems often require specialized handling procedures or modified cell designs that deviate from established lithium-ion battery production methods, increasing implementation costs and slowing industry adoption.
The inherent chemical instability at the lithium metal anode interface presents another major obstacle. Hybrid electrolytes must simultaneously protect the highly reactive lithium metal from parasitic reactions while facilitating uniform lithium deposition to prevent dendrite formation. This dual requirement creates a complex design challenge that few current formulations can adequately address.
Viscosity management represents a significant engineering hurdle in hybrid electrolyte development. Many polymer components that effectively suppress polysulfide shuttling also increase system viscosity, negatively impacting ion transport kinetics and rate capability. This trade-off between polysulfide suppression and electrochemical performance continues to limit practical applications.
Temperature sensitivity further complicates hybrid electrolyte design. Many promising systems exhibit dramatic performance variations across different operating temperatures, with particular challenges in low-temperature environments where ion mobility decreases substantially. This temperature dependence restricts the potential application scenarios for Li-S batteries equipped with current hybrid electrolytes.
Long-term stability issues persist across most hybrid electrolyte formulations. The gradual degradation of electrolyte components through reactions with polysulfides, exposure to high voltages, or mechanical stress during cycling leads to diminishing performance over time. This stability challenge directly impacts the commercial viability of Li-S technology.
Manufacturing scalability presents additional complications. Many laboratory-demonstrated hybrid electrolytes utilize complex synthesis procedures or expensive materials that prove challenging to scale for mass production. The transition from promising research results to commercially viable manufacturing processes remains a significant barrier to widespread adoption.
Compatibility with existing battery manufacturing infrastructure represents another practical challenge. Novel hybrid electrolyte systems often require specialized handling procedures or modified cell designs that deviate from established lithium-ion battery production methods, increasing implementation costs and slowing industry adoption.
Current Hybrid Electrolyte Design Approaches
01 Polymer-based hybrid electrolytes
Polymer-based hybrid electrolytes combine the advantages of solid polymer electrolytes with liquid components to enhance ionic conductivity while maintaining mechanical stability. These electrolytes typically incorporate polymers such as polyethylene oxide (PEO) or polyvinylidene fluoride (PVDF) with liquid electrolyte components. The polymer matrix provides structural support while the liquid phase facilitates lithium ion transport, addressing the shuttle effect in lithium-sulfur batteries and improving cycle life.- Solid-liquid hybrid electrolytes for lithium-sulfur batteries: Solid-liquid hybrid electrolytes combine the advantages of both solid and liquid electrolytes, offering improved ionic conductivity while suppressing the shuttle effect in lithium-sulfur batteries. These hybrid systems typically consist of a solid polymer or ceramic matrix infused with a liquid electrolyte component. This configuration helps to immobilize polysulfides, enhance lithium-ion transport, and improve the overall electrochemical performance and cycling stability of lithium-sulfur batteries.
- Ionic liquid-based hybrid electrolytes: Ionic liquid-based hybrid electrolytes offer unique advantages for lithium-sulfur batteries due to their negligible vapor pressure, wide electrochemical window, and ability to dissolve lithium salts. When combined with polymers or other materials to form hybrid electrolytes, ionic liquids can effectively suppress the polysulfide shuttle effect while maintaining good ionic conductivity. These electrolytes demonstrate enhanced thermal stability and safety characteristics compared to conventional liquid electrolytes, leading to improved battery performance and longevity.
- Polymer-based hybrid electrolytes with additives: Polymer-based hybrid electrolytes incorporating specific additives can significantly enhance the performance of lithium-sulfur batteries. These electrolytes typically combine a polymer matrix with liquid components and functional additives such as ceramic particles, metal-organic frameworks, or flame retardants. The additives help to improve ionic conductivity, mechanical strength, and thermal stability while creating physical barriers to prevent polysulfide migration. This approach results in better capacity retention, extended cycle life, and enhanced safety characteristics.
- Gel polymer electrolytes for lithium-sulfur batteries: Gel polymer electrolytes represent an important class of hybrid electrolytes for lithium-sulfur batteries, consisting of a polymer network swollen with liquid electrolyte. These electrolytes combine the cohesive properties of solids with the high ionic conductivity of liquids. The polymer network helps to trap polysulfides and prevent their migration, while the liquid component facilitates fast lithium-ion transport. Various polymers such as PEO, PVDF, and PAN are commonly used as the host matrix, offering different advantages in terms of mechanical properties and electrochemical stability.
- Ceramic-reinforced hybrid electrolytes: Ceramic-reinforced hybrid electrolytes incorporate inorganic ceramic particles into polymer or gel electrolyte systems to enhance the performance of lithium-sulfur batteries. The ceramic components, such as Al2O3, TiO2, or LLZO, improve mechanical strength, thermal stability, and lithium-ion conductivity while helping to immobilize polysulfides. These hybrid electrolytes create more efficient ion transport pathways and strengthen the electrode-electrolyte interface, resulting in reduced interfacial resistance, improved rate capability, and enhanced cycling performance of lithium-sulfur batteries.
02 Ionic liquid-based hybrid electrolytes
Ionic liquid-based hybrid electrolytes utilize room temperature ionic liquids combined with conventional electrolyte components to enhance the performance of lithium-sulfur batteries. These electrolytes offer high thermal stability, low volatility, and wide electrochemical windows. The ionic liquids help suppress polysulfide dissolution and migration, reducing capacity fade and improving cycling stability. Additionally, they can enhance the safety profile of the battery by reducing flammability risks.Expand Specific Solutions03 Gel polymer hybrid electrolytes
Gel polymer hybrid electrolytes combine the properties of solid and liquid electrolytes by trapping liquid electrolyte components within a gel polymer matrix. This approach provides improved ionic conductivity compared to solid polymer electrolytes while offering better mechanical stability than pure liquid systems. The gel structure helps contain polysulfides within the cathode region, mitigating the shuttle effect and enhancing capacity retention. Common gel-forming polymers include PVDF-HFP, PEO, and PMMA.Expand Specific Solutions04 Ceramic-reinforced hybrid electrolytes
Ceramic-reinforced hybrid electrolytes incorporate inorganic ceramic particles into polymer or liquid electrolyte systems to enhance mechanical strength and electrochemical performance. These ceramic fillers, such as Al2O3, SiO2, or LLZO, can improve the lithium-ion transport properties while providing physical barriers to polysulfide diffusion. The ceramic components also enhance the thermal stability and safety of the electrolyte system, making them particularly valuable for high-energy lithium-sulfur battery applications.Expand Specific Solutions05 Functional additive-enhanced hybrid electrolytes
Functional additive-enhanced hybrid electrolytes incorporate specific chemical additives to address the unique challenges of lithium-sulfur batteries. These additives include lithium nitrate, polysulfide mediators, and fluorinated compounds that form protective interfaces on the lithium anode or trap polysulfides at the cathode. By strategically combining these functional additives with conventional electrolyte systems, battery performance metrics such as coulombic efficiency, cycle life, and rate capability can be significantly improved while minimizing capacity fade.Expand Specific Solutions
Leading Companies and Research Institutions in Hybrid Electrolytes
The lithium-sulfur battery hybrid electrolyte market is in an early growth phase, characterized by intensive R&D activities and emerging commercialization efforts. Major industrial players like LG Energy Solution, Samsung SDI, and Johnson Matthey are investing heavily in this technology, recognizing its potential to deliver higher energy density than conventional lithium-ion batteries. Academic institutions including Central South University, KAIST, and University of Michigan are advancing fundamental research, while specialized companies like PolyPlus Battery and Nanotek Instruments focus on commercialization pathways. The market is expected to grow significantly as technical challenges around electrolyte stability and sulfur utilization are addressed. Current technology readiness level is transitioning from laboratory to early commercial applications, with automotive companies like Volkswagen showing interest in adoption for next-generation electric vehicles.
LG Chem Ltd.
Technical Solution: LG Chem has developed advanced hybrid electrolytes for lithium-sulfur batteries combining ionic liquids with conventional organic solvents. Their proprietary formulation incorporates lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) salt in a mixture of 1,3-dioxolane (DOL) and 1,2-dimethoxyethane (DME), enhanced with lithium nitrate (LiNO3) as a protective additive for the lithium metal anode. This electrolyte system effectively addresses the polysulfide shuttle effect through the formation of a stable solid electrolyte interphase (SEI) layer. LG Chem's hybrid electrolyte also features fluorinated ether components that enhance the solubility of lithium polysulfides while maintaining high ionic conductivity (>5 mS/cm at room temperature). Their recent innovations include incorporating polymer components like poly(ethylene oxide) to create gel-polymer hybrid electrolytes that provide mechanical stability while maintaining excellent electrochemical performance across wide temperature ranges.
Strengths: Superior ionic conductivity and electrochemical stability window (>4V vs Li/Li+). Effective suppression of polysulfide shuttling through optimized SEI formation. Weaknesses: Higher production costs compared to conventional electrolytes. Potential scalability challenges for mass production due to complex formulation processes.
SAMSUNG SDI CO LTD
Technical Solution: Samsung SDI has pioneered a multi-functional hybrid electrolyte system for lithium-sulfur batteries that combines solid and liquid components. Their approach utilizes a solid polymer matrix based on modified polyethylene oxide (PEO) infused with an optimized liquid electrolyte containing lithium salts and functional additives. The solid component provides mechanical support and helps contain polysulfides, while the liquid component ensures high ionic conductivity. Samsung's proprietary electrolyte incorporates lithium bis(fluorosulfonyl)imide (LiFSI) salt and lithium nitrate in a carefully balanced solvent mixture of 1,2-dimethoxyethane and 1,3-dioxolane. This formulation creates a stable interface on both the sulfur cathode and lithium anode surfaces. Additionally, Samsung has integrated nano-sized ceramic fillers (Al2O3, SiO2) into their hybrid electrolyte to enhance mechanical properties and create tortuous pathways that physically restrict polysulfide migration, effectively addressing the shuttle effect that typically plagues lithium-sulfur systems.
Strengths: Excellent thermal stability across wide temperature range (-20°C to 60°C). Superior polysulfide trapping capability through physical and chemical mechanisms. Weaknesses: Complex manufacturing process requiring precise control of multiple components. Potential long-term stability issues under extreme cycling conditions.
Key Patents and Innovations in Hybrid Electrolyte Technology
Methods For Stabilizing A Garnet-Electron Pair Donor Hybrid Electrolyte For A Lithium-Sulfur Battery
PatentPendingUS20240372150A1
Innovation
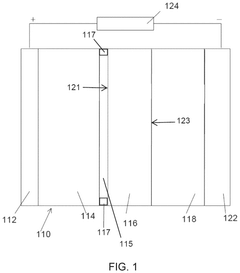
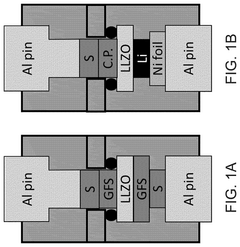
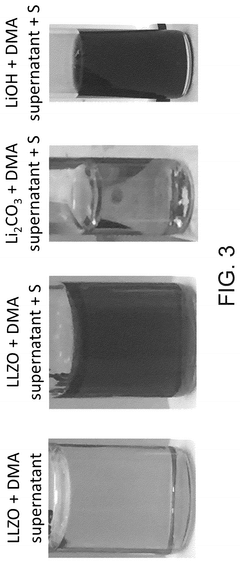
- A hybrid electrolyte system is developed, comprising a solid-state electrolyte with an acid-treated surface and a liquid electrolyte containing an alkali metal salt and an electron pair donor solvent, such as N,N-dimethylacetamide, to stabilize the interface and improve ionic transport.
Hybrid GEL polymer electrolytes and catholytes for lithium-sulfur batteries
PatentWO2024118412A1
Innovation
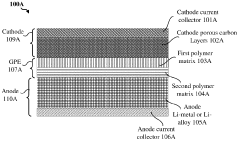
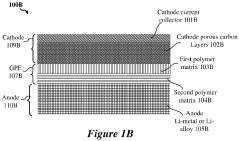
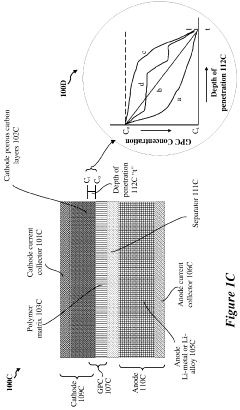
- A hybrid gel polymer electrolyte system is introduced, comprising a first polymer matrix trapping lithium-polysulfides and a second matrix confining them, along with a liquid fluorinated ether electrolyte dispersed in both matrices, which acts as a separator to prevent polysulfide migration and enhance lithium-ion conductivity.
Environmental Impact and Sustainability Considerations
The development of hybrid electrolytes for lithium-sulfur batteries must be evaluated not only for performance but also for their environmental impact and sustainability profile. Current lithium-ion battery technologies rely heavily on materials with significant environmental footprints, including cobalt and nickel, which face ethical mining concerns and resource scarcity. Lithium-sulfur batteries offer a promising alternative with sulfur being abundant, inexpensive, and less environmentally harmful, but the electrolyte components require careful consideration.
Conventional electrolytes for lithium-sulfur batteries often contain fluorinated salts and toxic organic solvents that pose environmental risks throughout their lifecycle. These components can lead to harmful emissions during production, potential leakage during use, and challenges in recycling and disposal. Hybrid electrolytes, which combine different types of electrolyte systems, present an opportunity to address these concerns while maintaining or improving battery performance.
Recent research has focused on developing greener alternatives for electrolyte components. Water-based or aqueous components in hybrid electrolytes can reduce the dependency on volatile organic solvents. Similarly, ionic liquids, though currently expensive, offer non-flammability and lower toxicity. Bio-derived solvents and polymers from renewable sources are emerging as sustainable options for the organic components of hybrid electrolytes.
Life cycle assessment (LCA) studies indicate that hybrid electrolytes can potentially reduce the carbon footprint of lithium-sulfur batteries by 15-30% compared to conventional electrolytes. This reduction stems from lower energy requirements during production and the use of more environmentally benign materials. However, comprehensive LCA data specific to various hybrid electrolyte formulations remains limited, highlighting a critical research gap.
The recyclability of hybrid electrolytes presents both challenges and opportunities. The multi-component nature of these systems can complicate separation processes, but their design can incorporate features that facilitate easier disassembly and material recovery. Some polymer-based components in hybrid electrolytes show promising recyclability characteristics, with potential recovery rates exceeding 70% in laboratory settings.
Regulatory frameworks worldwide are increasingly emphasizing battery sustainability. The European Union's Battery Directive and similar initiatives in North America and Asia are driving the industry toward greener battery technologies. Hybrid electrolytes that align with these regulatory trends will likely gain market advantage, particularly as carbon pricing mechanisms and extended producer responsibility policies become more prevalent.
Future research directions should prioritize developing hybrid electrolytes with components that are not only high-performing but also biodegradable, derived from renewable sources, and manufactured through energy-efficient processes. Balancing these sustainability considerations with the technical requirements of lithium-sulfur batteries represents a key challenge for advancing this promising technology toward commercial viability and environmental responsibility.
Conventional electrolytes for lithium-sulfur batteries often contain fluorinated salts and toxic organic solvents that pose environmental risks throughout their lifecycle. These components can lead to harmful emissions during production, potential leakage during use, and challenges in recycling and disposal. Hybrid electrolytes, which combine different types of electrolyte systems, present an opportunity to address these concerns while maintaining or improving battery performance.
Recent research has focused on developing greener alternatives for electrolyte components. Water-based or aqueous components in hybrid electrolytes can reduce the dependency on volatile organic solvents. Similarly, ionic liquids, though currently expensive, offer non-flammability and lower toxicity. Bio-derived solvents and polymers from renewable sources are emerging as sustainable options for the organic components of hybrid electrolytes.
Life cycle assessment (LCA) studies indicate that hybrid electrolytes can potentially reduce the carbon footprint of lithium-sulfur batteries by 15-30% compared to conventional electrolytes. This reduction stems from lower energy requirements during production and the use of more environmentally benign materials. However, comprehensive LCA data specific to various hybrid electrolyte formulations remains limited, highlighting a critical research gap.
The recyclability of hybrid electrolytes presents both challenges and opportunities. The multi-component nature of these systems can complicate separation processes, but their design can incorporate features that facilitate easier disassembly and material recovery. Some polymer-based components in hybrid electrolytes show promising recyclability characteristics, with potential recovery rates exceeding 70% in laboratory settings.
Regulatory frameworks worldwide are increasingly emphasizing battery sustainability. The European Union's Battery Directive and similar initiatives in North America and Asia are driving the industry toward greener battery technologies. Hybrid electrolytes that align with these regulatory trends will likely gain market advantage, particularly as carbon pricing mechanisms and extended producer responsibility policies become more prevalent.
Future research directions should prioritize developing hybrid electrolytes with components that are not only high-performing but also biodegradable, derived from renewable sources, and manufactured through energy-efficient processes. Balancing these sustainability considerations with the technical requirements of lithium-sulfur batteries represents a key challenge for advancing this promising technology toward commercial viability and environmental responsibility.
Safety Standards and Performance Metrics for Li-S Electrolytes
The development of lithium-sulfur (Li-S) batteries necessitates rigorous safety standards and performance metrics for hybrid electrolytes, as these components significantly influence both operational safety and electrochemical performance. Current safety standards for Li-S electrolytes primarily focus on thermal stability, with requirements that electrolytes maintain stability at temperatures ranging from -20°C to 60°C under normal operating conditions, and resist decomposition up to 150°C during thermal runaway scenarios.
Flammability metrics represent another critical safety parameter, with industry standards increasingly demanding non-flammable or flame-retardant electrolyte formulations. Modern hybrid electrolytes incorporating fluorinated solvents or ionic liquids have demonstrated significant improvements in this area, with flash points exceeding 100°C compared to conventional carbonate-based systems (typically 30-40°C).
Toxicity assessments have become increasingly stringent, with regulatory frameworks requiring comprehensive hazard evaluations according to GHS (Globally Harmonized System) classifications. Hybrid electrolytes must achieve at least Category 4 or lower toxicity ratings to meet current market expectations, with particular emphasis on reduced volatile organic compound (VOC) content.
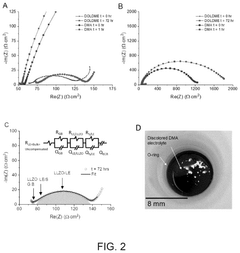
Performance metrics for Li-S electrolytes focus primarily on ionic conductivity, with minimum thresholds of 1-5 mS/cm at room temperature being essential for practical applications. The polysulfide shuttle inhibition capability represents a specialized metric unique to Li-S systems, quantified through shuttle current measurements during extended cycling, with values below 0.05 mA/cm² considered acceptable for commercial viability.
Electrochemical stability windows must exceed 3V (vs. Li/Li+) to accommodate the Li-S redox chemistry, while interface stability metrics evaluate the formation and stability of the solid electrolyte interphase (SEI) layer, typically assessed through impedance spectroscopy measurements over hundreds of cycles.
Cycle life requirements have intensified, with current standards demanding electrolyte stability supporting at least 500 cycles with capacity retention above 80%. Temperature performance metrics specify functionality across -20°C to 60°C, with conductivity retention of at least 50% at temperature extremes compared to room temperature values.
These standards continue to evolve as Li-S technology advances toward commercialization, with increasing emphasis on sustainability metrics including recyclability and environmental impact assessments becoming integral to next-generation electrolyte development frameworks.
Flammability metrics represent another critical safety parameter, with industry standards increasingly demanding non-flammable or flame-retardant electrolyte formulations. Modern hybrid electrolytes incorporating fluorinated solvents or ionic liquids have demonstrated significant improvements in this area, with flash points exceeding 100°C compared to conventional carbonate-based systems (typically 30-40°C).
Toxicity assessments have become increasingly stringent, with regulatory frameworks requiring comprehensive hazard evaluations according to GHS (Globally Harmonized System) classifications. Hybrid electrolytes must achieve at least Category 4 or lower toxicity ratings to meet current market expectations, with particular emphasis on reduced volatile organic compound (VOC) content.
Performance metrics for Li-S electrolytes focus primarily on ionic conductivity, with minimum thresholds of 1-5 mS/cm at room temperature being essential for practical applications. The polysulfide shuttle inhibition capability represents a specialized metric unique to Li-S systems, quantified through shuttle current measurements during extended cycling, with values below 0.05 mA/cm² considered acceptable for commercial viability.
Electrochemical stability windows must exceed 3V (vs. Li/Li+) to accommodate the Li-S redox chemistry, while interface stability metrics evaluate the formation and stability of the solid electrolyte interphase (SEI) layer, typically assessed through impedance spectroscopy measurements over hundreds of cycles.
Cycle life requirements have intensified, with current standards demanding electrolyte stability supporting at least 500 cycles with capacity retention above 80%. Temperature performance metrics specify functionality across -20°C to 60°C, with conductivity retention of at least 50% at temperature extremes compared to room temperature values.
These standards continue to evolve as Li-S technology advances toward commercialization, with increasing emphasis on sustainability metrics including recyclability and environmental impact assessments becoming integral to next-generation electrolyte development frameworks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!