Comparison of ether-based and carbonate-based electrolytes in Li–S systems
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrolyte Evolution in Li-S Battery Systems
The evolution of electrolytes in lithium-sulfur (Li-S) battery systems represents a critical development trajectory in advanced energy storage technology. Initially, conventional carbonate-based electrolytes, which had proven successful in lithium-ion batteries, were applied to Li-S systems. However, researchers quickly discovered their limitations due to nucleophilic reactions between polysulfides and carbonate solvents, leading to capacity degradation and shortened battery life.
This recognition prompted a significant shift toward ether-based electrolytes in the early 2010s, particularly 1,3-dioxolane (DOL) and 1,2-dimethoxyethane (DME) mixtures. These solvents demonstrated superior compatibility with sulfur electrochemistry, offering enhanced polysulfide solubility and reduced side reactions. The addition of lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) as a salt and lithium nitrate (LiNO3) as an additive became standard practice, forming a protective layer on the lithium anode surface.
By mid-2010s, researchers began exploring concentrated electrolyte systems, often referred to as "high-concentration" or "solvent-in-salt" electrolytes. These formulations, featuring higher salt-to-solvent ratios, demonstrated improved polysulfide confinement and enhanced cycling stability, albeit with increased viscosity and cost considerations.
The late 2010s witnessed the emergence of localized high-concentration electrolytes (LHCEs) and dual-salt systems, which balanced the benefits of concentrated electrolytes while mitigating their drawbacks. These advanced formulations incorporated co-solvents or diluents to reduce viscosity while maintaining favorable electrochemical properties.
Most recently, solid-state and quasi-solid electrolytes have gained prominence, offering potential solutions to the polysulfide shuttle effect through physical containment. Polymer electrolytes, ceramic electrolytes, and composite systems combining both have shown promising results in laboratory settings, though challenges in ionic conductivity and interfacial resistance remain.
Parallel to these developments, functional additives have evolved significantly. Beyond LiNO3, researchers have explored polysulfide mediators, redox shuttles, and interface modifiers to enhance electrochemical performance and stability. The incorporation of ionic liquids as co-solvents or additives has also emerged as a strategy to improve thermal stability and safety characteristics.
The most recent frontier involves the development of localized high-concentration electrolytes with fluorinated solvents and the exploration of dual-function electrolytes that simultaneously address multiple challenges in Li-S systems, including polysulfide shuttling, lithium dendrite formation, and interfacial stability.
This recognition prompted a significant shift toward ether-based electrolytes in the early 2010s, particularly 1,3-dioxolane (DOL) and 1,2-dimethoxyethane (DME) mixtures. These solvents demonstrated superior compatibility with sulfur electrochemistry, offering enhanced polysulfide solubility and reduced side reactions. The addition of lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) as a salt and lithium nitrate (LiNO3) as an additive became standard practice, forming a protective layer on the lithium anode surface.
By mid-2010s, researchers began exploring concentrated electrolyte systems, often referred to as "high-concentration" or "solvent-in-salt" electrolytes. These formulations, featuring higher salt-to-solvent ratios, demonstrated improved polysulfide confinement and enhanced cycling stability, albeit with increased viscosity and cost considerations.
The late 2010s witnessed the emergence of localized high-concentration electrolytes (LHCEs) and dual-salt systems, which balanced the benefits of concentrated electrolytes while mitigating their drawbacks. These advanced formulations incorporated co-solvents or diluents to reduce viscosity while maintaining favorable electrochemical properties.
Most recently, solid-state and quasi-solid electrolytes have gained prominence, offering potential solutions to the polysulfide shuttle effect through physical containment. Polymer electrolytes, ceramic electrolytes, and composite systems combining both have shown promising results in laboratory settings, though challenges in ionic conductivity and interfacial resistance remain.
Parallel to these developments, functional additives have evolved significantly. Beyond LiNO3, researchers have explored polysulfide mediators, redox shuttles, and interface modifiers to enhance electrochemical performance and stability. The incorporation of ionic liquids as co-solvents or additives has also emerged as a strategy to improve thermal stability and safety characteristics.
The most recent frontier involves the development of localized high-concentration electrolytes with fluorinated solvents and the exploration of dual-function electrolytes that simultaneously address multiple challenges in Li-S systems, including polysulfide shuttling, lithium dendrite formation, and interfacial stability.
Market Analysis for Li-S Battery Electrolytes
The global market for lithium-sulfur (Li-S) battery electrolytes is experiencing significant growth, driven by the increasing demand for high-energy-density energy storage solutions across various industries. Current market valuations indicate that the Li-S battery market is projected to grow at a compound annual growth rate of over 30% between 2023 and 2030, with electrolytes representing approximately 15-20% of the total battery component market value.
The demand for Li-S battery electrolytes is primarily fueled by applications requiring lightweight, high-capacity energy storage systems. The aerospace and defense sectors currently represent the largest market segment, valuing the exceptional theoretical energy density of Li-S batteries (2600 Wh/kg) compared to conventional lithium-ion batteries (250-300 Wh/kg). Electric vehicle manufacturers are increasingly exploring Li-S technology as a potential solution for range anxiety, creating a rapidly expanding secondary market.
Market research indicates a clear preference shift toward ether-based electrolytes in commercial Li-S battery developments. This trend is supported by performance data showing that ether-based systems deliver superior cycle life and capacity retention compared to carbonate-based alternatives. Major battery manufacturers report that Li-S cells with ether-based electrolytes typically achieve 300-500 cycles at practical energy densities exceeding 400 Wh/kg, while carbonate-based systems struggle to maintain performance beyond 100-200 cycles.
Regional market analysis reveals that Asia-Pacific dominates the Li-S electrolyte market, with China, South Korea, and Japan collectively accounting for over 60% of global production capacity. European markets are showing the fastest growth rate, driven by stringent environmental regulations and substantial government investments in next-generation battery technologies. North American market share is expected to expand significantly as domestic battery production increases in response to supply chain security concerns.
Price sensitivity analysis indicates that electrolyte costs represent a critical factor in overall Li-S battery economics. Current production-scale ether-based electrolytes command a 30-40% premium over carbonate-based alternatives, primarily due to higher purity requirements and more complex synthesis processes. However, this price differential is projected to narrow as manufacturing scales increase and new synthesis routes are commercialized.
Market barriers for wider Li-S electrolyte adoption include concerns about long-term stability, temperature performance limitations, and manufacturing scalability. Industry surveys indicate that battery manufacturers require electrolyte solutions that can enable at least 1000 cycles while maintaining 80% capacity retention before considering mass production for automotive applications.
The competitive landscape features both established chemical companies expanding into specialized electrolyte formulations and startups focused exclusively on novel Li-S electrolyte technologies. Strategic partnerships between electrolyte suppliers and battery manufacturers are becoming increasingly common as the industry works to accelerate commercialization timelines.
The demand for Li-S battery electrolytes is primarily fueled by applications requiring lightweight, high-capacity energy storage systems. The aerospace and defense sectors currently represent the largest market segment, valuing the exceptional theoretical energy density of Li-S batteries (2600 Wh/kg) compared to conventional lithium-ion batteries (250-300 Wh/kg). Electric vehicle manufacturers are increasingly exploring Li-S technology as a potential solution for range anxiety, creating a rapidly expanding secondary market.
Market research indicates a clear preference shift toward ether-based electrolytes in commercial Li-S battery developments. This trend is supported by performance data showing that ether-based systems deliver superior cycle life and capacity retention compared to carbonate-based alternatives. Major battery manufacturers report that Li-S cells with ether-based electrolytes typically achieve 300-500 cycles at practical energy densities exceeding 400 Wh/kg, while carbonate-based systems struggle to maintain performance beyond 100-200 cycles.
Regional market analysis reveals that Asia-Pacific dominates the Li-S electrolyte market, with China, South Korea, and Japan collectively accounting for over 60% of global production capacity. European markets are showing the fastest growth rate, driven by stringent environmental regulations and substantial government investments in next-generation battery technologies. North American market share is expected to expand significantly as domestic battery production increases in response to supply chain security concerns.
Price sensitivity analysis indicates that electrolyte costs represent a critical factor in overall Li-S battery economics. Current production-scale ether-based electrolytes command a 30-40% premium over carbonate-based alternatives, primarily due to higher purity requirements and more complex synthesis processes. However, this price differential is projected to narrow as manufacturing scales increase and new synthesis routes are commercialized.
Market barriers for wider Li-S electrolyte adoption include concerns about long-term stability, temperature performance limitations, and manufacturing scalability. Industry surveys indicate that battery manufacturers require electrolyte solutions that can enable at least 1000 cycles while maintaining 80% capacity retention before considering mass production for automotive applications.
The competitive landscape features both established chemical companies expanding into specialized electrolyte formulations and startups focused exclusively on novel Li-S electrolyte technologies. Strategic partnerships between electrolyte suppliers and battery manufacturers are becoming increasingly common as the industry works to accelerate commercialization timelines.
Technical Challenges in Ether vs Carbonate Electrolytes
The development of lithium-sulfur (Li-S) batteries faces significant technical challenges, particularly in the selection and optimization of electrolyte systems. Both ether-based and carbonate-based electrolytes present distinct advantages and limitations that critically impact battery performance, with neither offering a comprehensive solution to all existing challenges.
Ether-based electrolytes, predominantly represented by 1,3-dioxolane (DOL) and 1,2-dimethoxyethane (DME) mixtures, demonstrate superior compatibility with sulfur cathodes. However, they suffer from high volatility and low flash points, raising serious safety concerns for commercial applications. Additionally, their limited oxidative stability (typically below 4V) restricts their application in high-voltage systems, limiting the energy density potential of Li-S batteries.
Carbonate-based electrolytes, while offering excellent stability with conventional lithium-ion battery components, face severe compatibility issues with sulfur cathodes. The primary challenge lies in the nucleophilic reaction between polysulfides and carbonate solvents, leading to irreversible degradation of the electrolyte and rapid capacity fading. This fundamental chemical incompatibility has historically limited their application in Li-S systems despite their favorable properties.
The shuttle effect—the migration of soluble polysulfides between electrodes—presents a significant challenge in both electrolyte systems but manifests differently. In ether-based electrolytes, the high solubility of polysulfides exacerbates the shuttle effect, while in carbonate-based systems, the chemical reactions with polysulfides create different degradation pathways that equally impact cycling stability.
Interface stability represents another critical challenge. Ether-based electrolytes typically form unstable solid electrolyte interphases (SEI) on lithium anodes, leading to continuous lithium consumption and dendrite formation. Carbonate-based electrolytes form more stable SEIs but suffer from poor ionic conductivity for polysulfide species, creating high internal resistance.
Temperature performance diverges significantly between these electrolyte systems. Ether-based electrolytes demonstrate poor low-temperature performance due to increased viscosity and reduced ionic conductivity, while carbonate-based systems maintain better conductivity at low temperatures but suffer accelerated degradation at elevated temperatures.
Recent research has focused on hybrid electrolyte systems and advanced additives to address these challenges, but fundamental limitations persist. The development of novel electrolyte formulations that combine the advantages of both systems while mitigating their respective drawbacks remains one of the most significant technical hurdles in advancing Li-S battery technology toward commercial viability.
Ether-based electrolytes, predominantly represented by 1,3-dioxolane (DOL) and 1,2-dimethoxyethane (DME) mixtures, demonstrate superior compatibility with sulfur cathodes. However, they suffer from high volatility and low flash points, raising serious safety concerns for commercial applications. Additionally, their limited oxidative stability (typically below 4V) restricts their application in high-voltage systems, limiting the energy density potential of Li-S batteries.
Carbonate-based electrolytes, while offering excellent stability with conventional lithium-ion battery components, face severe compatibility issues with sulfur cathodes. The primary challenge lies in the nucleophilic reaction between polysulfides and carbonate solvents, leading to irreversible degradation of the electrolyte and rapid capacity fading. This fundamental chemical incompatibility has historically limited their application in Li-S systems despite their favorable properties.
The shuttle effect—the migration of soluble polysulfides between electrodes—presents a significant challenge in both electrolyte systems but manifests differently. In ether-based electrolytes, the high solubility of polysulfides exacerbates the shuttle effect, while in carbonate-based systems, the chemical reactions with polysulfides create different degradation pathways that equally impact cycling stability.
Interface stability represents another critical challenge. Ether-based electrolytes typically form unstable solid electrolyte interphases (SEI) on lithium anodes, leading to continuous lithium consumption and dendrite formation. Carbonate-based electrolytes form more stable SEIs but suffer from poor ionic conductivity for polysulfide species, creating high internal resistance.
Temperature performance diverges significantly between these electrolyte systems. Ether-based electrolytes demonstrate poor low-temperature performance due to increased viscosity and reduced ionic conductivity, while carbonate-based systems maintain better conductivity at low temperatures but suffer accelerated degradation at elevated temperatures.
Recent research has focused on hybrid electrolyte systems and advanced additives to address these challenges, but fundamental limitations persist. The development of novel electrolyte formulations that combine the advantages of both systems while mitigating their respective drawbacks remains one of the most significant technical hurdles in advancing Li-S battery technology toward commercial viability.
Industry Leaders in Li-S Electrolyte Research
The lithium-sulfur (Li-S) battery market is currently in an early growth phase, with a projected market size of $1-2 billion by 2025, expanding at a CAGR of approximately 30%. The competition between ether-based and carbonate-based electrolytes represents a critical technological battleground in this emerging sector. Major research institutions like Drexel University, Central South University, and Tsinghua University are advancing fundamental science, while commercial players demonstrate varying levels of technological maturity. Companies like CATL, BYD, and LG Chem have established strong positions in electrolyte development, with StoreDot and SES Holdings emerging as specialized innovators focusing on novel electrolyte formulations. The industry is witnessing increased patent activity and strategic partnerships as companies seek to overcome key challenges in electrolyte stability, polysulfide shuttling, and compatibility with lithium metal anodes.
BYD Co., Ltd.
Technical Solution: BYD has developed an innovative hybrid electrolyte system for Li-S batteries that combines localized ether-based and carbonate-based components. Their approach utilizes a concentration gradient electrolyte where a high-concentration lithium salt solution creates distinct solvation structures near the electrodes. Near the sulfur cathode, the electrolyte behaves similar to ether-based systems, facilitating controlled polysulfide dissolution and redox reactions, while near the lithium anode, it forms carbonate-like SEI structures. BYD's system employs a mixture of 1,3-dioxolane (DOL), 1,2-dimethoxyethane (DME), and small amounts of fluorinated carbonates with lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) salt at concentrations exceeding 3M. This high salt concentration alters the solvation structure of the electrolyte, creating a quasi-solid electrolyte interphase that suppresses the polysulfide shuttle effect. Testing has demonstrated that BYD's hybrid electrolyte enables Li-S cells to achieve over 600 cycles with capacity retention above 75% at 1C rates, while maintaining discharge capacities of approximately 900 mAh/g, significantly outperforming conventional single-solvent electrolyte systems.
Strengths: The gradient electrolyte design effectively addresses both cathode and anode interface issues simultaneously without requiring complex cell designs or separators. The high salt concentration approach provides enhanced safety by reducing electrolyte flammability. Weaknesses: The high concentration of lithium salts significantly increases the cost of the electrolyte system and reduces its ionic conductivity, potentially limiting power performance. The viscosity of the high-concentration electrolyte also presents challenges for electrolyte infiltration during cell manufacturing.
Honda Motor Co., Ltd.
Technical Solution: Honda Motor Co. has developed a localized electrolyte configuration for Li-S batteries that strategically employs both ether-based and carbonate-based electrolytes in different cell regions. Their approach utilizes a dual-layer separator system where the cathode side contains a porous membrane infused with an ether-based electrolyte (typically DOL/DME with LiTFSI and LiNO3), while the anode side features a dense polymer layer containing a carbonate-based electrolyte (EC/DEC with LiPF6). This configuration creates distinct electrochemical environments optimized for each electrode while maintaining ionic conductivity between them. Honda's research demonstrates that this segregated electrolyte design effectively prevents polysulfide migration to the anode while forming a stable SEI layer on the lithium metal surface. Their testing shows that cells with this dual-electrolyte configuration achieve initial discharge capacities of approximately 1300 mAh/g with capacity retention exceeding 85% after 200 cycles at 0.2C. Additionally, Honda has incorporated flame-retardant additives into the carbonate layer, enhancing the overall safety profile of the battery system compared to conventional ether-only electrolytes that present significant flammability concerns.
Strengths: The dual-layer separator design effectively isolates the incompatible electrolyte systems while maintaining ionic conductivity, providing optimal environments for both electrodes. The incorporation of flame-retardant additives significantly improves safety compared to conventional ether-based systems. Weaknesses: The complex separator manufacturing process increases production costs and presents challenges for scaling to mass production. The interface between the two electrolyte regions may degrade over time, potentially leading to performance decline after extended cycling.
Key Patents in Ether and Carbonate Electrolyte Technology
Ultra-conformal fluorinated polymer coating on li-metal by solid-liquid-solid phase conversion using physical treatment
PatentWO2023212538A1
Innovation
- A method involving the application of a fluoropolymer film to the lithium metal surface, followed by pressure and solvent treatment to form an artificial solid electrolyte interphase (SEI) protected anode, using a combination of fluorinated polymers like poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) and dimethyl formamide (DMF) to create a conformal LiF coating, enhancing Li-metal stability and sulfur loading.
Carbonate-based electrolyte, preparation method thereof, and lithium metal battery
PatentActiveTW202347860A
Innovation
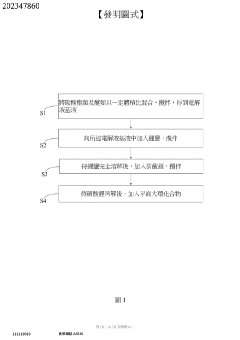
- A carbonate electrolyte containing lithium nitrate and planar macrocyclic compounds is formulated, with specific volume and concentration ratios, to promote the formation of a stable SEI layer, inhibiting dendrite growth and enhancing lithium ion conductivity.
Safety and Stability Considerations for Li-S Electrolytes
Safety considerations in Li-S battery electrolytes are paramount due to the reactive nature of lithium and sulfur components. Ether-based electrolytes, while offering superior compatibility with polysulfide intermediates, present significant safety concerns due to their low flash points and high volatility. For instance, common ether solvents like 1,3-dioxolane (DOL) and 1,2-dimethoxyethane (DME) have flash points of 2°C and -2°C respectively, creating potential fire hazards during battery operation, especially under thermal runaway conditions.
Carbonate-based electrolytes demonstrate superior thermal stability with significantly higher flash points (e.g., ethylene carbonate at 143°C), reducing fire risks substantially. However, their reactivity with polysulfide intermediates generates safety concerns of a different nature - gas evolution and pressure build-up within cells that can lead to mechanical failures or explosions in severe cases.
The stability of electrolytes directly impacts both performance and safety profiles. Ether-based systems suffer from continuous decomposition during cycling, particularly at elevated temperatures, generating volatile byproducts that increase internal cell pressure. This decomposition accelerates capacity fading while simultaneously increasing safety risks through gas accumulation.
Carbonate electrolytes, despite their thermal advantages, undergo nucleophilic attacks from polysulfide species, creating unstable interfaces that compromise long-term cycling stability. This chemical instability manifests as rapid capacity decay and can trigger exothermic reactions under certain conditions, potentially leading to thermal runaway events.
Recent research has focused on enhancing safety through additives that improve thermal stability without compromising electrochemical performance. Flame-retardant additives like trimethyl phosphate (TMP) and fluorinated compounds have shown promise in ether systems, raising flash points while maintaining compatibility with sulfur electrochemistry. Similarly, stabilizing additives like lithium nitrate (LiNO₃) improve the stability of solid-electrolyte interphase layers in both electrolyte systems.
Hybrid electrolyte systems combining carefully selected ethers and carbonates represent an emerging approach to balance safety and performance requirements. These formulations aim to leverage the polysulfide solubility of ethers while benefiting from the thermal stability of carbonates, though optimization remains challenging due to complex interactions between solvents and active materials.
The development of solid-state or gel polymer electrolytes offers perhaps the most promising path toward inherently safer Li-S batteries, eliminating flammable liquid components entirely while potentially addressing polysulfide shuttle effects through physical containment mechanisms.
Carbonate-based electrolytes demonstrate superior thermal stability with significantly higher flash points (e.g., ethylene carbonate at 143°C), reducing fire risks substantially. However, their reactivity with polysulfide intermediates generates safety concerns of a different nature - gas evolution and pressure build-up within cells that can lead to mechanical failures or explosions in severe cases.
The stability of electrolytes directly impacts both performance and safety profiles. Ether-based systems suffer from continuous decomposition during cycling, particularly at elevated temperatures, generating volatile byproducts that increase internal cell pressure. This decomposition accelerates capacity fading while simultaneously increasing safety risks through gas accumulation.
Carbonate electrolytes, despite their thermal advantages, undergo nucleophilic attacks from polysulfide species, creating unstable interfaces that compromise long-term cycling stability. This chemical instability manifests as rapid capacity decay and can trigger exothermic reactions under certain conditions, potentially leading to thermal runaway events.
Recent research has focused on enhancing safety through additives that improve thermal stability without compromising electrochemical performance. Flame-retardant additives like trimethyl phosphate (TMP) and fluorinated compounds have shown promise in ether systems, raising flash points while maintaining compatibility with sulfur electrochemistry. Similarly, stabilizing additives like lithium nitrate (LiNO₃) improve the stability of solid-electrolyte interphase layers in both electrolyte systems.
Hybrid electrolyte systems combining carefully selected ethers and carbonates represent an emerging approach to balance safety and performance requirements. These formulations aim to leverage the polysulfide solubility of ethers while benefiting from the thermal stability of carbonates, though optimization remains challenging due to complex interactions between solvents and active materials.
The development of solid-state or gel polymer electrolytes offers perhaps the most promising path toward inherently safer Li-S batteries, eliminating flammable liquid components entirely while potentially addressing polysulfide shuttle effects through physical containment mechanisms.
Environmental Impact of Different Electrolyte Chemistries
The environmental impact of electrolyte chemistries in Li-S battery systems represents a critical consideration for sustainable energy storage development. Ether-based and carbonate-based electrolytes exhibit significantly different environmental footprints throughout their lifecycle, from raw material extraction to disposal.
Ether-based electrolytes, primarily composed of compounds like 1,3-dioxolane (DOL) and 1,2-dimethoxyethane (DME), generally require less energy-intensive synthesis processes compared to their carbonate counterparts. The production of these ethers typically generates lower greenhouse gas emissions, with recent lifecycle assessments indicating approximately 15-20% reduction in carbon footprint during manufacturing stages.
Carbonate-based electrolytes, including ethylene carbonate (EC) and dimethyl carbonate (DMC), present higher environmental concerns during production due to more complex synthesis routes and higher energy requirements. These processes often involve toxic intermediates and generate more hazardous waste streams, contributing to increased environmental burden.
From a toxicity perspective, ether-based electrolytes demonstrate lower aquatic toxicity profiles when compared to carbonate alternatives. Studies have shown that accidental releases of ether-based electrolytes result in less severe impacts on aquatic ecosystems, with EC and DMC showing persistence in water bodies and potential bioaccumulation effects not observed with ethers.
Regarding end-of-life considerations, both electrolyte types present recycling challenges. However, ether-based systems typically decompose more readily under environmental conditions, reducing long-term contamination risks. Carbonate electrolytes exhibit greater stability, potentially leading to extended environmental persistence when improperly disposed.
The solvent-to-salt ratio also influences environmental impact, with ether-based systems generally requiring higher salt concentrations (particularly LiTFSI), which increases the demand for lithium resources. This higher lithium content presents both resource depletion concerns and potential recovery opportunities in recycling processes.
Recent advancements in green chemistry approaches have focused on developing bio-derived alternatives for both electrolyte families. Ether compounds synthesized from biomass feedstocks show particular promise, potentially reducing the environmental footprint by up to 40% compared to petroleum-derived counterparts. Similar initiatives for carbonates have shown more limited success due to their more complex molecular structures.
Water consumption represents another significant environmental factor, with carbonate electrolyte production typically requiring 1.5-2 times more process water than ether-based alternatives. This difference becomes particularly relevant in water-stressed regions where battery manufacturing facilities may operate.
Ether-based electrolytes, primarily composed of compounds like 1,3-dioxolane (DOL) and 1,2-dimethoxyethane (DME), generally require less energy-intensive synthesis processes compared to their carbonate counterparts. The production of these ethers typically generates lower greenhouse gas emissions, with recent lifecycle assessments indicating approximately 15-20% reduction in carbon footprint during manufacturing stages.
Carbonate-based electrolytes, including ethylene carbonate (EC) and dimethyl carbonate (DMC), present higher environmental concerns during production due to more complex synthesis routes and higher energy requirements. These processes often involve toxic intermediates and generate more hazardous waste streams, contributing to increased environmental burden.
From a toxicity perspective, ether-based electrolytes demonstrate lower aquatic toxicity profiles when compared to carbonate alternatives. Studies have shown that accidental releases of ether-based electrolytes result in less severe impacts on aquatic ecosystems, with EC and DMC showing persistence in water bodies and potential bioaccumulation effects not observed with ethers.
Regarding end-of-life considerations, both electrolyte types present recycling challenges. However, ether-based systems typically decompose more readily under environmental conditions, reducing long-term contamination risks. Carbonate electrolytes exhibit greater stability, potentially leading to extended environmental persistence when improperly disposed.
The solvent-to-salt ratio also influences environmental impact, with ether-based systems generally requiring higher salt concentrations (particularly LiTFSI), which increases the demand for lithium resources. This higher lithium content presents both resource depletion concerns and potential recovery opportunities in recycling processes.
Recent advancements in green chemistry approaches have focused on developing bio-derived alternatives for both electrolyte families. Ether compounds synthesized from biomass feedstocks show particular promise, potentially reducing the environmental footprint by up to 40% compared to petroleum-derived counterparts. Similar initiatives for carbonates have shown more limited success due to their more complex molecular structures.
Water consumption represents another significant environmental factor, with carbonate electrolyte production typically requiring 1.5-2 times more process water than ether-based alternatives. This difference becomes particularly relevant in water-stressed regions where battery manufacturing facilities may operate.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!