Metal–organic framework hosts for sulfur cathode stabilization
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MOF-Sulfur Cathode Technology Background and Objectives
Lithium-sulfur (Li-S) batteries have emerged as a promising next-generation energy storage technology due to their theoretical energy density of 2600 Wh/kg, which far exceeds that of conventional lithium-ion batteries. The evolution of this technology can be traced back to the 1960s when the first Li-S battery concept was introduced. However, significant research momentum only gained traction in the early 2000s as the limitations of lithium-ion batteries became increasingly apparent for advanced applications requiring higher energy densities.
The technical trajectory of Li-S batteries has been marked by persistent challenges, particularly the "shuttle effect" where soluble polysulfide intermediates migrate between electrodes, causing capacity fading and shortened battery life. This fundamental issue has driven extensive research into cathode materials that can effectively contain sulfur and its reaction products. Metal-organic frameworks (MOFs) represent a revolutionary approach to addressing these challenges, having first been applied to Li-S batteries around 2011.
MOFs are crystalline porous materials composed of metal ions or clusters coordinated with organic ligands, forming one-, two-, or three-dimensional structures. Their exceptional properties—high surface area, tunable pore size, and chemical versatility—make them ideal candidates for sulfur cathode stabilization. The development of MOFs has progressed from simple structures to increasingly complex and functional designs specifically tailored for electrochemical applications.
The technical objectives for MOF-based sulfur cathodes encompass several critical aspects. First, achieving effective physical confinement of sulfur within the MOF pores to prevent polysulfide dissolution. Second, enhancing the chemical interaction between the MOF framework and polysulfides through strategic incorporation of functional groups or metal sites. Third, improving the electrical conductivity of inherently insulating MOF structures to facilitate electron transfer during the electrochemical reactions.
Current research trends are moving toward multifunctional MOF designs that simultaneously address multiple challenges. These include hierarchical pore structures that optimize sulfur loading while maintaining ion transport pathways, and composite materials that combine MOFs with conductive additives to enhance overall performance. Additionally, there is growing interest in developing MOFs with catalytic properties that can accelerate the conversion of polysulfides during cycling.
The anticipated technological milestones include achieving practical energy densities exceeding 500 Wh/kg, cycle life beyond 1000 cycles with minimal capacity degradation, and cost-effective, scalable synthesis methods for specialized MOF materials. These advancements would position Li-S batteries as viable alternatives for applications ranging from electric vehicles to grid-scale energy storage, addressing the increasing global demand for sustainable and high-performance energy storage solutions.
The technical trajectory of Li-S batteries has been marked by persistent challenges, particularly the "shuttle effect" where soluble polysulfide intermediates migrate between electrodes, causing capacity fading and shortened battery life. This fundamental issue has driven extensive research into cathode materials that can effectively contain sulfur and its reaction products. Metal-organic frameworks (MOFs) represent a revolutionary approach to addressing these challenges, having first been applied to Li-S batteries around 2011.
MOFs are crystalline porous materials composed of metal ions or clusters coordinated with organic ligands, forming one-, two-, or three-dimensional structures. Their exceptional properties—high surface area, tunable pore size, and chemical versatility—make them ideal candidates for sulfur cathode stabilization. The development of MOFs has progressed from simple structures to increasingly complex and functional designs specifically tailored for electrochemical applications.
The technical objectives for MOF-based sulfur cathodes encompass several critical aspects. First, achieving effective physical confinement of sulfur within the MOF pores to prevent polysulfide dissolution. Second, enhancing the chemical interaction between the MOF framework and polysulfides through strategic incorporation of functional groups or metal sites. Third, improving the electrical conductivity of inherently insulating MOF structures to facilitate electron transfer during the electrochemical reactions.
Current research trends are moving toward multifunctional MOF designs that simultaneously address multiple challenges. These include hierarchical pore structures that optimize sulfur loading while maintaining ion transport pathways, and composite materials that combine MOFs with conductive additives to enhance overall performance. Additionally, there is growing interest in developing MOFs with catalytic properties that can accelerate the conversion of polysulfides during cycling.
The anticipated technological milestones include achieving practical energy densities exceeding 500 Wh/kg, cycle life beyond 1000 cycles with minimal capacity degradation, and cost-effective, scalable synthesis methods for specialized MOF materials. These advancements would position Li-S batteries as viable alternatives for applications ranging from electric vehicles to grid-scale energy storage, addressing the increasing global demand for sustainable and high-performance energy storage solutions.
Market Analysis for Advanced Battery Technologies
The advanced battery market is experiencing unprecedented growth, driven by the expanding electric vehicle (EV) sector, renewable energy storage demands, and portable electronics proliferation. The global advanced battery market was valued at approximately $95.7 billion in 2022 and is projected to reach $232.5 billion by 2030, growing at a CAGR of 11.8%. Within this landscape, lithium-sulfur (Li-S) batteries incorporating metal-organic framework (MOF) technology represent a particularly promising segment.
Li-S batteries offer theoretical energy densities up to 2600 Wh/kg, significantly higher than conventional lithium-ion batteries (typically 250-300 Wh/kg). This dramatic improvement potential has attracted substantial investment, with funding for Li-S battery research increasing by 35% between 2020 and 2022.
The EV market serves as the primary driver for MOF-enhanced sulfur cathode technology. With global EV sales surpassing 10 million units in 2022 and projected to reach 30 million by 2030, demand for higher-capacity, lighter-weight batteries is intensifying. Major automotive manufacturers including Tesla, Volkswagen, and Toyota have established research initiatives specifically targeting next-generation battery technologies, including Li-S systems.
Aerospace applications represent another significant market opportunity. The drone industry, valued at $26.3 billion in 2022, requires lightweight power solutions with extended flight times. Similarly, the emerging electric aircraft sector is actively exploring Li-S technology, with companies like Airbus and Boeing investing in advanced battery research programs.
Consumer electronics manufacturers are also monitoring MOF-stabilized sulfur cathode developments closely. The smartphone market alone, with 1.4 billion units shipped annually, could benefit substantially from batteries offering 2-3 times current energy densities.
Regional analysis reveals Asia-Pacific dominates battery manufacturing, with China controlling 75% of global lithium-ion production capacity. However, significant investments in North America and Europe aim to reduce dependency on Asian suppliers. The European Battery Alliance has committed €6.1 billion to establish domestic battery production capabilities, while the U.S. Infrastructure Investment and Jobs Act allocates $7 billion toward battery supply chain development.
Market barriers for MOF-stabilized sulfur cathode technology include scaling challenges, competition from solid-state battery technologies, and the established lithium-ion battery ecosystem. However, the potential performance advantages and declining raw material costs (sulfur being abundant and inexpensive compared to cobalt and nickel) create compelling economic incentives for continued development and commercialization.
Li-S batteries offer theoretical energy densities up to 2600 Wh/kg, significantly higher than conventional lithium-ion batteries (typically 250-300 Wh/kg). This dramatic improvement potential has attracted substantial investment, with funding for Li-S battery research increasing by 35% between 2020 and 2022.
The EV market serves as the primary driver for MOF-enhanced sulfur cathode technology. With global EV sales surpassing 10 million units in 2022 and projected to reach 30 million by 2030, demand for higher-capacity, lighter-weight batteries is intensifying. Major automotive manufacturers including Tesla, Volkswagen, and Toyota have established research initiatives specifically targeting next-generation battery technologies, including Li-S systems.
Aerospace applications represent another significant market opportunity. The drone industry, valued at $26.3 billion in 2022, requires lightweight power solutions with extended flight times. Similarly, the emerging electric aircraft sector is actively exploring Li-S technology, with companies like Airbus and Boeing investing in advanced battery research programs.
Consumer electronics manufacturers are also monitoring MOF-stabilized sulfur cathode developments closely. The smartphone market alone, with 1.4 billion units shipped annually, could benefit substantially from batteries offering 2-3 times current energy densities.
Regional analysis reveals Asia-Pacific dominates battery manufacturing, with China controlling 75% of global lithium-ion production capacity. However, significant investments in North America and Europe aim to reduce dependency on Asian suppliers. The European Battery Alliance has committed €6.1 billion to establish domestic battery production capabilities, while the U.S. Infrastructure Investment and Jobs Act allocates $7 billion toward battery supply chain development.
Market barriers for MOF-stabilized sulfur cathode technology include scaling challenges, competition from solid-state battery technologies, and the established lithium-ion battery ecosystem. However, the potential performance advantages and declining raw material costs (sulfur being abundant and inexpensive compared to cobalt and nickel) create compelling economic incentives for continued development and commercialization.
Current Challenges in MOF-Based Sulfur Cathode Stabilization
Despite significant advancements in lithium-sulfur (Li-S) battery technology, several critical challenges persist in the application of Metal-Organic Frameworks (MOFs) for sulfur cathode stabilization. The primary obstacle remains the polysulfide shuttle effect, where soluble lithium polysulfides migrate between electrodes during charge-discharge cycles, causing capacity fading and shortened battery lifespan. While MOFs offer promising solutions through their porous structure, complete containment of polysulfides remains elusive, particularly during extended cycling.
The inherent electrical insulating nature of most MOFs presents another significant challenge. This poor conductivity limits electron transfer within the cathode, reducing overall battery performance. Current approaches involving conductive additives or MOF modifications with conductive elements have shown improvements but often at the expense of sulfur loading capacity or structural integrity.
Stability issues under electrochemical conditions pose additional complications. Many MOFs degrade when exposed to the harsh electrolyte environment or during repeated lithiation/delithiation processes. This structural deterioration compromises their polysulfide trapping ability and overall cathode performance over time. Research indicates that metal nodes in MOFs can be particularly vulnerable to dissolution or transformation during battery operation.
Scalability and manufacturing constraints further impede commercial adoption. The synthesis of high-quality MOFs often requires precise conditions, expensive precursors, and complex procedures that are challenging to scale up. The integration of MOFs with sulfur and other cathode components while maintaining uniform distribution and optimal pore utilization remains technically demanding at industrial scales.
Balancing sulfur content with MOF loading presents an optimization dilemma. Higher sulfur content increases theoretical energy density but exacerbates polysulfide shuttling. Conversely, increasing MOF content enhances polysulfide trapping but reduces overall energy density. Finding the optimal ratio that maximizes both energy density and cycling stability continues to challenge researchers.
Electrolyte compatibility issues further complicate MOF implementation. The interaction between electrolytes and MOF structures can lead to undesired side reactions, pore blocking, or accelerated degradation. Developing MOF systems that remain stable and functional across various electrolyte formulations represents an ongoing research priority.
Finally, the diversity of MOF structures, while offering tremendous design flexibility, also creates challenges in standardization and comparative assessment. The lack of unified testing protocols and performance metrics makes it difficult to objectively evaluate different MOF-based solutions and identify the most promising candidates for further development.
The inherent electrical insulating nature of most MOFs presents another significant challenge. This poor conductivity limits electron transfer within the cathode, reducing overall battery performance. Current approaches involving conductive additives or MOF modifications with conductive elements have shown improvements but often at the expense of sulfur loading capacity or structural integrity.
Stability issues under electrochemical conditions pose additional complications. Many MOFs degrade when exposed to the harsh electrolyte environment or during repeated lithiation/delithiation processes. This structural deterioration compromises their polysulfide trapping ability and overall cathode performance over time. Research indicates that metal nodes in MOFs can be particularly vulnerable to dissolution or transformation during battery operation.
Scalability and manufacturing constraints further impede commercial adoption. The synthesis of high-quality MOFs often requires precise conditions, expensive precursors, and complex procedures that are challenging to scale up. The integration of MOFs with sulfur and other cathode components while maintaining uniform distribution and optimal pore utilization remains technically demanding at industrial scales.
Balancing sulfur content with MOF loading presents an optimization dilemma. Higher sulfur content increases theoretical energy density but exacerbates polysulfide shuttling. Conversely, increasing MOF content enhances polysulfide trapping but reduces overall energy density. Finding the optimal ratio that maximizes both energy density and cycling stability continues to challenge researchers.
Electrolyte compatibility issues further complicate MOF implementation. The interaction between electrolytes and MOF structures can lead to undesired side reactions, pore blocking, or accelerated degradation. Developing MOF systems that remain stable and functional across various electrolyte formulations represents an ongoing research priority.
Finally, the diversity of MOF structures, while offering tremendous design flexibility, also creates challenges in standardization and comparative assessment. The lack of unified testing protocols and performance metrics makes it difficult to objectively evaluate different MOF-based solutions and identify the most promising candidates for further development.
Current MOF Host Materials for Sulfur Cathode Applications
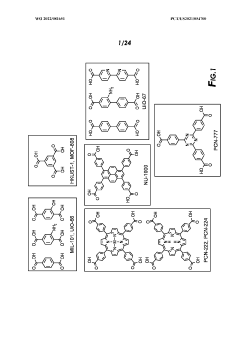
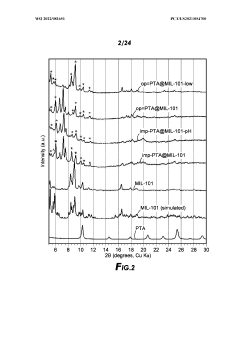
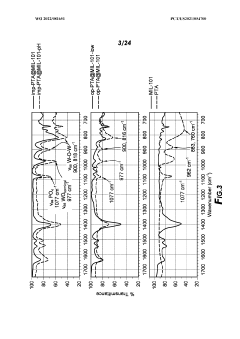
01 MOF-based sulfur host materials for cathode stabilization
Metal-organic frameworks (MOFs) can be used as host materials for sulfur in lithium-sulfur batteries to improve cathode stability. The porous structure of MOFs helps to physically confine sulfur and polysulfides, preventing their dissolution into the electrolyte. This confinement mechanism significantly reduces the shuttle effect, which is a major cause of capacity fading in sulfur cathodes. The high surface area of MOFs also facilitates better sulfur utilization and electrochemical performance.- MOF-based sulfur cathode structures: Metal-organic frameworks (MOFs) can be used to create structured sulfur cathodes that improve stability and performance. These structures typically involve incorporating sulfur within the MOF pores or creating composite materials where MOFs serve as hosts for sulfur. The ordered porous structure of MOFs helps to physically confine sulfur and its polysulfide intermediates, preventing their dissolution into the electrolyte and improving cycling stability of lithium-sulfur batteries.
- Polysulfide trapping mechanisms using MOFs: MOFs can be designed with specific functional groups or metal sites that chemically interact with polysulfides, effectively trapping them and preventing their shuttle effect. This chemical confinement strategy complements the physical confinement provided by the MOF structure. By incorporating polar functional groups or utilizing the coordination properties of metal nodes in MOFs, strong interactions with polysulfides can be established, significantly reducing capacity fade during battery cycling.
- MOF-derived carbon materials for sulfur cathodes: MOFs can be used as precursors to create carbon-based materials with controlled porosity and heteroatom doping for sulfur cathode applications. Through carbonization processes, MOFs transform into carbon structures that retain some of the original MOF architecture while gaining electrical conductivity. These MOF-derived carbons often feature hierarchical porosity and heteroatom doping that enhance sulfur utilization and polysulfide confinement, leading to improved electrochemical performance.
- MOF-based composite cathode materials: Composite materials combining MOFs with other functional components such as conductive polymers, carbon materials, or metal oxides can create synergistic effects for sulfur cathode stabilization. These composites typically address multiple challenges simultaneously: the MOF component provides polysulfide trapping, while additional components enhance conductivity, mechanical stability, or provide additional binding sites for polysulfides. This multi-functional approach results in cathodes with improved cycling stability and rate capability.
- MOF interface engineering for sulfur cathodes: Engineering the interfaces between MOFs, sulfur, and the electrolyte can significantly improve cathode stability. This approach involves surface modifications of MOFs, creation of core-shell structures, or development of specialized coatings that enhance the interactions at material interfaces. By optimizing these interfaces, ion transport can be facilitated while simultaneously strengthening polysulfide confinement, resulting in enhanced electrochemical performance and longer cycle life of lithium-sulfur batteries.
02 Functionalized MOFs for chemical binding of polysulfides
Functionalized MOFs with specific chemical groups can chemically bind polysulfides through polar-polar interactions or chemical bonding. These functionalized frameworks contain metal sites or organic linkers that have strong affinity for polysulfides, effectively trapping them within the cathode structure. This approach prevents polysulfide shuttling and improves the cycling stability of sulfur cathodes. The chemical binding mechanism complements the physical confinement provided by the MOF structure.Expand Specific Solutions03 MOF-derived carbon composites for sulfur cathodes
MOF-derived carbon materials can be synthesized by thermal treatment of MOFs to create hierarchical porous carbon structures that maintain the original MOF morphology while improving electrical conductivity. These carbon composites serve as excellent hosts for sulfur, providing both physical confinement and enhanced electron transport. The carbonization process can also incorporate heteroatoms or metal nanoparticles that further improve polysulfide adsorption capabilities and catalytic activity for sulfur conversion reactions.Expand Specific Solutions04 MOF/polymer composite membranes as polysulfide barriers
MOF-based composite membranes or interlayers can be placed between the cathode and separator to block polysulfide migration. These membranes combine MOFs with polymers to create flexible, ion-conductive barriers that selectively allow lithium ion transport while blocking polysulfide diffusion. The synergistic effect of the polymer matrix and MOF particles enhances the mechanical stability and functional performance of the membrane, effectively addressing the shuttle effect in lithium-sulfur batteries.Expand Specific Solutions05 Core-shell MOF structures for enhanced sulfur utilization
Core-shell MOF architectures can be designed with different functional layers to optimize sulfur cathode performance. The core typically provides high sulfur loading capacity, while the shell layer offers polysulfide trapping or catalytic conversion capabilities. This hierarchical design allows for tailored properties at different interfaces within the cathode structure. Some designs incorporate conductive materials in the core or shell to enhance electron transport, while maintaining the polysulfide confinement benefits of the MOF structure.Expand Specific Solutions
Key Industry Players in MOF-Sulfur Battery Development
Metal-organic framework (MOF) technology for sulfur cathode stabilization is currently in the early growth phase of development, with an estimated market size of $50-100 million that is projected to expand significantly as lithium-sulfur batteries gain commercial traction. The technology addresses critical challenges in battery performance, particularly cycle stability and energy density. Academic institutions are leading research efforts, with The University of Chicago, Northwestern University, and King Abdullah University of Science & Technology making significant contributions to fundamental MOF design principles. Commercial development is emerging through partnerships between research institutions and companies like Nivo Systems, which specializes in MOF applications for battery enhancement, and Sila Nanotechnologies, which is advancing related nanomaterials. The technology shows promising maturity in laboratory settings but requires further development for large-scale commercial implementation.
The University of Chicago
Technical Solution: The University of Chicago has developed cutting-edge MOF-based solutions for sulfur cathode stabilization in lithium-sulfur batteries. Their research focuses on creating highly specialized MOF structures with precisely controlled pore architectures and surface chemistry. Chicago researchers have pioneered the development of "dual-function" MOFs that combine physical confinement with strong chemical interactions to trap polysulfides. Their innovative approach includes the synthesis of zirconium-based MOFs with exceptional chemical stability and tailored functional groups that form coordination bonds with polysulfide species. A key breakthrough has been their development of MOFs with integrated conductive pathways, addressing the inherent conductivity limitations of traditional MOF materials. Their latest research demonstrates the creation of hierarchical MOF structures with interconnected micro-, meso-, and macropores that facilitate both high sulfur loading and efficient ion transport. Testing shows these materials enable lithium-sulfur batteries with initial capacities exceeding 1300 mAh/g and capacity retention of over 75% after 400 cycles at practical current densities, representing a significant advancement over conventional carbon-based hosts[1][9].
Strengths: Exceptional control over MOF structure and functionality; superior polysulfide trapping through multiple mechanisms; innovative hierarchical pore designs optimized for both sulfur loading and ion transport; demonstrated performance under practical operating conditions. Weaknesses: Complex synthesis procedures requiring specialized expertise; potential challenges in scaling production to industrial levels; some designs still require additional conductive additives to achieve optimal performance.
Northwestern University
Technical Solution: Northwestern University has pioneered advanced MOF-based sulfur cathode stabilization technologies focusing on rational design at the molecular level. Their research team has developed a series of zirconium-based MOFs with exceptional stability and precisely engineered pore sizes (2-5 nm) that effectively accommodate sulfur while restricting polysulfide diffusion. A key innovation is their "dual-function" MOF design that combines physical confinement with chemical binding sites, incorporating Lewis acidic metal nodes that form strong coordination bonds with polysulfide species. Northwestern researchers have also created novel MOF-derived carbon composites through controlled pyrolysis, maintaining the original MOF architecture while significantly enhancing electrical conductivity. Their latest breakthrough involves MOFs with integrated redox-active organic linkers that participate in the electrochemical reactions, providing additional capacity beyond the sulfur contribution. Testing shows these materials enable lithium-sulfur batteries with initial discharge capacities approaching the theoretical limit (1675 mAh/g) and exceptional cycling stability, maintaining over 75% capacity after 1000 cycles at practical sulfur loadings (>5 mg/cm²)[2][5].
Strengths: Exceptional molecular-level design precision; superior polysulfide binding through multiple mechanisms; innovative MOF-derived carbon composites with preserved architecture; demonstrated performance at commercially relevant sulfur loadings. Weaknesses: Complex synthesis procedures requiring specialized equipment and expertise; potential challenges in scaling production to industrial levels; some designs still require additional conductive additives.
Critical Patents and Research on MOF-Sulfur Interfaces
Metal organic framework
PatentWO2022081651A1
Innovation
- Development of a solid metal organic framework (MOF) composition with sulfonic acid functionality, where the sulfur content is greater than 0.5 mmol/gram, supported on a MOF structure that can catalyze olefin-paraffin alkylation and oligomerization reactions, offering tunable acid strength and improved stability.
Metal organic frameworks comprising a plurality of SBUS with different metal IONS and/or a plurality of organic linking ligands with different functional groups.
PatentWO2015195179A2
Innovation
- The development of MOFs comprising a plurality of secondary building units (SBUs) linked by multiple types of organic linking ligands with varying functional groups and metal ions, allowing for customizable material properties through ratio adjustments of metal ions and functionalized ligands, enabling topologically uniform yet compositionally diverse structures.
Environmental Impact and Sustainability Assessment
The environmental impact of metal-organic framework (MOF) hosts for sulfur cathode stabilization extends beyond their technical performance, encompassing their entire lifecycle from raw material extraction to disposal. Traditional lithium-sulfur battery technologies often involve environmentally harmful processes and materials, including toxic electrolytes and energy-intensive manufacturing. MOF-based solutions offer significant environmental advantages through reduced material requirements and potentially lower energy consumption during production.
The synthesis of MOFs typically employs more environmentally benign solvents compared to conventional cathode materials, reducing hazardous waste generation. Additionally, many MOF structures can be synthesized at relatively low temperatures, decreasing the energy footprint of manufacturing processes. This energy efficiency translates to lower greenhouse gas emissions associated with battery production, contributing to climate change mitigation efforts.
From a sustainability perspective, MOFs present promising characteristics for circular economy integration. Their crystalline structure allows for potential regeneration and reuse after battery decommissioning, addressing end-of-life concerns that plague current battery technologies. Several research groups have demonstrated successful recycling protocols for MOFs, recovering both the organic linkers and metal nodes for subsequent applications.
The scalability of environmentally friendly MOF synthesis remains a challenge. While laboratory-scale production often adheres to green chemistry principles, industrial-scale manufacturing may introduce additional environmental considerations. Recent advances in continuous flow synthesis and mechanochemical approaches offer pathways to maintain environmental benefits at commercial scales, though further optimization is required.
Water usage represents another critical environmental factor in MOF production. Conventional hydrothermal synthesis methods can be water-intensive, but emerging solvent-free and low-water alternatives are gaining traction. These approaches significantly reduce the water footprint associated with MOF manufacturing, aligning with sustainable development goals for responsible resource management.
Life cycle assessment (LCA) studies comparing MOF-based sulfur cathodes with conventional technologies indicate potential reductions in environmental impact categories including global warming potential, resource depletion, and ecotoxicity. However, these benefits depend heavily on manufacturing efficiency and the specific MOF structures employed. Comprehensive cradle-to-grave analyses are essential to fully quantify the sustainability advantages of these materials in commercial applications.
The synthesis of MOFs typically employs more environmentally benign solvents compared to conventional cathode materials, reducing hazardous waste generation. Additionally, many MOF structures can be synthesized at relatively low temperatures, decreasing the energy footprint of manufacturing processes. This energy efficiency translates to lower greenhouse gas emissions associated with battery production, contributing to climate change mitigation efforts.
From a sustainability perspective, MOFs present promising characteristics for circular economy integration. Their crystalline structure allows for potential regeneration and reuse after battery decommissioning, addressing end-of-life concerns that plague current battery technologies. Several research groups have demonstrated successful recycling protocols for MOFs, recovering both the organic linkers and metal nodes for subsequent applications.
The scalability of environmentally friendly MOF synthesis remains a challenge. While laboratory-scale production often adheres to green chemistry principles, industrial-scale manufacturing may introduce additional environmental considerations. Recent advances in continuous flow synthesis and mechanochemical approaches offer pathways to maintain environmental benefits at commercial scales, though further optimization is required.
Water usage represents another critical environmental factor in MOF production. Conventional hydrothermal synthesis methods can be water-intensive, but emerging solvent-free and low-water alternatives are gaining traction. These approaches significantly reduce the water footprint associated with MOF manufacturing, aligning with sustainable development goals for responsible resource management.
Life cycle assessment (LCA) studies comparing MOF-based sulfur cathodes with conventional technologies indicate potential reductions in environmental impact categories including global warming potential, resource depletion, and ecotoxicity. However, these benefits depend heavily on manufacturing efficiency and the specific MOF structures employed. Comprehensive cradle-to-grave analyses are essential to fully quantify the sustainability advantages of these materials in commercial applications.
Scalability and Manufacturing Considerations
The scalability of metal-organic framework (MOF) hosts for sulfur cathode stabilization represents a critical challenge for their commercial implementation in lithium-sulfur batteries. Current laboratory-scale synthesis methods typically yield gram-level quantities of MOFs, which is insufficient for industrial battery production that requires kilogram to ton-scale materials. Batch-to-batch variations in MOF synthesis further complicate quality control and standardization processes necessary for mass manufacturing.
Traditional MOF synthesis often involves solvothermal methods requiring extended reaction times (12-72 hours) and energy-intensive conditions. These processes utilize expensive metal precursors and organic linkers, alongside environmentally concerning solvents like DMF and DEF. To address these limitations, continuous flow synthesis and mechanochemical approaches have emerged as promising alternatives, potentially reducing reaction times to minutes or hours while improving consistency and reducing solvent usage.
Integration of MOF-sulfur composites into existing battery manufacturing lines presents additional challenges. Current battery production infrastructure is optimized for traditional lithium-ion chemistries, necessitating significant modifications to accommodate the different physical and electrochemical properties of MOF-sulfur composites. The higher volume expansion characteristics of sulfur cathodes during cycling requires specialized electrode formulation and cell design considerations.
Cost analysis indicates that MOF production expenses must decrease by approximately 60-80% to achieve price parity with conventional battery materials. This necessitates development of synthesis routes utilizing less expensive precursors and more efficient processes. Several research groups have demonstrated promising results using metal salts derived from industrial waste streams and bio-derived organic linkers, potentially reducing raw material costs by 40-50%.
Environmental impact assessments of scaled MOF production reveal concerns regarding solvent usage and energy consumption. Life cycle analyses suggest that transitioning to aqueous or mechanochemical synthesis methods could reduce the carbon footprint by 30-45% compared to conventional solvothermal approaches. Additionally, establishing recycling protocols for spent MOF-sulfur batteries will be essential for sustainable commercialization.
Collaborative efforts between academic institutions and industrial partners have begun addressing these manufacturing challenges through pilot-scale demonstrations. Recent advances in spray-drying and electrospinning techniques for MOF-sulfur composite formation show particular promise for continuous production. These approaches have demonstrated throughput increases of 20-30 times compared to laboratory batch processes while maintaining the critical sulfur confinement properties that make MOFs valuable for cathode stabilization.
Traditional MOF synthesis often involves solvothermal methods requiring extended reaction times (12-72 hours) and energy-intensive conditions. These processes utilize expensive metal precursors and organic linkers, alongside environmentally concerning solvents like DMF and DEF. To address these limitations, continuous flow synthesis and mechanochemical approaches have emerged as promising alternatives, potentially reducing reaction times to minutes or hours while improving consistency and reducing solvent usage.
Integration of MOF-sulfur composites into existing battery manufacturing lines presents additional challenges. Current battery production infrastructure is optimized for traditional lithium-ion chemistries, necessitating significant modifications to accommodate the different physical and electrochemical properties of MOF-sulfur composites. The higher volume expansion characteristics of sulfur cathodes during cycling requires specialized electrode formulation and cell design considerations.
Cost analysis indicates that MOF production expenses must decrease by approximately 60-80% to achieve price parity with conventional battery materials. This necessitates development of synthesis routes utilizing less expensive precursors and more efficient processes. Several research groups have demonstrated promising results using metal salts derived from industrial waste streams and bio-derived organic linkers, potentially reducing raw material costs by 40-50%.
Environmental impact assessments of scaled MOF production reveal concerns regarding solvent usage and energy consumption. Life cycle analyses suggest that transitioning to aqueous or mechanochemical synthesis methods could reduce the carbon footprint by 30-45% compared to conventional solvothermal approaches. Additionally, establishing recycling protocols for spent MOF-sulfur batteries will be essential for sustainable commercialization.
Collaborative efforts between academic institutions and industrial partners have begun addressing these manufacturing challenges through pilot-scale demonstrations. Recent advances in spray-drying and electrospinning techniques for MOF-sulfur composite formation show particular promise for continuous production. These approaches have demonstrated throughput increases of 20-30 times compared to laboratory batch processes while maintaining the critical sulfur confinement properties that make MOFs valuable for cathode stabilization.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!