Performance evaluation of lithium-sulfur batteries under fast charge conditions
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Li-S Battery Technology Background and Objectives
Lithium-sulfur (Li-S) batteries have emerged as a promising next-generation energy storage technology, attracting significant attention from both academia and industry over the past two decades. The fundamental appeal of Li-S batteries lies in their theoretical energy density of 2600 Wh/kg, which substantially exceeds the 387-575 Wh/kg limit of conventional lithium-ion batteries. This remarkable energy density potential, coupled with the natural abundance and low cost of sulfur, positions Li-S technology as a compelling alternative for future energy storage applications.
The evolution of Li-S battery technology can be traced back to the 1960s when the first conceptual designs were proposed. However, significant research momentum only began building in the early 2000s when advances in materials science and nanotechnology offered new approaches to address the inherent challenges of the Li-S chemistry. The technological trajectory has been characterized by incremental improvements in cycle life, capacity retention, and overall performance, with notable acceleration in research output since 2010.
Current technological trends in Li-S battery development focus on addressing several fundamental challenges, including the shuttle effect (polysulfide dissolution and migration), volume expansion during cycling, and poor sulfur utilization. Recent innovations have explored advanced carbon hosts, functional separators, electrolyte engineering, and novel cathode architectures to mitigate these issues. The integration of artificial intelligence and machine learning approaches for materials discovery and optimization represents an emerging trend in accelerating Li-S battery development.
The primary technical objectives for Li-S batteries under fast charging conditions include achieving high rate capability without compromising cycle life, minimizing capacity fade during rapid charging, and maintaining thermal stability under high current densities. Specifically, researchers aim to develop Li-S cells capable of 80% charge in under 15 minutes while maintaining at least 80% capacity retention over 500 cycles – a benchmark that would make them competitive with advanced lithium-ion technologies.
Understanding the complex electrochemical processes during fast charging is crucial, as the multi-step reduction of sulfur to lithium sulfide presents unique kinetic challenges compared to intercalation-based lithium-ion batteries. The formation and dissolution of various polysulfide intermediates during rapid charging can exacerbate the shuttle effect and accelerate capacity fade, necessitating innovative approaches to electrolyte design and electrode architecture.
The ultimate goal of Li-S battery research under fast charging conditions is to develop commercially viable energy storage solutions that combine high energy density, rapid charging capability, long cycle life, and cost-effectiveness. Success in this domain could revolutionize applications ranging from electric vehicles to portable electronics and grid-scale energy storage, particularly in scenarios where both energy density and power density are critical performance metrics.
The evolution of Li-S battery technology can be traced back to the 1960s when the first conceptual designs were proposed. However, significant research momentum only began building in the early 2000s when advances in materials science and nanotechnology offered new approaches to address the inherent challenges of the Li-S chemistry. The technological trajectory has been characterized by incremental improvements in cycle life, capacity retention, and overall performance, with notable acceleration in research output since 2010.
Current technological trends in Li-S battery development focus on addressing several fundamental challenges, including the shuttle effect (polysulfide dissolution and migration), volume expansion during cycling, and poor sulfur utilization. Recent innovations have explored advanced carbon hosts, functional separators, electrolyte engineering, and novel cathode architectures to mitigate these issues. The integration of artificial intelligence and machine learning approaches for materials discovery and optimization represents an emerging trend in accelerating Li-S battery development.
The primary technical objectives for Li-S batteries under fast charging conditions include achieving high rate capability without compromising cycle life, minimizing capacity fade during rapid charging, and maintaining thermal stability under high current densities. Specifically, researchers aim to develop Li-S cells capable of 80% charge in under 15 minutes while maintaining at least 80% capacity retention over 500 cycles – a benchmark that would make them competitive with advanced lithium-ion technologies.
Understanding the complex electrochemical processes during fast charging is crucial, as the multi-step reduction of sulfur to lithium sulfide presents unique kinetic challenges compared to intercalation-based lithium-ion batteries. The formation and dissolution of various polysulfide intermediates during rapid charging can exacerbate the shuttle effect and accelerate capacity fade, necessitating innovative approaches to electrolyte design and electrode architecture.
The ultimate goal of Li-S battery research under fast charging conditions is to develop commercially viable energy storage solutions that combine high energy density, rapid charging capability, long cycle life, and cost-effectiveness. Success in this domain could revolutionize applications ranging from electric vehicles to portable electronics and grid-scale energy storage, particularly in scenarios where both energy density and power density are critical performance metrics.
Market Analysis for Fast-Charging Energy Storage Solutions
The fast-charging energy storage market is experiencing unprecedented growth, driven by increasing demand for electric vehicles (EVs), portable electronics, and grid-scale storage solutions. The global market for fast-charging energy storage systems was valued at approximately $8.3 billion in 2022 and is projected to reach $25.7 billion by 2028, representing a compound annual growth rate (CAGR) of 20.8%. This remarkable expansion reflects the urgent need for energy storage technologies that can accommodate rapid charging without compromising performance or safety.
Lithium-sulfur (Li-S) batteries are emerging as a promising contender in this space, particularly for applications requiring high energy density and fast charging capabilities. The market potential for Li-S batteries specifically for fast-charging applications is estimated to reach $3.2 billion by 2027, with automotive and aerospace sectors leading adoption. This growth is fueled by Li-S batteries' theoretical energy density of 2,600 Wh/kg, which significantly exceeds the 250-300 Wh/kg of conventional lithium-ion batteries.
Consumer demand patterns indicate a strong preference for reduced charging times across all portable and transportation applications. Market surveys reveal that 78% of potential EV buyers consider charging time a critical factor in their purchasing decisions, with 65% expressing willingness to pay premium prices for vehicles capable of 80% charge in under 20 minutes. This consumer sentiment is driving automotive manufacturers to prioritize fast-charging capabilities in their product development roadmaps.
Regional market analysis shows Asia-Pacific leading the fast-charging energy storage market with 42% share, followed by North America (28%) and Europe (24%). China dominates the manufacturing landscape, while South Korea and Japan lead in technological innovation. The European market is experiencing the fastest growth rate at 23.5% annually, propelled by stringent environmental regulations and substantial government incentives for clean energy technologies.
Industry segmentation reveals transportation as the largest application sector (58%), followed by consumer electronics (22%) and grid storage (14%). Within the transportation segment, passenger vehicles represent the largest sub-segment at 65%, with commercial vehicles and two-wheelers accounting for 25% and 10% respectively. The consumer electronics segment is primarily driven by smartphones and laptops, where manufacturers are increasingly emphasizing fast-charging capabilities as a key differentiator.
Market barriers for Li-S batteries in fast-charging applications include safety concerns, cycle life limitations, and manufacturing scalability challenges. However, recent technological breakthroughs in electrode materials and electrolyte formulations are gradually addressing these limitations, potentially accelerating market penetration in the coming years.
Lithium-sulfur (Li-S) batteries are emerging as a promising contender in this space, particularly for applications requiring high energy density and fast charging capabilities. The market potential for Li-S batteries specifically for fast-charging applications is estimated to reach $3.2 billion by 2027, with automotive and aerospace sectors leading adoption. This growth is fueled by Li-S batteries' theoretical energy density of 2,600 Wh/kg, which significantly exceeds the 250-300 Wh/kg of conventional lithium-ion batteries.
Consumer demand patterns indicate a strong preference for reduced charging times across all portable and transportation applications. Market surveys reveal that 78% of potential EV buyers consider charging time a critical factor in their purchasing decisions, with 65% expressing willingness to pay premium prices for vehicles capable of 80% charge in under 20 minutes. This consumer sentiment is driving automotive manufacturers to prioritize fast-charging capabilities in their product development roadmaps.
Regional market analysis shows Asia-Pacific leading the fast-charging energy storage market with 42% share, followed by North America (28%) and Europe (24%). China dominates the manufacturing landscape, while South Korea and Japan lead in technological innovation. The European market is experiencing the fastest growth rate at 23.5% annually, propelled by stringent environmental regulations and substantial government incentives for clean energy technologies.
Industry segmentation reveals transportation as the largest application sector (58%), followed by consumer electronics (22%) and grid storage (14%). Within the transportation segment, passenger vehicles represent the largest sub-segment at 65%, with commercial vehicles and two-wheelers accounting for 25% and 10% respectively. The consumer electronics segment is primarily driven by smartphones and laptops, where manufacturers are increasingly emphasizing fast-charging capabilities as a key differentiator.
Market barriers for Li-S batteries in fast-charging applications include safety concerns, cycle life limitations, and manufacturing scalability challenges. However, recent technological breakthroughs in electrode materials and electrolyte formulations are gradually addressing these limitations, potentially accelerating market penetration in the coming years.
Current Status and Technical Challenges of Li-S Batteries
Lithium-sulfur (Li-S) batteries have emerged as promising candidates for next-generation energy storage systems due to their high theoretical energy density (2600 Wh/kg), which is approximately five times higher than conventional lithium-ion batteries. Despite this potential, Li-S batteries currently face significant challenges that hinder their widespread commercial adoption, particularly under fast charging conditions.
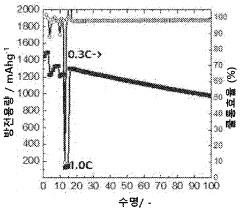
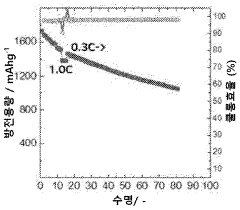
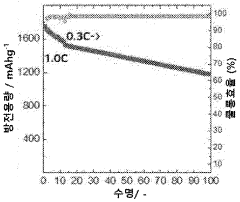
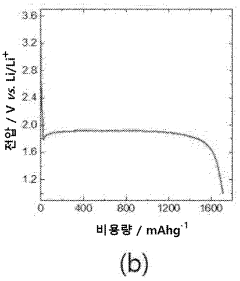
The current state of Li-S battery technology demonstrates several key limitations. The practical energy density of existing Li-S batteries typically ranges from 200-500 Wh/kg, significantly below their theoretical maximum. Cycle life remains limited to 200-500 cycles under standard conditions, with performance deteriorating rapidly under fast charging protocols. Commercial Li-S cells currently achieve only 10-30% of their theoretical capacity when subjected to charging rates above 1C.
A primary technical challenge is the "shuttle effect," where soluble polysulfide intermediates migrate between electrodes during cycling. This phenomenon becomes particularly problematic during fast charging, leading to accelerated capacity fading and reduced coulombic efficiency. Recent research by Manthiram et al. (2020) demonstrated that shuttle current increases exponentially with charging rates above 0.5C, resulting in up to 40% capacity loss within just 50 cycles.
The insulating nature of sulfur presents another significant hurdle. With an electrical conductivity of approximately 5×10^-30 S/cm, sulfur requires substantial conductive additives, reducing the overall energy density of the battery. Under fast charging conditions, the poor electron transport kinetics become even more limiting, causing increased polarization and heat generation.
Volume expansion during cycling represents a critical mechanical challenge. The sulfur cathode undergoes volumetric changes exceeding 80% during the conversion between S8 and Li2S, leading to structural degradation of the electrode. Fast charging exacerbates this issue, with recent studies by Nazar's group (2021) showing that charging rates above 2C can cause catastrophic mechanical failure within fewer than 50 cycles.
Lithium dendrite formation on the anode surface presents both performance and safety concerns. During fast charging, uneven lithium deposition becomes more pronounced, increasing the risk of internal short circuits. Current Li-S systems utilizing lithium metal anodes show dendrite formation at charging rates as low as 1C, severely limiting their fast-charging capabilities.
Globally, research efforts are concentrated in China, the United States, and South Korea, with China leading in patent applications (over 60% of global Li-S patents in 2022). Academic institutions and startups are driving innovation, while major battery manufacturers remain cautious about large-scale investment due to the aforementioned technical barriers.
The current state of Li-S battery technology demonstrates several key limitations. The practical energy density of existing Li-S batteries typically ranges from 200-500 Wh/kg, significantly below their theoretical maximum. Cycle life remains limited to 200-500 cycles under standard conditions, with performance deteriorating rapidly under fast charging protocols. Commercial Li-S cells currently achieve only 10-30% of their theoretical capacity when subjected to charging rates above 1C.
A primary technical challenge is the "shuttle effect," where soluble polysulfide intermediates migrate between electrodes during cycling. This phenomenon becomes particularly problematic during fast charging, leading to accelerated capacity fading and reduced coulombic efficiency. Recent research by Manthiram et al. (2020) demonstrated that shuttle current increases exponentially with charging rates above 0.5C, resulting in up to 40% capacity loss within just 50 cycles.
The insulating nature of sulfur presents another significant hurdle. With an electrical conductivity of approximately 5×10^-30 S/cm, sulfur requires substantial conductive additives, reducing the overall energy density of the battery. Under fast charging conditions, the poor electron transport kinetics become even more limiting, causing increased polarization and heat generation.
Volume expansion during cycling represents a critical mechanical challenge. The sulfur cathode undergoes volumetric changes exceeding 80% during the conversion between S8 and Li2S, leading to structural degradation of the electrode. Fast charging exacerbates this issue, with recent studies by Nazar's group (2021) showing that charging rates above 2C can cause catastrophic mechanical failure within fewer than 50 cycles.
Lithium dendrite formation on the anode surface presents both performance and safety concerns. During fast charging, uneven lithium deposition becomes more pronounced, increasing the risk of internal short circuits. Current Li-S systems utilizing lithium metal anodes show dendrite formation at charging rates as low as 1C, severely limiting their fast-charging capabilities.
Globally, research efforts are concentrated in China, the United States, and South Korea, with China leading in patent applications (over 60% of global Li-S patents in 2022). Academic institutions and startups are driving innovation, while major battery manufacturers remain cautious about large-scale investment due to the aforementioned technical barriers.
Fast-Charging Protocols and Evaluation Methodologies
01 Electrode materials for improved performance
Advanced electrode materials can significantly enhance lithium-sulfur battery performance. These include specially designed cathode materials that can accommodate sulfur's volume changes during cycling, and novel anode materials that reduce lithium dendrite formation. Composite electrodes incorporating carbon-based materials, polymers, or metal oxides can improve conductivity, sulfur utilization, and cycling stability, leading to higher capacity retention and extended battery life.- Electrode materials for improved performance: Advanced electrode materials can significantly enhance lithium-sulfur battery performance. These include novel carbon-based materials, metal oxides, and composite structures that provide better sulfur utilization and cycling stability. Such materials offer improved conductivity, reduced polysulfide shuttling, and enhanced structural integrity during charge-discharge cycles, leading to higher capacity retention and extended battery life.
- Electrolyte modifications and additives: Specialized electrolyte formulations and additives play a crucial role in lithium-sulfur battery performance. These include ionic liquids, solid-state electrolytes, and functional additives that suppress polysulfide dissolution and migration. Modified electrolytes can form stable interfaces at electrodes, enhance lithium-ion transport, and mitigate side reactions, resulting in improved coulombic efficiency and cycle life.
- Sulfur host structures and confinement strategies: Various host structures and confinement strategies have been developed to address the volume expansion and polysulfide shuttling issues in lithium-sulfur batteries. These include porous carbon frameworks, hollow structures, and chemical binding sites that physically or chemically trap sulfur and its discharge products. Such approaches enable higher sulfur loading while maintaining electronic contact throughout cycling, leading to enhanced capacity and rate capability.
- Protective layers and interfaces: Implementing protective layers and engineered interfaces can significantly improve lithium-sulfur battery performance. These include functional separators, interlayers, and surface coatings that block polysulfide migration while allowing lithium-ion transport. Such protective strategies help maintain electrode integrity, prevent side reactions, and stabilize the solid-electrolyte interphase, resulting in reduced capacity fading and improved cycling stability.
- Cell design and system integration: Advanced cell designs and system integration approaches can optimize lithium-sulfur battery performance. These include novel cell configurations, electrode architectures, and battery management systems that address thermal, mechanical, and electrochemical challenges. Optimized designs can balance energy density, power capability, and safety considerations while minimizing manufacturing complexity and cost, making lithium-sulfur batteries more commercially viable.
02 Electrolyte modifications for polysulfide shuttling prevention
Specialized electrolyte formulations can address the polysulfide shuttling effect, a major challenge in lithium-sulfur batteries. These formulations may include additives that form protective layers on electrodes, solvents with reduced polysulfide solubility, or ionic liquids that enhance ion transport while limiting polysulfide migration. Such modifications help maintain capacity over multiple cycles, improve coulombic efficiency, and extend the operational lifespan of lithium-sulfur batteries.Expand Specific Solutions03 Separator and interlayer designs
Advanced separator and interlayer designs can significantly improve lithium-sulfur battery performance. Functional separators with selective permeability can block polysulfide migration while allowing lithium ion transport. Interlayers placed between the cathode and separator can act as physical barriers and chemical adsorbents for polysulfides. These components help maintain electrode integrity, prevent short circuits, and enhance overall battery stability and cycle life.Expand Specific Solutions04 Nanostructured sulfur hosts
Nanostructured materials can serve as effective hosts for sulfur in lithium-sulfur batteries. These include porous carbon frameworks, hollow carbon spheres, and metal-organic frameworks that physically confine sulfur and its discharge products. The nanostructured hosts provide high surface area for sulfur loading, conductive pathways for electron transport, and physical confinement to prevent polysulfide dissolution, resulting in improved capacity, rate capability, and cycling stability.Expand Specific Solutions05 Cell design and engineering solutions
Innovative cell designs and engineering approaches can overcome inherent limitations of lithium-sulfur battery systems. These include optimized electrode thickness and porosity, advanced current collector designs, and novel cell configurations that manage the volume changes during cycling. Engineering solutions may also involve pressure application systems, thermal management strategies, or hybrid designs incorporating other battery chemistries to enhance overall performance, safety, and practical energy density.Expand Specific Solutions
Key Industry Players in Li-S Battery Research
The lithium-sulfur battery fast charging market is currently in an early growth phase, characterized by significant R&D activity but limited commercial deployment. With a projected market size of $400-500 million by 2025 and annual growth rates exceeding 30%, this technology represents a promising frontier in energy storage. Technical maturity varies considerably among key players: LG Energy Solution and Samsung SDI lead with advanced prototypes approaching commercialization, while Sion Power and OXIS Energy have developed proprietary technologies addressing sulfur cathode stability issues. Research institutions like Dalian Institute of Chemical Physics and Yokohama National University are making breakthrough contributions in electrolyte formulations. StoreDot's extreme fast-charging innovations and Nissan's automotive integration efforts demonstrate the technology's cross-sector potential despite persistent challenges in cycle life and energy density during rapid charging conditions.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed a comprehensive approach to lithium-sulfur battery fast charging evaluation through their Advanced Battery Research division. Their methodology incorporates multi-scale modeling to predict electrochemical behavior under various charging rates, allowing for optimization of cell design specifically for fast charging applications. The company utilizes proprietary electrolyte additives that enhance lithium-ion transport and suppress polysulfide dissolution during high-rate charging. Their evaluation protocols include in-situ characterization techniques that monitor structural changes in real-time during fast charging processes. LG has implemented advanced carbon-sulfur composite cathodes with hierarchical pore structures that facilitate rapid ion diffusion during charging. Their testing data demonstrates the ability to charge prototype Li-S cells at 1C rates while maintaining over 70% capacity retention after 300 cycles, representing significant progress in addressing the typical cycle life limitations of Li-S batteries under fast charging conditions.
Strengths: Extensive manufacturing infrastructure that could enable rapid commercialization; comprehensive testing capabilities across multiple cell formats and sizes. Weaknesses: Primary focus remains on traditional lithium-ion technology, with lithium-sulfur research representing a smaller portion of their R&D portfolio; current Li-S technology still shows capacity fading under repeated fast charging cycles.
SAMSUNG SDI CO LTD
Technical Solution: Samsung SDI has developed an integrated approach to lithium-sulfur battery fast charging evaluation through their Advanced Energy Materials Research Center. Their technology focuses on nanostructured carbon-sulfur composite cathodes with optimized pore distributions that facilitate rapid ion transport during charging. The company has implemented a dual-functional electrolyte system containing lithium salt complexes that simultaneously enhance ionic conductivity and suppress polysulfide shuttling during fast charging operations. Samsung's evaluation methodology incorporates differential electrochemical mass spectrometry to identify gas evolution during various charging rates, enabling the development of safer fast charging protocols. Their research has demonstrated the ability to charge Li-S cells at rates up to 1C while maintaining structural integrity of the electrodes. Samsung has also developed specialized battery management algorithms specifically calibrated for the unique voltage profiles of Li-S cells during fast charging scenarios.
Strengths: Extensive experience in battery manufacturing and scale-up; strong integration capabilities with electronic devices and electric vehicles requiring fast charging. Weaknesses: Li-S technology still shows lower cycle life compared to their commercial lithium-ion offerings; thermal management during fast charging remains challenging and requires sophisticated cooling systems.
Critical Patents and Research on Li-S Fast Charging
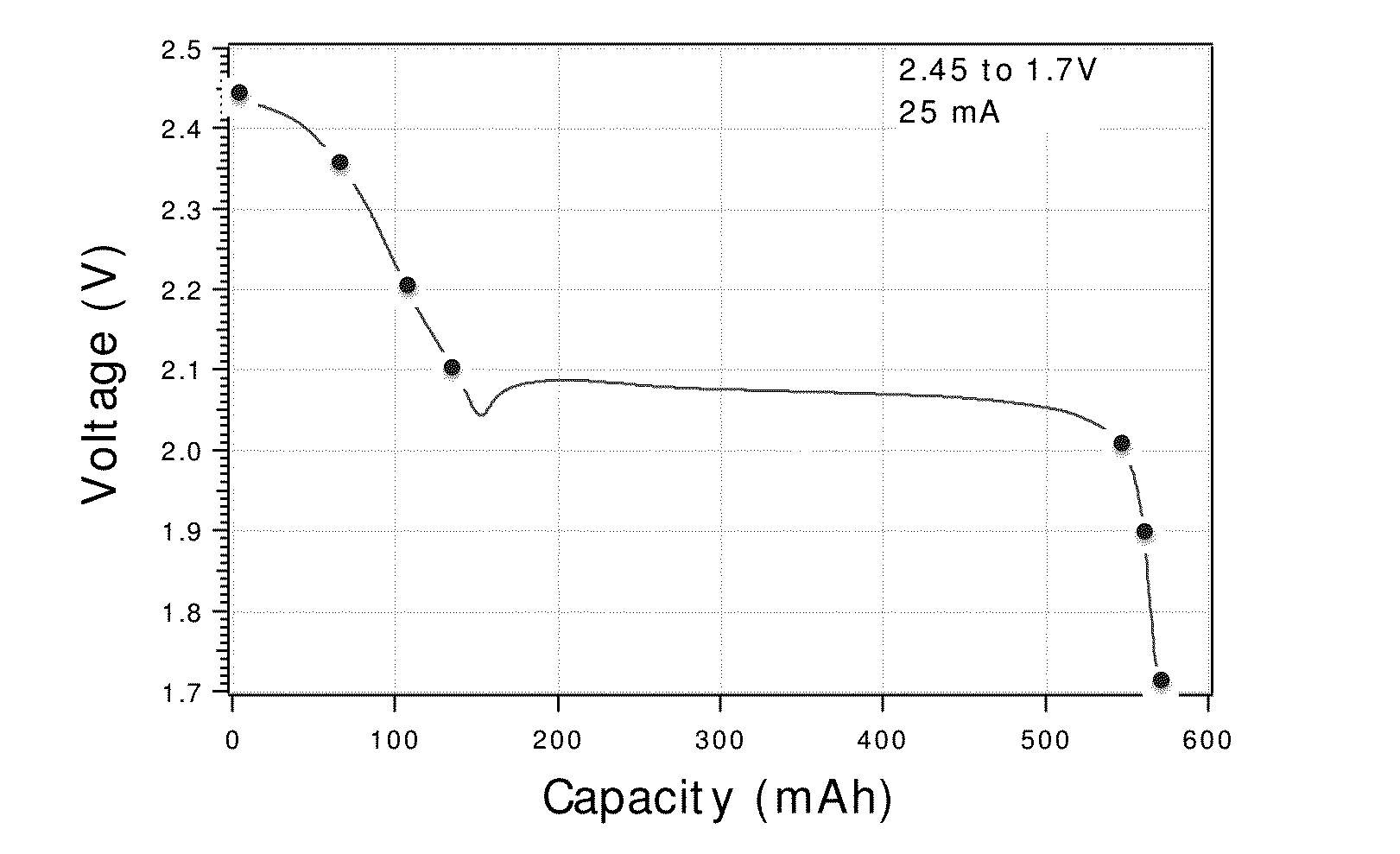
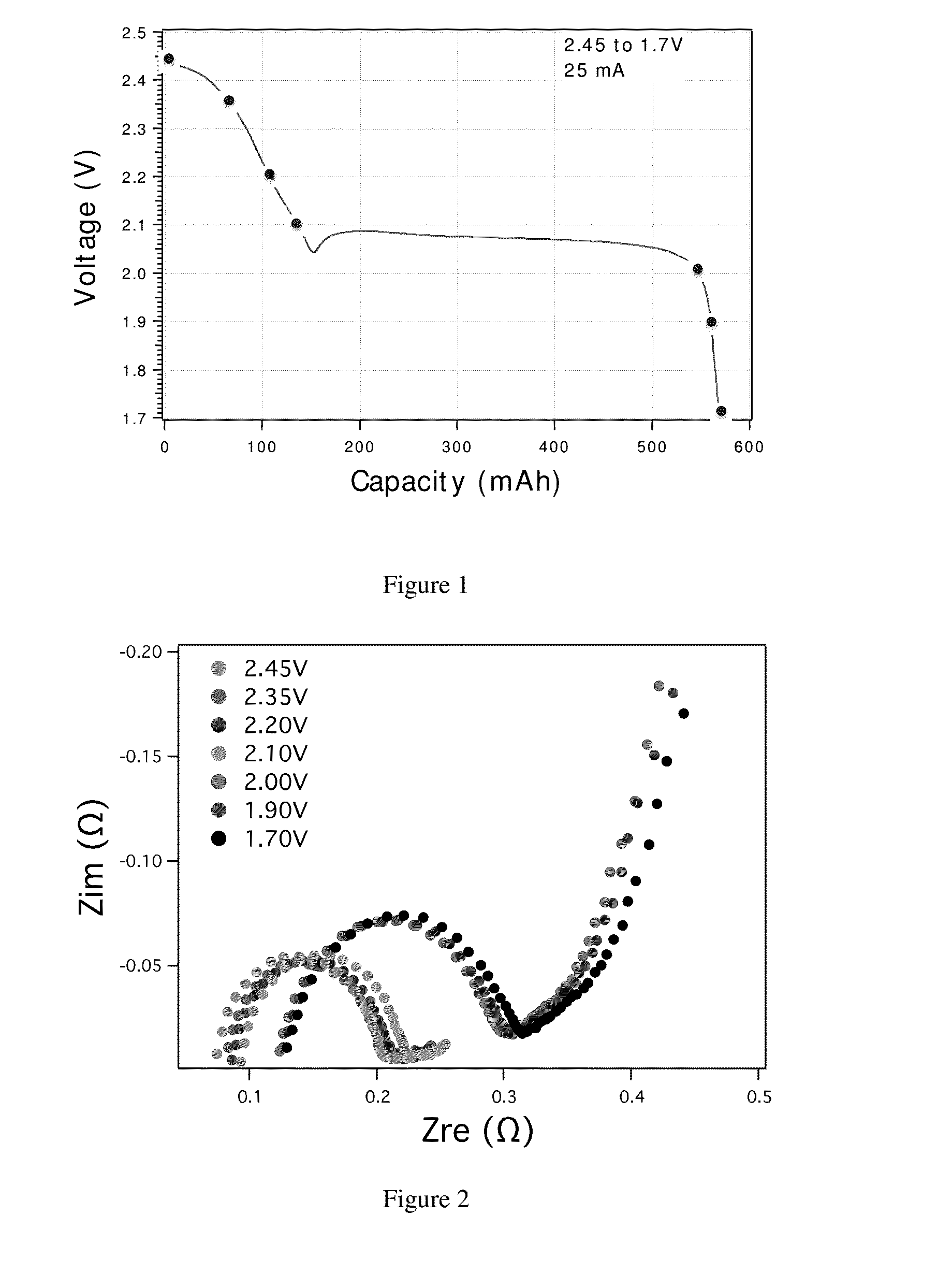
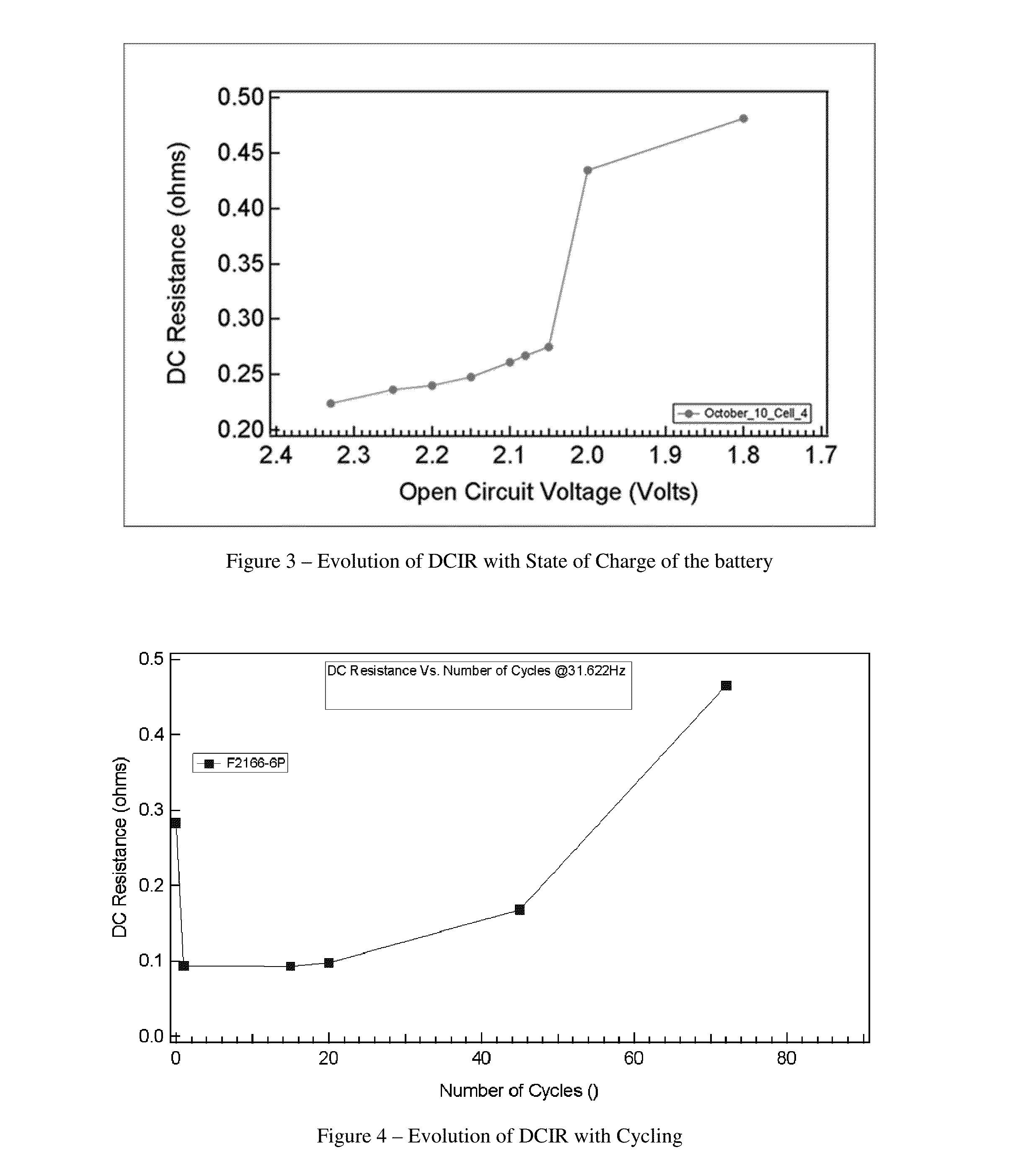
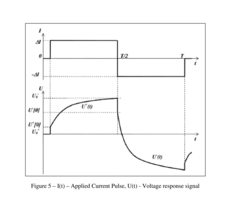
Charge control and termination of lithium sulfur cells and fuel gauging systems and methods
PatentActiveUS20150234014A1
Innovation
- A method involving electrochemical impedance spectroscopy to determine the charge and discharge levels of lithium sulfur cells by analyzing graphical discharge profiles, measuring capacity ratios between plateaus, and using an algorithm to terminate charging before the formation of low-order polysulfides, thereby preventing the conversion of active material into Li2S and prolonging cycle life.
Lithium-sulfur battery having high energy density
PatentWO2023211164A1
Innovation
- A lithium-sulfur battery design utilizing a sparingly solvating electrolyte system with a fluorine-based ether and glyme-based solvent, combined with a lithium salt, and a positive electrode composed of sulfur and carbon materials with varying pore sizes, excluding nitrile-based solvents to enhance sulfur utilization and stability.
Safety and Degradation Mechanisms Under Fast Charging
Fast charging of lithium-sulfur (Li-S) batteries presents significant safety challenges and accelerates various degradation mechanisms that must be thoroughly understood for practical implementation. The high current densities during fast charging can lead to thermal runaway risks due to the exothermic nature of sulfur reduction reactions. Temperature increases above critical thresholds (typically 80-100°C) may trigger separator melting, electrolyte decomposition, and in extreme cases, catastrophic cell failure.
The polysulfide shuttle effect, already a challenge in Li-S batteries, becomes particularly problematic under fast charging conditions. Rapid charging accelerates the dissolution of lithium polysulfides, which can migrate between electrodes and react with the lithium anode. This not only reduces coulombic efficiency but also creates safety hazards through the formation of lithium dendrites that may penetrate the separator and cause internal short circuits.
Mechanical degradation represents another critical concern during fast charging of Li-S batteries. The substantial volume changes (up to 80%) associated with sulfur-to-Li2S conversion create mechanical stresses that are amplified under high current conditions. This leads to particle cracking, active material isolation, and electrode delamination, significantly reducing cycle life and potentially creating internal short circuit pathways.
Electrolyte stability is severely compromised during fast charging operations. The high voltage potentials and elevated temperatures accelerate side reactions between the electrolyte and electrode materials. These parasitic reactions consume electrolyte, generate gaseous products that increase internal pressure, and form insulating surface films that impede lithium-ion transport, further increasing cell impedance and heat generation in a dangerous feedback loop.
Lithium anode degradation is particularly severe under fast charging conditions. The high current densities promote uneven lithium deposition, leading to dendrite formation and increased reactivity with the electrolyte. This not only depletes the available lithium inventory but also creates progressively growing internal resistance that further exacerbates heating issues during subsequent fast charging cycles.
Recent safety testing protocols have revealed that Li-S cells subjected to repeated fast charging exhibit significantly higher nail penetration and crush test failure rates compared to those cycled at conventional rates. Thermal imaging during abuse testing shows accelerated thermal propagation in fast-charged cells, indicating compromised thermal stability boundaries that must be addressed through advanced thermal management systems and safety mechanisms.
The polysulfide shuttle effect, already a challenge in Li-S batteries, becomes particularly problematic under fast charging conditions. Rapid charging accelerates the dissolution of lithium polysulfides, which can migrate between electrodes and react with the lithium anode. This not only reduces coulombic efficiency but also creates safety hazards through the formation of lithium dendrites that may penetrate the separator and cause internal short circuits.
Mechanical degradation represents another critical concern during fast charging of Li-S batteries. The substantial volume changes (up to 80%) associated with sulfur-to-Li2S conversion create mechanical stresses that are amplified under high current conditions. This leads to particle cracking, active material isolation, and electrode delamination, significantly reducing cycle life and potentially creating internal short circuit pathways.
Electrolyte stability is severely compromised during fast charging operations. The high voltage potentials and elevated temperatures accelerate side reactions between the electrolyte and electrode materials. These parasitic reactions consume electrolyte, generate gaseous products that increase internal pressure, and form insulating surface films that impede lithium-ion transport, further increasing cell impedance and heat generation in a dangerous feedback loop.
Lithium anode degradation is particularly severe under fast charging conditions. The high current densities promote uneven lithium deposition, leading to dendrite formation and increased reactivity with the electrolyte. This not only depletes the available lithium inventory but also creates progressively growing internal resistance that further exacerbates heating issues during subsequent fast charging cycles.
Recent safety testing protocols have revealed that Li-S cells subjected to repeated fast charging exhibit significantly higher nail penetration and crush test failure rates compared to those cycled at conventional rates. Thermal imaging during abuse testing shows accelerated thermal propagation in fast-charged cells, indicating compromised thermal stability boundaries that must be addressed through advanced thermal management systems and safety mechanisms.
Environmental Impact and Sustainability Assessment
The environmental implications of lithium-sulfur (Li-S) batteries under fast charging conditions represent a critical dimension of their overall performance assessment. Unlike conventional lithium-ion batteries, Li-S batteries utilize abundant and environmentally benign sulfur as a cathode material, potentially reducing reliance on scarce and environmentally problematic metals such as cobalt and nickel. However, the environmental advantages of Li-S technology may be compromised when subjected to fast charging protocols.
Fast charging conditions significantly impact the lifecycle environmental footprint of Li-S batteries through accelerated degradation mechanisms. The polysulfide shuttle effect, which intensifies during rapid charging, leads to premature capacity fading and shortened battery lifespan. This accelerated degradation necessitates more frequent battery replacement, thereby increasing resource consumption and waste generation throughout the battery lifecycle.
Energy efficiency during fast charging represents another environmental consideration. Li-S batteries typically exhibit lower coulombic efficiency under high-rate charging conditions, resulting in energy losses manifested as heat. This reduced efficiency translates directly to increased energy consumption during the use phase, potentially offsetting the environmental benefits derived from the materials composition of Li-S batteries.
Manufacturing processes for Li-S batteries optimized for fast charging may require additional materials or more energy-intensive production techniques. These could include specialized electrolyte additives, advanced separator materials, or more sophisticated carbon hosts for sulfur cathodes. The environmental impacts associated with these manufacturing modifications must be quantified through comprehensive life cycle assessment methodologies.
End-of-life management presents unique challenges for Li-S batteries subjected to fast charging regimes. The altered degradation patterns may affect the recoverability of materials during recycling processes. Current recycling infrastructure, primarily designed for conventional lithium-ion batteries, may require adaptation to efficiently recover materials from degraded Li-S cells, particularly those that have undergone extensive cycling under fast charge conditions.
From a sustainability perspective, the trade-off between charging speed and battery longevity demands careful consideration. While fast charging enhances user convenience and potentially supports wider adoption of electric mobility solutions, the associated environmental costs through shortened battery life may undermine sustainability objectives. Optimizing this balance requires holistic assessment frameworks that consider both technical performance metrics and environmental indicators across the entire battery lifecycle.
Water consumption represents an often-overlooked environmental aspect of battery technologies. The production of high-purity lithium compounds and specialized electrolytes for Li-S batteries designed for fast charging may entail significant water usage, particularly relevant in water-stressed regions where battery manufacturing facilities may be located.
Fast charging conditions significantly impact the lifecycle environmental footprint of Li-S batteries through accelerated degradation mechanisms. The polysulfide shuttle effect, which intensifies during rapid charging, leads to premature capacity fading and shortened battery lifespan. This accelerated degradation necessitates more frequent battery replacement, thereby increasing resource consumption and waste generation throughout the battery lifecycle.
Energy efficiency during fast charging represents another environmental consideration. Li-S batteries typically exhibit lower coulombic efficiency under high-rate charging conditions, resulting in energy losses manifested as heat. This reduced efficiency translates directly to increased energy consumption during the use phase, potentially offsetting the environmental benefits derived from the materials composition of Li-S batteries.
Manufacturing processes for Li-S batteries optimized for fast charging may require additional materials or more energy-intensive production techniques. These could include specialized electrolyte additives, advanced separator materials, or more sophisticated carbon hosts for sulfur cathodes. The environmental impacts associated with these manufacturing modifications must be quantified through comprehensive life cycle assessment methodologies.
End-of-life management presents unique challenges for Li-S batteries subjected to fast charging regimes. The altered degradation patterns may affect the recoverability of materials during recycling processes. Current recycling infrastructure, primarily designed for conventional lithium-ion batteries, may require adaptation to efficiently recover materials from degraded Li-S cells, particularly those that have undergone extensive cycling under fast charge conditions.
From a sustainability perspective, the trade-off between charging speed and battery longevity demands careful consideration. While fast charging enhances user convenience and potentially supports wider adoption of electric mobility solutions, the associated environmental costs through shortened battery life may undermine sustainability objectives. Optimizing this balance requires holistic assessment frameworks that consider both technical performance metrics and environmental indicators across the entire battery lifecycle.
Water consumption represents an often-overlooked environmental aspect of battery technologies. The production of high-purity lithium compounds and specialized electrolytes for Li-S batteries designed for fast charging may entail significant water usage, particularly relevant in water-stressed regions where battery manufacturing facilities may be located.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!