Lithium-sulfur battery performance improvement through cathode design
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Li-S Battery Evolution and Objectives
Lithium-sulfur (Li-S) batteries have emerged as a promising next-generation energy storage technology since their theoretical inception in the 1960s. The evolution of Li-S battery technology has been marked by significant advancements in understanding the complex electrochemical processes involved in sulfur cathodes. Initially, these batteries faced severe limitations including rapid capacity fading, low Coulombic efficiency, and short cycle life, primarily due to the dissolution of lithium polysulfides and their shuttle effect.
The 1990s witnessed the first major breakthrough when researchers began exploring carbon-sulfur composite cathodes to enhance conductivity and contain polysulfides. By the early 2000s, nanostructured carbon materials were introduced as sulfur hosts, marking a pivotal shift in cathode design philosophy. The period between 2010 and 2020 saw exponential growth in research focused on advanced cathode architectures, including hierarchical porous carbons, graphene-based materials, and metal-organic frameworks.
Current Li-S battery technology demonstrates energy densities of 300-400 Wh/kg, significantly surpassing conventional lithium-ion batteries (150-260 Wh/kg). However, this remains well below the theoretical maximum of 2,600 Wh/kg that Li-S chemistry promises. The disparity between theoretical potential and practical performance underscores the critical need for innovative cathode design strategies.
The primary objective of contemporary Li-S battery research is to develop cathode materials and architectures that can simultaneously address multiple challenges: enhancing sulfur utilization, suppressing polysulfide shuttling, accommodating volume expansion during cycling, and improving electronic conductivity. These improvements must be achieved while maintaining high sulfur loading to ensure commercially viable energy densities.
Recent technological trajectories indicate a shift toward multifunctional cathode designs that integrate physical confinement, chemical binding, and catalytic conversion of polysulfides. The integration of advanced characterization techniques, including in-situ and operando methods, has accelerated understanding of reaction mechanisms and degradation pathways, enabling more targeted cathode optimization strategies.
Looking forward, the field aims to achieve Li-S batteries with energy densities exceeding 500 Wh/kg, cycle life beyond 1,000 cycles, and Coulombic efficiencies consistently above 99%. These ambitious targets necessitate breakthrough innovations in cathode materials and architectures. The ultimate goal remains commercialization of Li-S technology for applications ranging from electric vehicles to grid-scale energy storage, where their theoretical advantages in energy density, cost, and sustainability could revolutionize the energy storage landscape.
The 1990s witnessed the first major breakthrough when researchers began exploring carbon-sulfur composite cathodes to enhance conductivity and contain polysulfides. By the early 2000s, nanostructured carbon materials were introduced as sulfur hosts, marking a pivotal shift in cathode design philosophy. The period between 2010 and 2020 saw exponential growth in research focused on advanced cathode architectures, including hierarchical porous carbons, graphene-based materials, and metal-organic frameworks.
Current Li-S battery technology demonstrates energy densities of 300-400 Wh/kg, significantly surpassing conventional lithium-ion batteries (150-260 Wh/kg). However, this remains well below the theoretical maximum of 2,600 Wh/kg that Li-S chemistry promises. The disparity between theoretical potential and practical performance underscores the critical need for innovative cathode design strategies.
The primary objective of contemporary Li-S battery research is to develop cathode materials and architectures that can simultaneously address multiple challenges: enhancing sulfur utilization, suppressing polysulfide shuttling, accommodating volume expansion during cycling, and improving electronic conductivity. These improvements must be achieved while maintaining high sulfur loading to ensure commercially viable energy densities.
Recent technological trajectories indicate a shift toward multifunctional cathode designs that integrate physical confinement, chemical binding, and catalytic conversion of polysulfides. The integration of advanced characterization techniques, including in-situ and operando methods, has accelerated understanding of reaction mechanisms and degradation pathways, enabling more targeted cathode optimization strategies.
Looking forward, the field aims to achieve Li-S batteries with energy densities exceeding 500 Wh/kg, cycle life beyond 1,000 cycles, and Coulombic efficiencies consistently above 99%. These ambitious targets necessitate breakthrough innovations in cathode materials and architectures. The ultimate goal remains commercialization of Li-S technology for applications ranging from electric vehicles to grid-scale energy storage, where their theoretical advantages in energy density, cost, and sustainability could revolutionize the energy storage landscape.
Market Analysis for Next-Gen Battery Technologies
The global battery market is experiencing a significant shift towards next-generation technologies, with lithium-sulfur (Li-S) batteries emerging as a promising alternative to conventional lithium-ion batteries. The market for advanced battery technologies is projected to reach $168 billion by 2030, with Li-S batteries potentially capturing 15-20% of this market due to their theoretical energy density advantages.
Current market analysis indicates that electric vehicles (EVs) represent the largest potential application segment for Li-S batteries, driven by the need for longer range and lighter weight energy storage solutions. The EV market is growing at approximately 25% annually, creating substantial demand for batteries with higher energy density than current lithium-ion offerings. Li-S batteries, with theoretical energy densities up to 2,600 Wh/kg compared to lithium-ion's 260 Wh/kg, could revolutionize this sector if cathode design challenges are overcome.
Consumer electronics constitutes another significant market opportunity, valued at $90 billion annually, where the lightweight properties and potentially lower cost of Li-S batteries could provide competitive advantages. Market research indicates consumers are willing to pay a 15-20% premium for devices with substantially longer battery life, creating a clear value proposition for improved Li-S technology.
Grid storage applications represent a rapidly expanding market segment, growing at 32% annually, where cost-effectiveness is paramount. The abundant nature of sulfur as a cathode material offers potential cost advantages over cobalt and nickel-based cathodes, potentially reducing raw material costs by 70% compared to conventional lithium-ion batteries.
Market barriers for Li-S battery adoption primarily center around cathode design challenges, including the polysulfide shuttle effect and volume expansion issues. Industry surveys indicate that manufacturers require cycle life improvements of at least 300% and capacity retention improvements of 50% before considering mass production. These technical hurdles directly impact market penetration timelines, with most industry analysts projecting significant commercial adoption beginning in 2026-2028.
Geographically, Asia-Pacific dominates battery manufacturing capacity, with China, Japan, and South Korea accounting for 85% of global production. However, recent investments in North America and Europe suggest a shifting landscape, with these regions expected to increase their market share to 30% by 2030, creating new opportunities for Li-S battery manufacturing and cathode design innovation.
Current market analysis indicates that electric vehicles (EVs) represent the largest potential application segment for Li-S batteries, driven by the need for longer range and lighter weight energy storage solutions. The EV market is growing at approximately 25% annually, creating substantial demand for batteries with higher energy density than current lithium-ion offerings. Li-S batteries, with theoretical energy densities up to 2,600 Wh/kg compared to lithium-ion's 260 Wh/kg, could revolutionize this sector if cathode design challenges are overcome.
Consumer electronics constitutes another significant market opportunity, valued at $90 billion annually, where the lightweight properties and potentially lower cost of Li-S batteries could provide competitive advantages. Market research indicates consumers are willing to pay a 15-20% premium for devices with substantially longer battery life, creating a clear value proposition for improved Li-S technology.
Grid storage applications represent a rapidly expanding market segment, growing at 32% annually, where cost-effectiveness is paramount. The abundant nature of sulfur as a cathode material offers potential cost advantages over cobalt and nickel-based cathodes, potentially reducing raw material costs by 70% compared to conventional lithium-ion batteries.
Market barriers for Li-S battery adoption primarily center around cathode design challenges, including the polysulfide shuttle effect and volume expansion issues. Industry surveys indicate that manufacturers require cycle life improvements of at least 300% and capacity retention improvements of 50% before considering mass production. These technical hurdles directly impact market penetration timelines, with most industry analysts projecting significant commercial adoption beginning in 2026-2028.
Geographically, Asia-Pacific dominates battery manufacturing capacity, with China, Japan, and South Korea accounting for 85% of global production. However, recent investments in North America and Europe suggest a shifting landscape, with these regions expected to increase their market share to 30% by 2030, creating new opportunities for Li-S battery manufacturing and cathode design innovation.
Technical Barriers in Li-S Cathode Development
Despite significant advancements in lithium-sulfur (Li-S) battery technology, several critical technical barriers persist in cathode development that hinder widespread commercial adoption. The most prominent challenge is the polysulfide shuttle effect, where soluble lithium polysulfides formed during discharge migrate between electrodes, causing active material loss, capacity fading, and reduced coulombic efficiency. This phenomenon fundamentally limits cycle life and represents a primary obstacle to long-term stability.
The insulating nature of sulfur and its discharge products presents another significant barrier. With sulfur's electrical conductivity approximately 5×10^-30 S/cm at room temperature, electron transfer is severely restricted, resulting in poor reaction kinetics and underutilization of active materials. This limitation necessitates high carbon content in cathodes, which reduces overall energy density.
Volume expansion during cycling constitutes a third major challenge. The sulfur-to-Li2S conversion process involves approximately 80% volume expansion, causing mechanical stress that leads to structural degradation of the cathode, particle isolation, and eventual capacity loss. This expansion-contraction cycle progressively damages electrode integrity over multiple cycles.
The low sulfur loading in conventional cathode designs severely constrains practical energy density. While theoretical energy density calculations assume high sulfur content, practical implementations typically achieve only 50-70% of theoretical values due to the necessity for conductive additives, binders, and host materials to address other technical issues.
Lithium sulfide (Li2S) precipitation kinetics represent another significant barrier. The nucleation and growth of solid Li2S during discharge occurs non-uniformly, creating passivation layers that block further electrochemical reactions and limit capacity utilization. This precipitation process is highly sensitive to discharge rates, electrolyte composition, and cathode architecture.
The cathode-electrolyte interface stability remains problematic, with continuous reactions between polysulfides and electrolyte components leading to electrolyte depletion and impedance growth. This interface degradation accelerates with cycling and temperature fluctuations, contributing to performance decay.
Finally, manufacturing scalability presents considerable challenges. Current high-performance Li-S cathode designs often rely on complex nanostructured materials or multi-step fabrication processes that are difficult to scale economically. The trade-off between performance optimization and manufacturing feasibility represents a significant barrier to industrial implementation.
The insulating nature of sulfur and its discharge products presents another significant barrier. With sulfur's electrical conductivity approximately 5×10^-30 S/cm at room temperature, electron transfer is severely restricted, resulting in poor reaction kinetics and underutilization of active materials. This limitation necessitates high carbon content in cathodes, which reduces overall energy density.
Volume expansion during cycling constitutes a third major challenge. The sulfur-to-Li2S conversion process involves approximately 80% volume expansion, causing mechanical stress that leads to structural degradation of the cathode, particle isolation, and eventual capacity loss. This expansion-contraction cycle progressively damages electrode integrity over multiple cycles.
The low sulfur loading in conventional cathode designs severely constrains practical energy density. While theoretical energy density calculations assume high sulfur content, practical implementations typically achieve only 50-70% of theoretical values due to the necessity for conductive additives, binders, and host materials to address other technical issues.
Lithium sulfide (Li2S) precipitation kinetics represent another significant barrier. The nucleation and growth of solid Li2S during discharge occurs non-uniformly, creating passivation layers that block further electrochemical reactions and limit capacity utilization. This precipitation process is highly sensitive to discharge rates, electrolyte composition, and cathode architecture.
The cathode-electrolyte interface stability remains problematic, with continuous reactions between polysulfides and electrolyte components leading to electrolyte depletion and impedance growth. This interface degradation accelerates with cycling and temperature fluctuations, contributing to performance decay.
Finally, manufacturing scalability presents considerable challenges. Current high-performance Li-S cathode designs often rely on complex nanostructured materials or multi-step fabrication processes that are difficult to scale economically. The trade-off between performance optimization and manufacturing feasibility represents a significant barrier to industrial implementation.
Current Cathode Design Approaches
01 Electrode materials for improved performance
Advanced electrode materials can significantly enhance lithium-sulfur battery performance. These include specially designed cathode materials that can effectively contain sulfur and prevent polysulfide dissolution, as well as novel anode materials that improve lithium ion transport. Nanostructured carbon materials, metal oxides, and conductive polymers are commonly used to create composite electrodes with higher capacity, better cycling stability, and improved rate capability.- Electrode materials for improved performance: Advanced electrode materials can significantly enhance lithium-sulfur battery performance. These include specialized carbon-based materials, metal oxides, and composite structures that provide better sulfur utilization and cycling stability. The electrode materials help contain polysulfides, improve conductivity, and create stable interfaces, resulting in higher capacity retention and extended battery life.
- Electrolyte modifications and additives: Optimized electrolyte formulations play a crucial role in lithium-sulfur battery performance. By incorporating specific additives, solvents, and salts, the shuttle effect of polysulfides can be suppressed, and the lithium anode can be protected from degradation. These modifications improve ionic conductivity, enhance the electrochemical stability window, and facilitate better sulfur utilization during cycling.
- Protective coatings and interlayers: Implementing protective coatings and functional interlayers between electrodes can significantly enhance lithium-sulfur battery performance. These layers act as physical barriers to prevent polysulfide shuttling while maintaining good ionic conductivity. Various materials including polymers, ceramics, and composite structures can be used to create these protective interfaces, resulting in improved cycling stability and capacity retention.
- Nanostructured sulfur hosts: Nanostructured materials designed to host sulfur can dramatically improve lithium-sulfur battery performance. These host structures provide physical confinement of sulfur and its discharge products, enhance electronic conductivity, and facilitate electrolyte penetration. By controlling the architecture at the nanoscale, these hosts enable better utilization of active material, faster reaction kinetics, and improved cycling stability.
- Advanced cell design and manufacturing techniques: Innovative cell designs and manufacturing approaches can overcome traditional limitations of lithium-sulfur batteries. These include optimized electrode thickness ratios, novel cell configurations, and specialized production techniques that address volume expansion issues. Advanced manufacturing methods ensure uniform distribution of active materials, proper electrolyte infiltration, and effective pressure management, resulting in batteries with higher energy density and improved cycle life.
02 Electrolyte modifications and additives
Electrolyte composition plays a crucial role in lithium-sulfur battery performance. Modifications include the use of ionic liquids, solid-state electrolytes, and various additives that suppress the shuttle effect of polysulfides. These electrolyte systems can effectively enhance the ionic conductivity, interfacial stability, and overall electrochemical performance of lithium-sulfur batteries, leading to improved cycle life and energy density.Expand Specific Solutions03 Sulfur host structures and confinement strategies
Various host structures have been developed to effectively confine sulfur and its discharge products within the cathode. These include porous carbon frameworks, hollow carbon spheres, and metal-organic frameworks that physically trap polysulfides and prevent their dissolution into the electrolyte. Chemical binding approaches using polar materials that can form strong interactions with polysulfides further enhance the cycling stability and capacity retention of lithium-sulfur batteries.Expand Specific Solutions04 Interlayers and separator modifications
Functional interlayers and modified separators can effectively block polysulfide migration between electrodes. These components act as physical barriers and chemical adsorbents for soluble polysulfides, preventing the shuttle effect and reducing capacity fading. Carbon-based interlayers, polymer coatings, and ceramic-enhanced separators have shown significant improvements in battery performance by maintaining electrode integrity and preventing side reactions.Expand Specific Solutions05 Cell design and engineering approaches
Novel cell designs and engineering approaches can address fundamental challenges in lithium-sulfur batteries. These include optimized electrode thickness and porosity, advanced current collector designs, and innovative cell configurations that accommodate volume changes during cycling. Pressure-applying cell designs, lean electrolyte systems, and integrated battery management systems can significantly improve energy density, power capability, and operational safety of lithium-sulfur batteries.Expand Specific Solutions
Industry Leaders in Li-S Battery Research
The lithium-sulfur battery market is in an early growth phase, characterized by intensive R&D efforts to overcome performance limitations. Major players include established battery manufacturers like SAMSUNG SDI, LG Energy Solution, and LG Chem, who are leveraging their manufacturing expertise to commercialize this technology. Academic institutions (MIT, Cornell, Penn State) are driving fundamental research, while specialized companies like Lyten are developing proprietary cathode designs. The market is projected to grow significantly as technical challenges are addressed, with key innovations coming from collaborative efforts between industry and research institutions. Technical maturity varies, with companies like SAMSUNG SDI and Lyten demonstrating promising cathode designs that improve cycle life and energy density, though commercial viability at scale remains a challenge.
SAMSUNG SDI CO LTD
Technical Solution: Samsung SDI has developed innovative cathode designs for lithium-sulfur batteries focusing on hierarchical carbon-sulfur composites. Their approach involves encapsulating sulfur within mesoporous carbon structures with controlled pore sizes (2-50 nm) to physically confine polysulfides while maintaining high sulfur loading (>70 wt%). They've implemented a dual-layer cathode design with an inner sulfur-carbon composite core and an outer protective carbon layer that acts as a physical barrier against polysulfide diffusion[1]. Samsung has also pioneered the use of graphene-based materials as conductive additives in cathodes, creating 3D interconnected networks that enhance electron transport while providing additional polysulfide trapping sites through chemical bonding[2]. Their recent advancements include nitrogen and oxygen co-doped carbon hosts that form strong chemical bonds with lithium polysulfides, significantly reducing the shuttle effect.
Strengths: Superior polysulfide confinement through hierarchical pore structures; excellent electrical conductivity from graphene additives; high sulfur utilization (>80%) in initial cycles. Weaknesses: Complex manufacturing process increases production costs; gradual capacity decay still occurs over extended cycling (>300 cycles); energy density advantages diminish with the addition of carbon materials that don't contribute to capacity.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed a multi-functional cathode architecture for lithium-sulfur batteries centered around their proprietary "sulfur-trapping matrix" technology. This approach utilizes a core-shell structure where nano-sized sulfur particles (30-50 nm) are encapsulated within nitrogen-rich carbon frameworks derived from metal-organic frameworks (MOFs)[1]. The nitrogen functional groups create strong chemical interactions with lithium polysulfides, effectively suppressing their dissolution. LG has further enhanced this design by incorporating transition metal compounds (primarily cobalt and nickel oxides) as catalytic sites that accelerate the conversion reactions between soluble polysulfides and insoluble Li2S/S8[2]. Their latest innovation involves a gradient cathode structure with varying carbon-to-sulfur ratios throughout the electrode thickness, optimizing both sulfur utilization and ion transport kinetics. This design achieves sulfur loadings of 5-6 mg/cm² while maintaining high areal capacity (>5 mAh/cm²) over 500+ cycles.
Strengths: Excellent cycle stability with >80% capacity retention after 500 cycles; high sulfur utilization due to catalytic conversion reactions; scalable manufacturing process compatible with existing production lines. Weaknesses: Relatively low initial Coulombic efficiency (~75-80%); performance degradation at high discharge rates (>2C); reliance on expensive transition metal compounds increases overall cathode cost.
Key Patents in Sulfur Cathode Engineering
Lithium-sulfur battery and cathode therefore
PatentActiveEP2250688A2
Innovation
- Incorporating a metal oxide, such as CuO, Bi2O3, SnO, ZnO, or Mn2O3, into the sulfur cathode to trap polysulfides and using a polymeric material and inorganic additives in the separator to prevent migration, along with a barrier that ruptures to allow electrolyte contact, enhancing discharge efficiency and service life.
Cathode for lithium-sulfur batteries
PatentPendingUS20240145707A1
Innovation
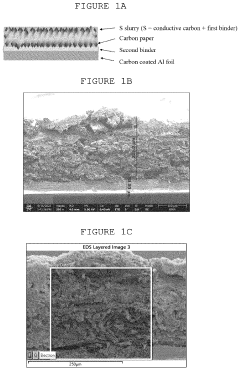
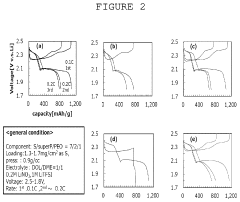
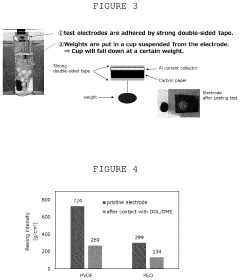
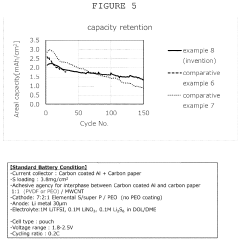
- A dual-layer sulfur cathode configuration is introduced, featuring a porous carbon layer as a host for sulfur active material, with a first binder to suppress polysulfide shuttle and a second binder for mechanical integrity, using polyethylene oxide and poly(vinylidene difluoride) to enhance adhesion and swelling properties.
Environmental Impact Assessment
The environmental impact of lithium-sulfur (Li-S) batteries represents a critical consideration in their development and implementation, particularly as cathode design innovations continue to advance performance metrics. Unlike conventional lithium-ion batteries that rely heavily on cobalt and nickel, Li-S batteries utilize sulfur as the primary cathode material, offering significant environmental advantages. Sulfur is abundantly available as a byproduct of petroleum refining processes, transforming what would otherwise be industrial waste into a valuable battery component.
The life cycle assessment of Li-S batteries with advanced cathode designs demonstrates reduced carbon footprints compared to traditional lithium-ion technologies. Studies indicate that the production phase of sulfur cathodes generates approximately 60% less greenhouse gas emissions than conventional cathode materials. This reduction stems primarily from the elimination of energy-intensive mining operations associated with cobalt and nickel extraction, which often occur in environmentally sensitive regions.
Water consumption metrics also favor Li-S technology, with manufacturing processes requiring approximately 40-50% less water than conventional lithium-ion battery production. This advantage becomes particularly significant in regions facing water scarcity challenges, where battery manufacturing facilities may compete with agricultural and municipal water needs.
The end-of-life considerations for Li-S batteries with engineered cathodes present both opportunities and challenges. The sulfur component is inherently less toxic than heavy metals used in conventional batteries, reducing leaching concerns in landfill environments. Additionally, recycling processes for sulfur cathodes can be less energy-intensive, with some innovative approaches achieving recovery rates exceeding 90% for both lithium and sulfur components.
However, certain cathode design approaches incorporating nanomaterials or complex carbon structures may introduce new environmental considerations. The production of carbon nanotubes or graphene-based materials often involves energy-intensive processes and potentially hazardous chemicals. Environmental risk assessments must therefore evaluate these trade-offs between performance enhancements and manufacturing impacts.
Land use impacts associated with Li-S battery production generally show improvement over conventional technologies. The sulfur supply chain typically requires less land disturbance than mining operations for cobalt and nickel, reducing habitat fragmentation and biodiversity impacts. This advantage becomes particularly pronounced when considering the ecological damage associated with cobalt mining in regions like the Democratic Republic of Congo.
The life cycle assessment of Li-S batteries with advanced cathode designs demonstrates reduced carbon footprints compared to traditional lithium-ion technologies. Studies indicate that the production phase of sulfur cathodes generates approximately 60% less greenhouse gas emissions than conventional cathode materials. This reduction stems primarily from the elimination of energy-intensive mining operations associated with cobalt and nickel extraction, which often occur in environmentally sensitive regions.
Water consumption metrics also favor Li-S technology, with manufacturing processes requiring approximately 40-50% less water than conventional lithium-ion battery production. This advantage becomes particularly significant in regions facing water scarcity challenges, where battery manufacturing facilities may compete with agricultural and municipal water needs.
The end-of-life considerations for Li-S batteries with engineered cathodes present both opportunities and challenges. The sulfur component is inherently less toxic than heavy metals used in conventional batteries, reducing leaching concerns in landfill environments. Additionally, recycling processes for sulfur cathodes can be less energy-intensive, with some innovative approaches achieving recovery rates exceeding 90% for both lithium and sulfur components.
However, certain cathode design approaches incorporating nanomaterials or complex carbon structures may introduce new environmental considerations. The production of carbon nanotubes or graphene-based materials often involves energy-intensive processes and potentially hazardous chemicals. Environmental risk assessments must therefore evaluate these trade-offs between performance enhancements and manufacturing impacts.
Land use impacts associated with Li-S battery production generally show improvement over conventional technologies. The sulfur supply chain typically requires less land disturbance than mining operations for cobalt and nickel, reducing habitat fragmentation and biodiversity impacts. This advantage becomes particularly pronounced when considering the ecological damage associated with cobalt mining in regions like the Democratic Republic of Congo.
Manufacturing Scalability Challenges
The scaling of lithium-sulfur battery cathode manufacturing from laboratory to industrial production presents significant challenges that must be addressed to enable commercial viability. Current laboratory-scale cathode preparation methods typically involve complex processes such as solution-based sulfur impregnation into carbon hosts, which are difficult to translate to mass production environments. These processes often require precise control of temperature, pressure, and chemical environments that become increasingly challenging to maintain consistently at larger scales.
Material handling represents another major obstacle in manufacturing scalability. Sulfur and its intermediates are highly reactive and sensitive to environmental conditions, necessitating specialized equipment and controlled atmospheres during production. The viscosity and rheological properties of sulfur-carbon slurries also change dramatically with scale, affecting coating uniformity and adhesion to current collectors when transitioning from small-scale to roll-to-roll processing.
Quality control becomes exponentially more complex at industrial scales. The homogeneity of sulfur distribution within carbon matrices, critical for optimal electrochemical performance, is difficult to maintain consistently across large production batches. Current analytical techniques suitable for laboratory samples may be inadequate for rapid, in-line quality assessment needed in high-volume manufacturing environments.
Cost considerations further complicate scaling efforts. While advanced cathode designs incorporating sophisticated carbon architectures and functional additives show promising performance in research settings, their production costs often prove prohibitive at industrial scales. The economic viability of lithium-sulfur batteries depends on developing cathode manufacturing processes that balance performance requirements with cost-effective production methods.
Environmental and safety concerns also present significant challenges. Sulfur processing generates hydrogen sulfide and other toxic compounds that require comprehensive containment and treatment systems at industrial scales. These safety measures add complexity and cost to manufacturing facilities, potentially offsetting the inherent cost advantages of sulfur as a cathode material.
The transition from batch processing common in laboratories to continuous manufacturing required for commercial production represents perhaps the most fundamental scaling challenge. Developing continuous processes for sulfur melt infiltration, carbon-sulfur composite formation, and electrode coating that maintain the delicate nanostructures critical for performance remains an active area of research with significant technical hurdles yet to be overcome.
Material handling represents another major obstacle in manufacturing scalability. Sulfur and its intermediates are highly reactive and sensitive to environmental conditions, necessitating specialized equipment and controlled atmospheres during production. The viscosity and rheological properties of sulfur-carbon slurries also change dramatically with scale, affecting coating uniformity and adhesion to current collectors when transitioning from small-scale to roll-to-roll processing.
Quality control becomes exponentially more complex at industrial scales. The homogeneity of sulfur distribution within carbon matrices, critical for optimal electrochemical performance, is difficult to maintain consistently across large production batches. Current analytical techniques suitable for laboratory samples may be inadequate for rapid, in-line quality assessment needed in high-volume manufacturing environments.
Cost considerations further complicate scaling efforts. While advanced cathode designs incorporating sophisticated carbon architectures and functional additives show promising performance in research settings, their production costs often prove prohibitive at industrial scales. The economic viability of lithium-sulfur batteries depends on developing cathode manufacturing processes that balance performance requirements with cost-effective production methods.
Environmental and safety concerns also present significant challenges. Sulfur processing generates hydrogen sulfide and other toxic compounds that require comprehensive containment and treatment systems at industrial scales. These safety measures add complexity and cost to manufacturing facilities, potentially offsetting the inherent cost advantages of sulfur as a cathode material.
The transition from batch processing common in laboratories to continuous manufacturing required for commercial production represents perhaps the most fundamental scaling challenge. Developing continuous processes for sulfur melt infiltration, carbon-sulfur composite formation, and electrode coating that maintain the delicate nanostructures critical for performance remains an active area of research with significant technical hurdles yet to be overcome.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!