Lithium anode protection in lithium-sulfur battery systems
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Anode Protection Background and Objectives
Lithium-sulfur (Li-S) batteries have emerged as a promising next-generation energy storage technology due to their theoretical energy density of 2600 Wh/kg, which far exceeds that of conventional lithium-ion batteries. The development of Li-S battery technology can be traced back to the 1960s, but significant research momentum has only built up in the past two decades as the demand for high-energy-density storage solutions has intensified across various sectors including electric vehicles, portable electronics, and grid storage.
The lithium metal anode is a critical component in Li-S battery systems, offering a theoretical specific capacity of 3860 mAh/g - approximately ten times higher than traditional graphite anodes. However, the practical implementation of lithium metal anodes faces substantial challenges that have hindered the commercialization of Li-S batteries. These challenges primarily stem from the high reactivity of lithium metal with electrolytes and the formation of dendritic structures during cycling.
The evolution of lithium anode protection strategies has progressed through several phases. Initial approaches focused on electrolyte modifications, followed by the development of artificial solid electrolyte interphase (SEI) layers. More recently, research has shifted toward advanced composite protective layers and three-dimensional host structures to accommodate volume changes and suppress dendrite growth.
Current technological trends in lithium anode protection include the exploration of nanomaterials, polymer composites, and ceramic-based protective layers. These approaches aim to create stable interfaces between the lithium metal and the electrolyte, thereby mitigating the issues of dendrite formation and parasitic reactions that lead to capacity fade and safety concerns.
The primary technical objectives for lithium anode protection in Li-S battery systems include: extending cycle life to over 1000 cycles with minimal capacity degradation; improving Coulombic efficiency to above 99.9%; preventing dendrite formation even at high current densities; and ensuring compatibility with high-sulfur-loading cathodes to maximize energy density at the cell level.
Additionally, researchers aim to develop protection strategies that are scalable and cost-effective for mass production, as commercial viability remains a significant hurdle for Li-S technology. This includes the development of protection methods that utilize earth-abundant materials and straightforward manufacturing processes.
The ultimate goal is to enable Li-S batteries that combine high energy density, long cycle life, and operational safety - a combination that would revolutionize energy storage capabilities across multiple applications and potentially accelerate the transition to renewable energy systems and electrified transportation.
The lithium metal anode is a critical component in Li-S battery systems, offering a theoretical specific capacity of 3860 mAh/g - approximately ten times higher than traditional graphite anodes. However, the practical implementation of lithium metal anodes faces substantial challenges that have hindered the commercialization of Li-S batteries. These challenges primarily stem from the high reactivity of lithium metal with electrolytes and the formation of dendritic structures during cycling.
The evolution of lithium anode protection strategies has progressed through several phases. Initial approaches focused on electrolyte modifications, followed by the development of artificial solid electrolyte interphase (SEI) layers. More recently, research has shifted toward advanced composite protective layers and three-dimensional host structures to accommodate volume changes and suppress dendrite growth.
Current technological trends in lithium anode protection include the exploration of nanomaterials, polymer composites, and ceramic-based protective layers. These approaches aim to create stable interfaces between the lithium metal and the electrolyte, thereby mitigating the issues of dendrite formation and parasitic reactions that lead to capacity fade and safety concerns.
The primary technical objectives for lithium anode protection in Li-S battery systems include: extending cycle life to over 1000 cycles with minimal capacity degradation; improving Coulombic efficiency to above 99.9%; preventing dendrite formation even at high current densities; and ensuring compatibility with high-sulfur-loading cathodes to maximize energy density at the cell level.
Additionally, researchers aim to develop protection strategies that are scalable and cost-effective for mass production, as commercial viability remains a significant hurdle for Li-S technology. This includes the development of protection methods that utilize earth-abundant materials and straightforward manufacturing processes.
The ultimate goal is to enable Li-S batteries that combine high energy density, long cycle life, and operational safety - a combination that would revolutionize energy storage capabilities across multiple applications and potentially accelerate the transition to renewable energy systems and electrified transportation.
Market Analysis for Li-S Battery Systems
The lithium-sulfur (Li-S) battery market is experiencing significant growth potential due to the technology's theoretical energy density advantages over conventional lithium-ion batteries. Current market projections indicate that the global Li-S battery market could reach $2.1 billion by 2030, with a compound annual growth rate exceeding 30% from 2023 to 2030. This rapid expansion is primarily driven by increasing demands for high-energy-density storage solutions in aerospace, defense, and electric vehicle applications.
The electric vehicle sector represents the largest potential market for Li-S batteries, with automotive manufacturers actively seeking alternatives to traditional lithium-ion technologies to extend driving ranges and reduce battery costs. Market research suggests that if lithium anode protection challenges are adequately addressed, Li-S batteries could potentially capture up to 15% of the premium electric vehicle battery market by 2035.
Consumer electronics manufacturers are also showing growing interest in Li-S technology, particularly for applications requiring lightweight power sources with extended operation times. The drone and portable electronics segments are expected to serve as early adoption markets, with several companies already incorporating prototype Li-S cells into their product development roadmaps.
From a geographical perspective, Asia-Pacific currently dominates Li-S battery research and development activities, with China, South Korea, and Japan leading commercial development efforts. North America and Europe follow closely, with significant investments in both academic research and industrial scale-up projects focused specifically on lithium anode protection technologies.
Market barriers remain substantial, with technical challenges related to lithium anode protection being the primary obstacle to widespread commercialization. End-user surveys indicate that cycle life limitations resulting from unprotected lithium anodes represent the most significant concern among potential adopters, followed by safety considerations and manufacturing scalability.
The competitive landscape includes both established battery manufacturers expanding into Li-S technology and specialized startups focused exclusively on overcoming lithium anode protection challenges. Strategic partnerships between material suppliers, cell manufacturers, and end-users are becoming increasingly common as the industry works to accelerate commercialization timelines.
Price sensitivity analysis reveals that Li-S batteries must achieve a production cost below $100/kWh to compete effectively with next-generation lithium-ion technologies in mass-market applications. Current production estimates place prototype Li-S cells at approximately $300-400/kWh, highlighting the need for continued innovation in lithium anode protection to reduce overall system costs while maintaining performance advantages.
The electric vehicle sector represents the largest potential market for Li-S batteries, with automotive manufacturers actively seeking alternatives to traditional lithium-ion technologies to extend driving ranges and reduce battery costs. Market research suggests that if lithium anode protection challenges are adequately addressed, Li-S batteries could potentially capture up to 15% of the premium electric vehicle battery market by 2035.
Consumer electronics manufacturers are also showing growing interest in Li-S technology, particularly for applications requiring lightweight power sources with extended operation times. The drone and portable electronics segments are expected to serve as early adoption markets, with several companies already incorporating prototype Li-S cells into their product development roadmaps.
From a geographical perspective, Asia-Pacific currently dominates Li-S battery research and development activities, with China, South Korea, and Japan leading commercial development efforts. North America and Europe follow closely, with significant investments in both academic research and industrial scale-up projects focused specifically on lithium anode protection technologies.
Market barriers remain substantial, with technical challenges related to lithium anode protection being the primary obstacle to widespread commercialization. End-user surveys indicate that cycle life limitations resulting from unprotected lithium anodes represent the most significant concern among potential adopters, followed by safety considerations and manufacturing scalability.
The competitive landscape includes both established battery manufacturers expanding into Li-S technology and specialized startups focused exclusively on overcoming lithium anode protection challenges. Strategic partnerships between material suppliers, cell manufacturers, and end-users are becoming increasingly common as the industry works to accelerate commercialization timelines.
Price sensitivity analysis reveals that Li-S batteries must achieve a production cost below $100/kWh to compete effectively with next-generation lithium-ion technologies in mass-market applications. Current production estimates place prototype Li-S cells at approximately $300-400/kWh, highlighting the need for continued innovation in lithium anode protection to reduce overall system costs while maintaining performance advantages.
Technical Challenges in Lithium Anode Protection
Lithium-sulfur (Li-S) batteries represent a promising next-generation energy storage technology due to their high theoretical energy density (2600 Wh/kg) and the natural abundance of sulfur. However, the lithium metal anode, while offering high specific capacity (3860 mAh/g), presents significant challenges that hinder the commercial viability of Li-S battery systems.
The primary challenge with lithium metal anodes is their high reactivity with electrolytes, leading to continuous SEI (Solid Electrolyte Interphase) formation and consumption of both lithium and electrolyte. This parasitic reaction not only reduces coulombic efficiency but also shortens battery lifespan. In Li-S systems specifically, this issue is exacerbated by the polysulfide shuttle effect, where dissolved polysulfides migrate to the lithium anode and react directly with lithium metal.
Dendrite formation represents another critical challenge. During cycling, lithium tends to deposit unevenly on the anode surface, forming needle-like structures that can penetrate the separator and cause catastrophic short circuits. The non-uniform lithium deposition is particularly problematic in Li-S batteries due to the complex electrolyte environment containing various sulfur species.
Volume expansion during cycling poses additional difficulties. Lithium metal undergoes significant volumetric changes (approximately 80%) during plating and stripping processes. This mechanical instability leads to pulverization of the SEI layer, exposing fresh lithium surfaces to the electrolyte and accelerating degradation processes.
The electrolyte compatibility issue further complicates lithium anode protection. Conventional carbonate-based electrolytes, while stable with intercalation anodes, react vigorously with lithium metal. Ether-based electrolytes commonly used in Li-S batteries offer better compatibility but still cannot completely prevent side reactions with lithium metal.
Interface stability between the lithium anode and solid-state electrolytes presents challenges for all-solid-state Li-S batteries. The high interfacial resistance and poor contact at the lithium/solid electrolyte interface limit ion transport and increase cell impedance. Additionally, the mechanical stress from volume changes can create voids at this interface, further degrading performance.
Self-discharge is particularly problematic in Li-S systems, where dissolved polysulfides can continuously react with the lithium anode even during storage periods. This parasitic reaction not only consumes active materials but also creates an increasingly resistive interface layer that impedes normal battery operation.
These technical challenges are interconnected and mutually reinforcing, creating a complex problem space that requires innovative, multifaceted protection strategies to enable practical Li-S battery systems with long cycle life and stable performance.
The primary challenge with lithium metal anodes is their high reactivity with electrolytes, leading to continuous SEI (Solid Electrolyte Interphase) formation and consumption of both lithium and electrolyte. This parasitic reaction not only reduces coulombic efficiency but also shortens battery lifespan. In Li-S systems specifically, this issue is exacerbated by the polysulfide shuttle effect, where dissolved polysulfides migrate to the lithium anode and react directly with lithium metal.
Dendrite formation represents another critical challenge. During cycling, lithium tends to deposit unevenly on the anode surface, forming needle-like structures that can penetrate the separator and cause catastrophic short circuits. The non-uniform lithium deposition is particularly problematic in Li-S batteries due to the complex electrolyte environment containing various sulfur species.
Volume expansion during cycling poses additional difficulties. Lithium metal undergoes significant volumetric changes (approximately 80%) during plating and stripping processes. This mechanical instability leads to pulverization of the SEI layer, exposing fresh lithium surfaces to the electrolyte and accelerating degradation processes.
The electrolyte compatibility issue further complicates lithium anode protection. Conventional carbonate-based electrolytes, while stable with intercalation anodes, react vigorously with lithium metal. Ether-based electrolytes commonly used in Li-S batteries offer better compatibility but still cannot completely prevent side reactions with lithium metal.
Interface stability between the lithium anode and solid-state electrolytes presents challenges for all-solid-state Li-S batteries. The high interfacial resistance and poor contact at the lithium/solid electrolyte interface limit ion transport and increase cell impedance. Additionally, the mechanical stress from volume changes can create voids at this interface, further degrading performance.
Self-discharge is particularly problematic in Li-S systems, where dissolved polysulfides can continuously react with the lithium anode even during storage periods. This parasitic reaction not only consumes active materials but also creates an increasingly resistive interface layer that impedes normal battery operation.
These technical challenges are interconnected and mutually reinforcing, creating a complex problem space that requires innovative, multifaceted protection strategies to enable practical Li-S battery systems with long cycle life and stable performance.
Current Protection Solutions for Lithium Anodes
01 Protective coatings for lithium anodes
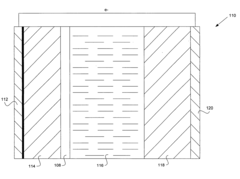
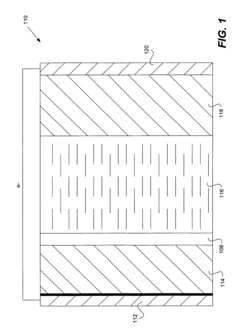
Various protective coatings can be applied to lithium anodes to prevent degradation and enhance battery performance. These coatings create a physical barrier between the lithium metal and the electrolyte, reducing unwanted reactions. Materials used for these protective layers include polymers, ceramics, and composite materials that allow lithium ion transport while blocking side reactions that lead to capacity loss and dendrite formation.- Solid electrolyte interface (SEI) formation for lithium anode protection: Formation of a stable solid electrolyte interface (SEI) layer on lithium metal anodes is crucial for protecting the anode from continuous reactions with the electrolyte. Various additives and electrolyte compositions can be used to form a more stable and uniform SEI layer, which prevents dendrite formation and reduces capacity loss during cycling. This protective layer acts as a barrier while still allowing lithium ion transport, significantly improving the cycling stability and safety of lithium metal batteries.
- Protective coatings and artificial layers for lithium anodes: Applying protective coatings or artificial layers directly onto lithium metal anodes can effectively prevent direct contact between the lithium and electrolyte. These coatings can be made from various materials including polymers, ceramics, or composite materials that are lithium-ion conductive but electronically insulating. Such protective layers help suppress dendrite growth, reduce side reactions, and improve the electrochemical performance and safety of lithium metal batteries by maintaining the structural integrity of the anode during cycling.
- Electrolyte modifications and additives for lithium anode stability: Modifying the electrolyte composition by incorporating specific additives can significantly enhance lithium anode protection. These additives can include fluorinated compounds, lithium salts, or organic molecules that contribute to forming more stable interfaces on the lithium surface. Advanced electrolyte formulations can regulate lithium deposition behavior, suppress dendrite growth, and reduce unwanted side reactions, thereby extending battery life and improving safety characteristics of lithium metal batteries.
- Structured and 3D lithium anodes for improved stability: Designing structured or three-dimensional lithium anodes can provide better mechanical stability and electrochemical performance. These designs include patterned lithium surfaces, 3D host structures, or composite architectures that guide uniform lithium deposition and reduce local current density hotspots. By controlling the deposition and stripping processes, these structured anodes can accommodate volume changes during cycling, minimize dendrite formation, and enhance the overall stability and lifespan of lithium metal batteries.
- Advanced separator technologies for lithium anode protection: Specialized separator technologies play a crucial role in protecting lithium anodes. These include functional separators with ceramic coatings, polymer-reinforced membranes, or composite separators with specific surface treatments. Advanced separators can physically block dendrite penetration, improve ion transport uniformity, and enhance thermal stability. By preventing internal short circuits and maintaining consistent ion flux across the electrode interface, these separator technologies significantly improve the safety and performance of lithium metal batteries.
02 Solid electrolyte interfaces (SEI) for lithium protection
Engineered solid electrolyte interfaces (SEI) can be formed on lithium anodes to provide protection against electrolyte reactions. These interfaces can be created through additives in the electrolyte that decompose to form a stable protective layer, or through pre-treatment of the lithium surface. A well-designed SEI layer allows lithium ions to pass through while preventing continuous electrolyte decomposition, thereby extending battery life and improving safety.Expand Specific Solutions03 Electrolyte modifications for lithium anode stability
Modified electrolyte formulations can significantly improve lithium anode protection. These modifications include incorporating additives that form stable interfaces with lithium, using concentrated electrolytes that reduce solvent activity, or employing localized high-concentration electrolytes. Such approaches minimize parasitic reactions between the lithium anode and electrolyte components, reducing dendrite formation and improving cycling efficiency.Expand Specific Solutions04 Artificial SEI layers and interlayers
Artificial SEI layers and interlayers can be deliberately applied to lithium anodes before battery assembly. These engineered protective layers are designed with specific properties to enhance lithium ion transport while blocking unwanted side reactions. Materials used include lithium-conducting ceramics, polymers, and composite structures that provide mechanical stability and chemical protection against electrolyte components, resulting in improved cycling performance and battery longevity.Expand Specific Solutions05 Three-dimensional and structured lithium anodes
Three-dimensional and structured lithium anode designs can inherently improve protection against degradation. These approaches include using lithium-host materials, 3D current collectors, and patterned lithium deposition structures. By controlling the spatial distribution of lithium and providing mechanical support, these designs help regulate lithium deposition/dissolution processes, reduce local current densities, and mitigate dendrite formation, leading to more stable cycling performance.Expand Specific Solutions
Key Industry Players in Li-S Battery Technology
The lithium-sulfur battery market is currently in an early growth phase, characterized by significant R&D investment but limited commercial deployment. The global market size is projected to expand rapidly, driven by demand for higher energy density solutions in electric vehicles and energy storage. Technologically, lithium anode protection remains a critical challenge, with varying maturity levels across competitors. Leading players include PolyPlus Battery with its protected lithium electrode (PLE™) technology, Sion Power with its Licerion® technology, and major battery manufacturers like CATL, LG Energy Solution, and Samsung SDI who are investing heavily in research. Academic-industrial partnerships are accelerating development, with institutions like South China University of Technology and Dalian Institute collaborating with commercial entities to overcome the persistent challenges of lithium dendrite formation and polysulfide shuttling.
PolyPlus Battery Co., Inc.
Technical Solution: PolyPlus has pioneered the Protected Lithium Electrode (PLE) technology specifically designed for lithium-sulfur battery systems. Their approach involves a solid-state lithium ion-conducting membrane that physically separates the lithium metal anode from the electrolyte while allowing lithium ions to pass through. This membrane acts as a protective layer preventing direct contact between lithium and polysulfides, effectively addressing the shuttle effect. The company has developed a proprietary ceramic-polymer composite membrane with high ionic conductivity and mechanical stability that can withstand the volume changes during cycling. Their technology also incorporates a specialized interface engineering approach that ensures stable contact between the lithium metal and the protective layer, minimizing interfacial resistance and preventing dendrite formation even at high current densities.
Strengths: Superior protection against polysulfide shuttling, excellent mechanical stability preventing dendrite penetration, and compatibility with various electrolyte systems. Weaknesses: Higher manufacturing costs compared to conventional approaches, potential challenges in scaling production, and possible limitations in power density due to increased internal resistance from the protective layer.
SAMSUNG SDI CO LTD
Technical Solution: Samsung SDI has developed an advanced lithium anode protection system for lithium-sulfur batteries utilizing a graphene-based protective layer. Their approach involves coating the lithium metal anode with a thin, flexible graphene oxide layer that has been chemically modified to enhance lithium-ion conductivity while blocking polysulfide migration. This protective layer is applied through a proprietary deposition process that ensures uniform coverage and strong adhesion to the lithium surface. Samsung's technology also incorporates functionalized nanoparticles embedded within the graphene layer that act as polysulfide traps, further preventing the shuttle effect. The company has additionally developed specialized electrolyte formulations containing lithium nitrate and other additives that work synergistically with the graphene coating to form a stable SEI layer. Recent testing has demonstrated that this protection system enables lithium-sulfur batteries to achieve over 400 cycles with capacity retention above 80%, representing a significant improvement over unprotected systems.
Strengths: Excellent mechanical flexibility of the graphene-based protection layer accommodating volume changes, high electronic conductivity improving overall cell performance, and compatibility with existing manufacturing processes. Weaknesses: Potential challenges in ensuring complete coverage of the lithium surface during scaling, sensitivity to moisture during manufacturing requiring stringent environmental controls, and possible increased costs associated with graphene materials.
Critical Patents in Lithium Anode Protection
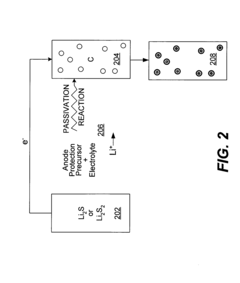
Intercalation anode protection for cells with dissolved lithium polysulfides
PatentInactiveUS20060194115A1
Innovation
- A surface coating is applied to the lithium intercalation anode to passivate redox reactions of polysulfides, allowing for lithium intercalation and de-intercalation while using a sulfur-based cathode and liquid electrolyte, enhancing the stability and performance of the battery cells.
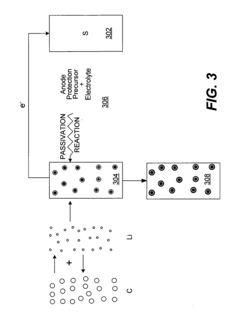
Lithium-sulfur battery
PatentInactiveUS20050042503A1
Innovation
- A lithium-sulfur battery design that forms a uniform and dense LiF passive layer on the lithium metal anode using a separator with controlled fluorine content and specific polymer composition, preventing polysulfide reaction and dendrite formation, thereby stabilizing the electrolyte and enhancing cycle efficiency.
Safety Standards and Regulations for Li-S Batteries
The regulatory landscape for lithium-sulfur (Li-S) battery systems is evolving rapidly as this technology advances toward commercialization. Current safety standards primarily designed for lithium-ion batteries require significant adaptation to address the unique characteristics and failure modes of Li-S systems, particularly concerning lithium anode protection mechanisms. The International Electrotechnical Commission (IEC) standards, including IEC 62660 and IEC 62619, provide baseline requirements but lack specific provisions for the polysulfide shuttle effect and lithium dendrite formation that are critical in Li-S battery safety.
Regulatory bodies including UL (Underwriters Laboratories), IEEE (Institute of Electrical and Electronics Engineers), and ISO (International Organization for Standardization) are actively developing specialized testing protocols for Li-S batteries. These protocols focus on thermal runaway prevention, electrolyte stability assessment, and anode protection evaluation under various operating conditions. The UN38.3 transportation regulations have also begun incorporating specific clauses addressing the unique safety concerns of lithium metal anodes used in Li-S systems.
In the European Union, the Battery Directive (2006/66/EC) and its upcoming revision will likely include specific provisions for Li-S technology, with particular emphasis on lithium anode protection requirements. Similarly, China's GB/T standards are being updated to include specific safety parameters for Li-S batteries, focusing on dendrite suppression techniques and protective layer integrity testing.
The automotive industry, through SAE International standards, is developing specialized requirements for Li-S batteries in electric vehicles, with emphasis on anode protection during fast charging and extreme temperature operations. These standards incorporate accelerated aging tests specifically designed to evaluate the long-term stability of protective layers on lithium anodes.
Emerging regulations are increasingly focusing on in-situ monitoring requirements for Li-S systems, mandating sensors capable of detecting early signs of anode degradation or protective layer failure. This represents a shift from the traditional pass/fail certification approach toward continuous safety monitoring throughout the battery lifecycle.
For research and development purposes, specialized laboratory safety protocols have been established by organizations like EUCAR and USABC, providing standardized methodologies for evaluating new lithium anode protection strategies. These protocols include specific hazard ratings for different protection approaches and establish minimum performance benchmarks for commercial viability.
As Li-S technology matures, regulatory convergence efforts are underway through international working groups to harmonize safety standards across different regions, ensuring consistent evaluation of lithium anode protection technologies regardless of manufacturing or deployment location.
Regulatory bodies including UL (Underwriters Laboratories), IEEE (Institute of Electrical and Electronics Engineers), and ISO (International Organization for Standardization) are actively developing specialized testing protocols for Li-S batteries. These protocols focus on thermal runaway prevention, electrolyte stability assessment, and anode protection evaluation under various operating conditions. The UN38.3 transportation regulations have also begun incorporating specific clauses addressing the unique safety concerns of lithium metal anodes used in Li-S systems.
In the European Union, the Battery Directive (2006/66/EC) and its upcoming revision will likely include specific provisions for Li-S technology, with particular emphasis on lithium anode protection requirements. Similarly, China's GB/T standards are being updated to include specific safety parameters for Li-S batteries, focusing on dendrite suppression techniques and protective layer integrity testing.
The automotive industry, through SAE International standards, is developing specialized requirements for Li-S batteries in electric vehicles, with emphasis on anode protection during fast charging and extreme temperature operations. These standards incorporate accelerated aging tests specifically designed to evaluate the long-term stability of protective layers on lithium anodes.
Emerging regulations are increasingly focusing on in-situ monitoring requirements for Li-S systems, mandating sensors capable of detecting early signs of anode degradation or protective layer failure. This represents a shift from the traditional pass/fail certification approach toward continuous safety monitoring throughout the battery lifecycle.
For research and development purposes, specialized laboratory safety protocols have been established by organizations like EUCAR and USABC, providing standardized methodologies for evaluating new lithium anode protection strategies. These protocols include specific hazard ratings for different protection approaches and establish minimum performance benchmarks for commercial viability.
As Li-S technology matures, regulatory convergence efforts are underway through international working groups to harmonize safety standards across different regions, ensuring consistent evaluation of lithium anode protection technologies regardless of manufacturing or deployment location.
Environmental Impact and Sustainability Considerations
The environmental impact of lithium-sulfur (Li-S) battery systems extends beyond their operational efficiency, encompassing the entire lifecycle from material extraction to disposal. Traditional lithium-ion batteries face significant environmental challenges due to cobalt mining, which is associated with habitat destruction, water pollution, and human rights concerns. Li-S batteries offer a promising alternative by eliminating cobalt and nickel, instead utilizing abundant and less environmentally harmful sulfur.
The protection strategies for lithium anodes in Li-S systems present varying environmental implications. Artificial solid electrolyte interphase (SEI) layers often involve fluorinated compounds that pose environmental risks due to their persistence and potential toxicity. Conversely, biomaterial-derived protective layers represent a more sustainable approach, utilizing renewable resources with lower environmental footprints.
Electrolyte additives used for anode protection require careful environmental assessment. While some additives enhance battery performance and longevity, potentially reducing waste, others contain volatile organic compounds or fluorinated substances that may contribute to air and water pollution if improperly managed. The development of green additives derived from sustainable sources is gaining momentum as an environmentally responsible alternative.
The manufacturing processes for protective coatings and modified separators also warrant environmental consideration. Energy-intensive deposition techniques contribute to carbon emissions, while solvent-based coating methods may release volatile organic compounds. Advancements in water-based processing and low-temperature techniques are emerging as more sustainable manufacturing approaches.
End-of-life management represents a critical environmental aspect of Li-S battery systems. The recyclability of lithium anodes and their protective layers significantly influences the overall sustainability profile. Current recycling processes for lithium metal are energy-intensive and often inefficient, highlighting the need for improved recovery methods. Designing protective layers with recyclability in mind could substantially enhance the circular economy potential of these battery systems.
Water consumption throughout the lifecycle of Li-S batteries with protected anodes presents another environmental consideration. From mining lithium to manufacturing protective materials and recycling spent batteries, water usage and potential contamination require careful management, particularly in water-stressed regions where lithium extraction often occurs.
The carbon footprint associated with lithium anode protection technologies varies considerably depending on material selection and manufacturing processes. Life cycle assessments indicate that while some protection strategies may increase initial environmental impacts, their contribution to extended battery life and improved performance could yield net environmental benefits through reduced replacement frequency and resource consumption.
The protection strategies for lithium anodes in Li-S systems present varying environmental implications. Artificial solid electrolyte interphase (SEI) layers often involve fluorinated compounds that pose environmental risks due to their persistence and potential toxicity. Conversely, biomaterial-derived protective layers represent a more sustainable approach, utilizing renewable resources with lower environmental footprints.
Electrolyte additives used for anode protection require careful environmental assessment. While some additives enhance battery performance and longevity, potentially reducing waste, others contain volatile organic compounds or fluorinated substances that may contribute to air and water pollution if improperly managed. The development of green additives derived from sustainable sources is gaining momentum as an environmentally responsible alternative.
The manufacturing processes for protective coatings and modified separators also warrant environmental consideration. Energy-intensive deposition techniques contribute to carbon emissions, while solvent-based coating methods may release volatile organic compounds. Advancements in water-based processing and low-temperature techniques are emerging as more sustainable manufacturing approaches.
End-of-life management represents a critical environmental aspect of Li-S battery systems. The recyclability of lithium anodes and their protective layers significantly influences the overall sustainability profile. Current recycling processes for lithium metal are energy-intensive and often inefficient, highlighting the need for improved recovery methods. Designing protective layers with recyclability in mind could substantially enhance the circular economy potential of these battery systems.
Water consumption throughout the lifecycle of Li-S batteries with protected anodes presents another environmental consideration. From mining lithium to manufacturing protective materials and recycling spent batteries, water usage and potential contamination require careful management, particularly in water-stressed regions where lithium extraction often occurs.
The carbon footprint associated with lithium anode protection technologies varies considerably depending on material selection and manufacturing processes. Life cycle assessments indicate that while some protection strategies may increase initial environmental impacts, their contribution to extended battery life and improved performance could yield net environmental benefits through reduced replacement frequency and resource consumption.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!