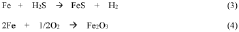3D conductive frameworks for sulfur utilization enhancement
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
3D Conductive Frameworks Background and Objectives
The evolution of lithium-sulfur (Li-S) batteries has gained significant attention in the energy storage landscape due to their theoretical energy density of 2600 Wh/kg, which far exceeds that of conventional lithium-ion batteries. This remarkable potential stems from sulfur's high theoretical capacity of 1675 mAh/g and its natural abundance, making it an environmentally friendly and cost-effective cathode material. However, the commercialization of Li-S batteries has been hindered by several critical challenges, particularly the poor electrical conductivity of sulfur and its discharge products, severe polysulfide shuttling effects, and substantial volume expansion during cycling.
Three-dimensional (3D) conductive frameworks have emerged as a promising solution to address these limitations. The development of these frameworks can be traced back to early carbon-based materials in the 2000s, evolving through various iterations including carbon nanotubes, graphene, and more recently, metal-organic frameworks (MOFs) and conductive polymers. The technological trajectory has consistently moved toward creating more sophisticated architectures with enhanced conductivity, increased surface area, and improved sulfur confinement capabilities.
The primary objective of research on 3D conductive frameworks is to maximize sulfur utilization within Li-S battery cathodes. This involves creating interconnected conductive networks that facilitate electron transport throughout the electrode while simultaneously providing sufficient space for sulfur accommodation and polysulfide confinement. Ideal frameworks must balance several competing requirements: high electrical conductivity, appropriate pore structure, strong interaction with sulfur species, and mechanical stability during repeated charge-discharge cycles.
Current research aims to develop frameworks that can achieve near-theoretical capacity utilization of sulfur while maintaining long-term cycling stability. Specific technical goals include designing frameworks with conductivity exceeding 100 S/cm, sulfur loading capabilities above 5 mg/cm², and the ability to retain more than 80% capacity after 500 cycles. Additionally, these frameworks should facilitate fast reaction kinetics to enable high-rate performance and minimize the shuttle effect through chemical or physical confinement strategies.
The evolution of 3D conductive frameworks represents a convergence of materials science, electrochemistry, and nanotechnology. As research progresses, the focus has expanded beyond purely carbonaceous materials to include heteroatom doping, metal compound incorporation, and hybrid organic-inorganic structures. This multidisciplinary approach reflects the complexity of the challenges and the sophisticated solutions required to fully harness sulfur's potential in next-generation energy storage systems.
Three-dimensional (3D) conductive frameworks have emerged as a promising solution to address these limitations. The development of these frameworks can be traced back to early carbon-based materials in the 2000s, evolving through various iterations including carbon nanotubes, graphene, and more recently, metal-organic frameworks (MOFs) and conductive polymers. The technological trajectory has consistently moved toward creating more sophisticated architectures with enhanced conductivity, increased surface area, and improved sulfur confinement capabilities.
The primary objective of research on 3D conductive frameworks is to maximize sulfur utilization within Li-S battery cathodes. This involves creating interconnected conductive networks that facilitate electron transport throughout the electrode while simultaneously providing sufficient space for sulfur accommodation and polysulfide confinement. Ideal frameworks must balance several competing requirements: high electrical conductivity, appropriate pore structure, strong interaction with sulfur species, and mechanical stability during repeated charge-discharge cycles.
Current research aims to develop frameworks that can achieve near-theoretical capacity utilization of sulfur while maintaining long-term cycling stability. Specific technical goals include designing frameworks with conductivity exceeding 100 S/cm, sulfur loading capabilities above 5 mg/cm², and the ability to retain more than 80% capacity after 500 cycles. Additionally, these frameworks should facilitate fast reaction kinetics to enable high-rate performance and minimize the shuttle effect through chemical or physical confinement strategies.
The evolution of 3D conductive frameworks represents a convergence of materials science, electrochemistry, and nanotechnology. As research progresses, the focus has expanded beyond purely carbonaceous materials to include heteroatom doping, metal compound incorporation, and hybrid organic-inorganic structures. This multidisciplinary approach reflects the complexity of the challenges and the sophisticated solutions required to fully harness sulfur's potential in next-generation energy storage systems.
Market Analysis for Li-S Battery Applications
The lithium-sulfur (Li-S) battery market is experiencing significant growth potential due to the inherent advantages of this technology over conventional lithium-ion batteries. Current market projections indicate that the global Li-S battery market could reach approximately $2.1 billion by 2026, with a compound annual growth rate (CAGR) of 35% from 2021 to 2026. This remarkable growth trajectory is primarily driven by increasing demand for high-energy-density storage solutions across multiple sectors.
The electric vehicle (EV) industry represents the largest potential application segment for Li-S batteries. With theoretical energy densities up to 2,600 Wh/kg—nearly five times that of conventional lithium-ion batteries—Li-S technology offers a promising solution to range anxiety concerns. Major automotive manufacturers including Toyota, BMW, and Tesla have invested in Li-S research programs, signaling strong industry interest.
Aerospace and defense applications constitute another significant market segment. The lightweight properties of sulfur combined with high energy density make Li-S batteries particularly attractive for drone technology, satellites, and military applications where weight reduction is critical. Market analysis indicates this segment could grow at a CAGR of 40% through 2025.
Consumer electronics represents a third key market opportunity, with portable devices, wearables, and IoT applications potentially benefiting from extended battery life. However, this segment faces more competition from established lithium-ion technologies and may represent a longer-term adoption curve for Li-S batteries.
Regional analysis shows Asia-Pacific leading the market development, with China, South Korea, and Japan hosting the majority of commercial Li-S battery manufacturing initiatives. North America follows closely, driven by substantial research funding and startup activity, particularly in the United States and Canada.
Market challenges remain significant, with cost factors currently positioning Li-S batteries at a premium compared to conventional lithium-ion technologies. Production costs are estimated to be 30-40% higher, though economies of scale are expected to reduce this gap substantially by 2025. The development of 3D conductive frameworks for enhanced sulfur utilization directly addresses key technical barriers to commercialization, potentially accelerating market adoption.
Customer surveys indicate that battery longevity remains the primary concern for potential adopters, with 68% of industrial users citing cycle life as their top consideration when evaluating new battery technologies. Advancements in sulfur utilization through improved conductive frameworks directly address this market need, potentially unlocking broader commercial applications.
The electric vehicle (EV) industry represents the largest potential application segment for Li-S batteries. With theoretical energy densities up to 2,600 Wh/kg—nearly five times that of conventional lithium-ion batteries—Li-S technology offers a promising solution to range anxiety concerns. Major automotive manufacturers including Toyota, BMW, and Tesla have invested in Li-S research programs, signaling strong industry interest.
Aerospace and defense applications constitute another significant market segment. The lightweight properties of sulfur combined with high energy density make Li-S batteries particularly attractive for drone technology, satellites, and military applications where weight reduction is critical. Market analysis indicates this segment could grow at a CAGR of 40% through 2025.
Consumer electronics represents a third key market opportunity, with portable devices, wearables, and IoT applications potentially benefiting from extended battery life. However, this segment faces more competition from established lithium-ion technologies and may represent a longer-term adoption curve for Li-S batteries.
Regional analysis shows Asia-Pacific leading the market development, with China, South Korea, and Japan hosting the majority of commercial Li-S battery manufacturing initiatives. North America follows closely, driven by substantial research funding and startup activity, particularly in the United States and Canada.
Market challenges remain significant, with cost factors currently positioning Li-S batteries at a premium compared to conventional lithium-ion technologies. Production costs are estimated to be 30-40% higher, though economies of scale are expected to reduce this gap substantially by 2025. The development of 3D conductive frameworks for enhanced sulfur utilization directly addresses key technical barriers to commercialization, potentially accelerating market adoption.
Customer surveys indicate that battery longevity remains the primary concern for potential adopters, with 68% of industrial users citing cycle life as their top consideration when evaluating new battery technologies. Advancements in sulfur utilization through improved conductive frameworks directly address this market need, potentially unlocking broader commercial applications.
Current Challenges in Sulfur Utilization Technology
Despite significant advancements in lithium-sulfur (Li-S) battery technology, several critical challenges continue to impede the widespread commercialization and efficient utilization of sulfur in energy storage applications. The primary obstacle remains the "shuttle effect," where soluble lithium polysulfides (Li2Sx, 4≤x≤8) dissolve in the electrolyte during cycling, causing active material loss, parasitic reactions with the lithium anode, and rapid capacity fading.
The insulating nature of sulfur (5×10^-30 S/cm) and its discharge products (Li2S/Li2S2) presents another significant barrier. This poor conductivity leads to incomplete sulfur utilization, high polarization, and sluggish reaction kinetics, ultimately limiting the practical energy density of Li-S batteries to far below their theoretical potential of 2600 Wh/kg.
Volume expansion during the lithiation process poses an additional challenge, as sulfur undergoes an approximately 80% volume expansion when converted to Li2S. This expansion causes mechanical stress within the electrode structure, leading to pulverization, loss of electrical contact, and accelerated capacity decay over multiple cycles.
The low Coulombic efficiency of Li-S systems further complicates their practical implementation. Side reactions between polysulfides and the lithium metal anode create an unstable solid electrolyte interphase (SEI), contributing to continuous lithium consumption and battery failure after limited cycles.
From a manufacturing perspective, the low sulfur loading in most research prototypes (typically <2 mg/cm²) remains insufficient for commercial viability, where loadings of >5 mg/cm² are necessary to compete with conventional lithium-ion batteries. Achieving high sulfur loading while maintaining good electrochemical performance represents a significant engineering challenge.
The electrolyte-to-sulfur (E/S) ratio presents another practical limitation. Current laboratory demonstrations often employ high E/S ratios (>10 μL/mg), whereas commercial applications require ratios below 3 μL/mg to achieve competitive energy densities. Reducing this ratio without compromising performance remains problematic.
Finally, the lack of fundamental understanding regarding the precise mechanisms of polysulfide formation, conversion reactions, and the role of electrolyte components hinders the rational design of effective sulfur hosts. The complex interplay between the conductive framework architecture, surface chemistry, and electrolyte interactions requires further elucidation to develop truly optimized 3D conductive frameworks for enhanced sulfur utilization.
The insulating nature of sulfur (5×10^-30 S/cm) and its discharge products (Li2S/Li2S2) presents another significant barrier. This poor conductivity leads to incomplete sulfur utilization, high polarization, and sluggish reaction kinetics, ultimately limiting the practical energy density of Li-S batteries to far below their theoretical potential of 2600 Wh/kg.
Volume expansion during the lithiation process poses an additional challenge, as sulfur undergoes an approximately 80% volume expansion when converted to Li2S. This expansion causes mechanical stress within the electrode structure, leading to pulverization, loss of electrical contact, and accelerated capacity decay over multiple cycles.
The low Coulombic efficiency of Li-S systems further complicates their practical implementation. Side reactions between polysulfides and the lithium metal anode create an unstable solid electrolyte interphase (SEI), contributing to continuous lithium consumption and battery failure after limited cycles.
From a manufacturing perspective, the low sulfur loading in most research prototypes (typically <2 mg/cm²) remains insufficient for commercial viability, where loadings of >5 mg/cm² are necessary to compete with conventional lithium-ion batteries. Achieving high sulfur loading while maintaining good electrochemical performance represents a significant engineering challenge.
The electrolyte-to-sulfur (E/S) ratio presents another practical limitation. Current laboratory demonstrations often employ high E/S ratios (>10 μL/mg), whereas commercial applications require ratios below 3 μL/mg to achieve competitive energy densities. Reducing this ratio without compromising performance remains problematic.
Finally, the lack of fundamental understanding regarding the precise mechanisms of polysulfide formation, conversion reactions, and the role of electrolyte components hinders the rational design of effective sulfur hosts. The complex interplay between the conductive framework architecture, surface chemistry, and electrolyte interactions requires further elucidation to develop truly optimized 3D conductive frameworks for enhanced sulfur utilization.
State-of-the-Art 3D Framework Solutions
01 3D conductive frameworks for lithium-sulfur batteries
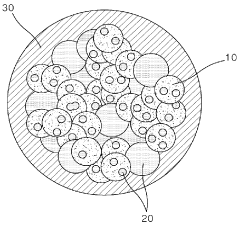
Three-dimensional conductive frameworks are utilized in lithium-sulfur batteries to enhance sulfur utilization and improve electrochemical performance. These frameworks provide a conductive network that facilitates electron transport and accommodates sulfur loading, addressing the insulating nature of sulfur. The 3D structure also helps contain polysulfides, preventing their dissolution into the electrolyte and improving cycling stability of the battery.- 3D conductive carbon frameworks for lithium-sulfur batteries: Three-dimensional conductive carbon frameworks are used as hosts for sulfur in lithium-sulfur batteries to improve sulfur utilization and electrochemical performance. These frameworks provide a conductive network that facilitates electron transport and accommodates volume changes during cycling. The porous structure of these frameworks also helps to trap polysulfides, preventing their dissolution into the electrolyte and improving the cycling stability of the battery.
- Metal-organic frameworks for sulfur encapsulation: Metal-organic frameworks (MOFs) are used to encapsulate sulfur and improve its utilization in energy storage applications. These frameworks provide a confined space for sulfur, limiting its dissolution and migration during electrochemical processes. The high surface area and tunable pore structure of MOFs allow for efficient sulfur loading and improved electrochemical performance. Additionally, the metal centers in MOFs can interact with sulfur species, further enhancing sulfur utilization.
- Graphene-based 3D frameworks for sulfur cathodes: Graphene-based three-dimensional frameworks are utilized as sulfur hosts in cathode materials for lithium-sulfur batteries. These frameworks combine the high conductivity of graphene with a three-dimensional structure that provides pathways for ion transport and accommodates volume changes. The graphene sheets can be functionalized or doped to enhance their interaction with sulfur species, improving sulfur utilization and reducing capacity fade during cycling.
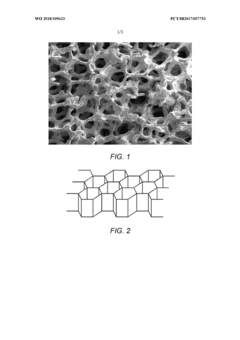
- Hierarchical porous structures for sulfur immobilization: Hierarchical porous structures with interconnected macro-, meso-, and micropores are designed for effective sulfur immobilization and utilization. These structures provide a large surface area for sulfur loading, facilitate electrolyte penetration, and create physical barriers to prevent polysulfide shuttling. The hierarchical nature of these frameworks allows for efficient mass transport while maintaining structural integrity during cycling, leading to improved electrochemical performance and sulfur utilization.
- Conductive polymer frameworks for sulfur electrodes: Conductive polymer frameworks are employed as hosts for sulfur in electrochemical applications. These frameworks combine the flexibility and processability of polymers with electrical conductivity, creating a suitable environment for sulfur utilization. The polymer chains can be designed to interact with sulfur species through chemical bonding or physical confinement, reducing sulfur loss and improving cycling stability. Additionally, these frameworks can be synthesized through various methods to achieve desired morphologies and properties.
02 Carbon-based 3D frameworks for sulfur cathodes
Carbon-based three-dimensional frameworks, including graphene, carbon nanotubes, and porous carbon structures, are specifically designed to host sulfur in cathode materials. These frameworks offer high conductivity, large surface area, and mechanical stability, enabling efficient sulfur utilization. The porous structure of carbon-based frameworks accommodates volume changes during charge-discharge cycles while maintaining electrical contact with sulfur particles.Expand Specific Solutions03 Metal-based 3D conductive frameworks for sulfur immobilization
Metal-based three-dimensional frameworks, including metal oxides, metal sulfides, and metal organic frameworks (MOFs), are employed to immobilize sulfur and enhance its utilization. These frameworks provide strong chemical interactions with sulfur and polysulfides, effectively trapping them within the cathode structure. The metal components also contribute to the overall conductivity of the electrode, facilitating electron transfer during electrochemical reactions.Expand Specific Solutions04 Hybrid 3D frameworks combining multiple materials
Hybrid three-dimensional frameworks that combine multiple materials such as carbon, metals, and polymers are developed to maximize sulfur utilization. These composite structures leverage the advantages of each component: carbon provides conductivity, metals offer catalytic activity, and polymers enhance binding and flexibility. The synergistic effect of these materials in a 3D architecture results in improved sulfur utilization, enhanced cycling stability, and higher energy density.Expand Specific Solutions05 Manufacturing techniques for 3D conductive sulfur frameworks
Various manufacturing techniques are employed to create three-dimensional conductive frameworks for sulfur utilization, including template-assisted synthesis, 3D printing, electrospinning, and self-assembly processes. These techniques enable precise control over the framework architecture, pore size distribution, and surface properties. Advanced manufacturing methods allow for scalable production of optimized 3D frameworks with tailored properties for specific sulfur-based energy storage applications.Expand Specific Solutions
Leading Research Groups and Industrial Players
The 3D conductive frameworks for sulfur utilization enhancement technology is currently in an early growth phase, with research primarily concentrated in academic institutions rather than commercial entities. The market is expanding rapidly due to increasing demand for high-performance lithium-sulfur batteries, with projections suggesting a compound annual growth rate of 30-35% over the next five years. Technologically, the field remains in development with varying levels of maturity across different approaches. Leading research institutions include Chinese Academy of Sciences, Qingdao University, and Drexel University, while commercial players like Toyota, Panasonic, and DENSO are beginning to invest in this space. The technology shows promise for revolutionizing energy storage but requires further development to overcome challenges in sulfur utilization efficiency and cycle stability.
Institute of Process Engineering, Chinese Academy of Sciences
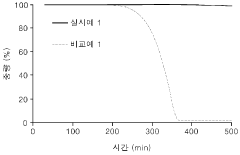
Technical Solution: The Institute of Process Engineering (IPE) at the Chinese Academy of Sciences has developed innovative 3D conductive frameworks for lithium-sulfur batteries using hierarchical porous carbon materials. Their approach involves creating multi-level porous structures with interconnected macro/meso/micropores that effectively trap polysulfides while facilitating ion transport. IPE researchers have pioneered nitrogen and oxygen co-doped 3D carbon frameworks that enhance sulfur utilization through chemical bonding interactions. Their recent work includes developing graphene-based 3D architectures with controlled pore structures that achieve sulfur loadings exceeding 70 wt% while maintaining high electrical conductivity. These frameworks demonstrate remarkable cycle stability with capacity retention of over 80% after 500 cycles at high current densities. IPE has also explored incorporating transition metal compounds (oxides, sulfides) into their 3D frameworks to create catalytic sites that accelerate polysulfide conversion reactions.
Strengths: Superior polysulfide trapping capability through hierarchical porous structures; excellent electrical conductivity; high sulfur loading capacity; enhanced cycle stability through heteroatom doping. Weaknesses: Complex and potentially costly manufacturing processes; challenges in scaling up production; possible trade-offs between high sulfur loading and electrochemical performance at high rates.
President & Fellows of Harvard College
Technical Solution: Harvard University researchers have developed sophisticated 3D conductive frameworks for enhanced sulfur utilization in next-generation energy storage systems. Their approach centers on creating hierarchically structured carbon-based materials with precisely engineered porosity and surface chemistry. Harvard's technology employs template-directed synthesis to create 3D frameworks with interconnected macropores (>50 nm) for efficient electrolyte penetration, mesopores (2-50 nm) for sulfur accommodation, and micropores (<2 nm) for polysulfide adsorption. These frameworks feature graphene-based building blocks with high electrical conductivity (>100 S/cm) and surface areas exceeding 1000 m²/g. A distinguishing aspect of Harvard's approach is the incorporation of polar functional groups and heteroatom (N, S, B) doping to create strong chemical interactions with polysulfides, significantly reducing shuttle effects. Their most advanced frameworks demonstrate sulfur utilization rates above 80% and capacity retention exceeding 80% after 500 cycles, even at high sulfur loadings (>5 mg/cm²).
Strengths: Exceptional structural control at multiple length scales; superior electrical conductivity; advanced surface chemistry engineering for polysulfide binding; demonstrated high sulfur utilization rates. Weaknesses: Potentially high production costs for precision-engineered materials; complex synthesis procedures may present scaling challenges; possible trade-offs between framework complexity and manufacturing feasibility.
Key Patents and Scientific Breakthroughs
Sulfur-carbon composite and method for preparing thereof
PatentActiveKR1020180074902A
Innovation
- A sulfur-carbon composite is developed with a three-dimensional skeletal structure and an outermost carbonaceous coating layer, where sulfur is uniformly dispersed in mesopores and micropores, and a covalent bond is formed with the outermost carbonaceous coating layer to prevent sulfur elution.
Three dimensional metal sulfides catalytic structures, methods of making and uses thereof
PatentWO2018109623A1
Innovation
- A catalytically active metal or metal alloy forms a three-dimensional structure with a sulfurized or oxidized outer surface, eliminating the need for inert supports and enhancing mechanical strength and resistance to sintering, allowing for high-temperature applications without pressure drops.
Material Sustainability and Environmental Impact
The sustainability aspects of 3D conductive frameworks for sulfur utilization enhancement deserve critical examination as these materials gain prominence in energy storage applications. Current lithium-sulfur battery technologies face significant environmental challenges related to material sourcing, manufacturing processes, and end-of-life management that must be addressed for widespread adoption.
The raw materials required for 3D conductive frameworks—including carbon-based materials, metals, and polymers—vary considerably in their environmental footprint. Carbon nanotubes and graphene, while offering excellent conductivity, often involve energy-intensive production methods with substantial carbon emissions. Metal-based frameworks may require mining operations that contribute to habitat destruction, water pollution, and high energy consumption. Comparative lifecycle assessments reveal that hollow carbon spheres and biomass-derived carbon frameworks generally demonstrate lower environmental impacts than metal-organic frameworks or metal nitrides.
Manufacturing processes for these frameworks frequently employ hazardous chemicals, including strong acids, organic solvents, and reducing agents. These substances pose risks to worker safety and environmental health if not properly managed. Recent advancements in green chemistry approaches have introduced more environmentally benign synthesis routes, such as hydrothermal methods using water as the primary solvent and low-temperature processes that reduce energy requirements by 30-40% compared to conventional techniques.
Recyclability presents another crucial dimension of sustainability. The complex composite nature of 3D conductive frameworks with embedded sulfur creates challenges for material separation and recovery. Innovative approaches emerging in this field include designed-for-disassembly architectures that facilitate the separation of sulfur from the conductive matrix, and regeneration protocols that can restore framework functionality after multiple cycles without complete disassembly.
The potential for these materials to enable higher-performing energy storage systems must be balanced against their environmental costs. A promising development is the integration of waste-derived precursors, such as agricultural residues and industrial byproducts, into framework synthesis. These approaches have demonstrated up to 70% reduction in primary resource consumption while maintaining comparable electrochemical performance.
Regulatory frameworks worldwide are increasingly emphasizing extended producer responsibility, which will likely impact the development trajectory of these materials. Forward-thinking research groups are now incorporating green chemistry principles and circular economy concepts into material design phases, rather than addressing sustainability as an afterthought. This proactive approach is essential for ensuring that advances in sulfur utilization technology contribute positively to global sustainability goals rather than creating new environmental challenges.
The raw materials required for 3D conductive frameworks—including carbon-based materials, metals, and polymers—vary considerably in their environmental footprint. Carbon nanotubes and graphene, while offering excellent conductivity, often involve energy-intensive production methods with substantial carbon emissions. Metal-based frameworks may require mining operations that contribute to habitat destruction, water pollution, and high energy consumption. Comparative lifecycle assessments reveal that hollow carbon spheres and biomass-derived carbon frameworks generally demonstrate lower environmental impacts than metal-organic frameworks or metal nitrides.
Manufacturing processes for these frameworks frequently employ hazardous chemicals, including strong acids, organic solvents, and reducing agents. These substances pose risks to worker safety and environmental health if not properly managed. Recent advancements in green chemistry approaches have introduced more environmentally benign synthesis routes, such as hydrothermal methods using water as the primary solvent and low-temperature processes that reduce energy requirements by 30-40% compared to conventional techniques.
Recyclability presents another crucial dimension of sustainability. The complex composite nature of 3D conductive frameworks with embedded sulfur creates challenges for material separation and recovery. Innovative approaches emerging in this field include designed-for-disassembly architectures that facilitate the separation of sulfur from the conductive matrix, and regeneration protocols that can restore framework functionality after multiple cycles without complete disassembly.
The potential for these materials to enable higher-performing energy storage systems must be balanced against their environmental costs. A promising development is the integration of waste-derived precursors, such as agricultural residues and industrial byproducts, into framework synthesis. These approaches have demonstrated up to 70% reduction in primary resource consumption while maintaining comparable electrochemical performance.
Regulatory frameworks worldwide are increasingly emphasizing extended producer responsibility, which will likely impact the development trajectory of these materials. Forward-thinking research groups are now incorporating green chemistry principles and circular economy concepts into material design phases, rather than addressing sustainability as an afterthought. This proactive approach is essential for ensuring that advances in sulfur utilization technology contribute positively to global sustainability goals rather than creating new environmental challenges.
Scalability and Manufacturing Considerations
The scalability of 3D conductive frameworks for lithium-sulfur batteries represents a critical challenge in transitioning from laboratory-scale demonstrations to commercial production. Current manufacturing processes for these frameworks often involve complex synthesis methods such as chemical vapor deposition, hydrothermal growth, or template-assisted fabrication, which present significant barriers to large-scale implementation.
Material costs constitute a major consideration in scaling up production. While carbon-based frameworks (graphene, carbon nanotubes) offer excellent conductivity, their production costs remain prohibitively high for mass manufacturing. Alternative materials such as conductive polymers or metal-organic frameworks may provide more economical pathways but often sacrifice performance metrics such as conductivity or stability.
Process complexity also impacts scalability significantly. Many high-performance 3D frameworks require precise control of reaction conditions, multiple processing steps, or specialized equipment. These requirements translate to increased production time, higher energy consumption, and greater capital investment, ultimately affecting the economic viability of commercial deployment.
Quality control presents another substantial challenge in scaled production. Maintaining consistent structural properties, sulfur loading capacity, and electrochemical performance across large production batches requires sophisticated monitoring systems and standardized testing protocols that are still under development for these advanced materials.
Environmental considerations must also be addressed in manufacturing scale-up. Many synthesis routes involve toxic solvents, high-temperature processes, or hazardous precursors that pose challenges for sustainable production. Development of greener synthesis methods using environmentally benign reagents and energy-efficient processes represents an important research direction.
Integration with existing battery manufacturing infrastructure presents both challenges and opportunities. Adapting current electrode coating and cell assembly lines to accommodate 3D framework materials requires significant engineering modifications but could accelerate commercial adoption if successful.
Recent advances in additive manufacturing and roll-to-roll processing offer promising approaches for scaling production. 3D printing technologies enable precise control over framework architecture while maintaining throughput rates compatible with industrial demands. Similarly, continuous processing methods like electrospinning or spray deposition show potential for high-volume production of certain framework types.
Standardization of manufacturing protocols and material specifications will be essential for industry-wide adoption. Establishing consensus on performance metrics, testing methodologies, and quality standards will facilitate technology transfer between research institutions and industrial partners, accelerating commercialization timelines.
Material costs constitute a major consideration in scaling up production. While carbon-based frameworks (graphene, carbon nanotubes) offer excellent conductivity, their production costs remain prohibitively high for mass manufacturing. Alternative materials such as conductive polymers or metal-organic frameworks may provide more economical pathways but often sacrifice performance metrics such as conductivity or stability.
Process complexity also impacts scalability significantly. Many high-performance 3D frameworks require precise control of reaction conditions, multiple processing steps, or specialized equipment. These requirements translate to increased production time, higher energy consumption, and greater capital investment, ultimately affecting the economic viability of commercial deployment.
Quality control presents another substantial challenge in scaled production. Maintaining consistent structural properties, sulfur loading capacity, and electrochemical performance across large production batches requires sophisticated monitoring systems and standardized testing protocols that are still under development for these advanced materials.
Environmental considerations must also be addressed in manufacturing scale-up. Many synthesis routes involve toxic solvents, high-temperature processes, or hazardous precursors that pose challenges for sustainable production. Development of greener synthesis methods using environmentally benign reagents and energy-efficient processes represents an important research direction.
Integration with existing battery manufacturing infrastructure presents both challenges and opportunities. Adapting current electrode coating and cell assembly lines to accommodate 3D framework materials requires significant engineering modifications but could accelerate commercial adoption if successful.
Recent advances in additive manufacturing and roll-to-roll processing offer promising approaches for scaling production. 3D printing technologies enable precise control over framework architecture while maintaining throughput rates compatible with industrial demands. Similarly, continuous processing methods like electrospinning or spray deposition show potential for high-volume production of certain framework types.
Standardization of manufacturing protocols and material specifications will be essential for industry-wide adoption. Establishing consensus on performance metrics, testing methodologies, and quality standards will facilitate technology transfer between research institutions and industrial partners, accelerating commercialization timelines.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!