Nano-confined sulfur cathodes for improved electrochemical kinetics
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nano-confined Sulfur Cathode Technology Background and Objectives
Lithium-sulfur (Li-S) batteries have emerged as a promising next-generation energy storage technology due to their theoretical energy density of 2600 Wh/kg, which far exceeds that of conventional lithium-ion batteries (typically 250-300 Wh/kg). The development of this technology can be traced back to the 1960s, but significant research momentum has only built up in the past two decades as the limitations of traditional lithium-ion batteries became increasingly apparent for applications demanding higher energy densities.
The evolution of sulfur cathode technology has progressed through several distinct phases. Initially, simple sulfur-carbon composites were utilized, which suffered from rapid capacity fading due to polysulfide shuttling. This led to the second phase focused on sulfur confinement strategies using porous carbon materials. The current phase emphasizes nano-confinement approaches, which represent a significant leap forward in addressing the fundamental challenges of sulfur cathodes.
Nano-confined sulfur cathodes specifically aim to tackle three critical issues: the insulating nature of sulfur and its discharge products (Li2S), the volume expansion during lithiation (up to 80%), and the dissolution of lithium polysulfides in the electrolyte. By confining sulfur within nanoscale structures, researchers seek to enhance electronic conductivity, accommodate volume changes, and physically restrict polysulfide diffusion.
The technological trajectory indicates a convergence toward multifunctional nano-architectures that not only physically confine sulfur but also catalytically enhance the electrochemical reaction kinetics. Recent advances in materials science, particularly in the synthesis of novel nanostructured materials with precisely controlled morphologies and surface properties, have accelerated progress in this field.
The primary technical objectives for nano-confined sulfur cathodes include achieving high sulfur loading (>5 mg/cm²) while maintaining high sulfur utilization, extending cycle life to >1000 cycles with minimal capacity degradation (<0.05% per cycle), improving rate capability for fast charging applications, and enhancing the electrochemical kinetics of sulfur redox reactions.
Looking forward, the field is moving toward integrating multiple strategies, including electrocatalysis, interface engineering, and advanced electrolyte systems, to synergistically address the challenges of sulfur cathodes. The ultimate goal is to develop commercially viable Li-S batteries with energy densities exceeding 500 Wh/kg at the cell level, which would revolutionize applications ranging from electric vehicles to grid-scale energy storage and portable electronics.
The evolution of sulfur cathode technology has progressed through several distinct phases. Initially, simple sulfur-carbon composites were utilized, which suffered from rapid capacity fading due to polysulfide shuttling. This led to the second phase focused on sulfur confinement strategies using porous carbon materials. The current phase emphasizes nano-confinement approaches, which represent a significant leap forward in addressing the fundamental challenges of sulfur cathodes.
Nano-confined sulfur cathodes specifically aim to tackle three critical issues: the insulating nature of sulfur and its discharge products (Li2S), the volume expansion during lithiation (up to 80%), and the dissolution of lithium polysulfides in the electrolyte. By confining sulfur within nanoscale structures, researchers seek to enhance electronic conductivity, accommodate volume changes, and physically restrict polysulfide diffusion.
The technological trajectory indicates a convergence toward multifunctional nano-architectures that not only physically confine sulfur but also catalytically enhance the electrochemical reaction kinetics. Recent advances in materials science, particularly in the synthesis of novel nanostructured materials with precisely controlled morphologies and surface properties, have accelerated progress in this field.
The primary technical objectives for nano-confined sulfur cathodes include achieving high sulfur loading (>5 mg/cm²) while maintaining high sulfur utilization, extending cycle life to >1000 cycles with minimal capacity degradation (<0.05% per cycle), improving rate capability for fast charging applications, and enhancing the electrochemical kinetics of sulfur redox reactions.
Looking forward, the field is moving toward integrating multiple strategies, including electrocatalysis, interface engineering, and advanced electrolyte systems, to synergistically address the challenges of sulfur cathodes. The ultimate goal is to develop commercially viable Li-S batteries with energy densities exceeding 500 Wh/kg at the cell level, which would revolutionize applications ranging from electric vehicles to grid-scale energy storage and portable electronics.
Market Analysis for High-Performance Battery Technologies
The global market for high-performance battery technologies is experiencing unprecedented growth, driven primarily by the rapid expansion of electric vehicles (EVs), renewable energy storage systems, and portable electronics. Within this landscape, lithium-sulfur (Li-S) batteries featuring nano-confined sulfur cathodes represent a promising next-generation technology poised to address current limitations in energy density and cycle life.
Market projections indicate that the advanced battery market will reach approximately $240 billion by 2027, with high-energy density solutions like Li-S batteries potentially capturing a significant portion of this growth. The compound annual growth rate (CAGR) for specialized high-performance batteries is estimated at 18.7% through 2030, substantially outpacing traditional lithium-ion technologies growing at 12.3%.
Demand signals from automotive manufacturers are particularly strong, with major players including Tesla, Volkswagen Group, and Toyota actively investing in next-generation battery technologies. The automotive sector represents the largest potential market for nano-confined sulfur cathode technology, accounting for nearly 60% of projected demand. This is followed by grid-scale energy storage (22%) and consumer electronics (15%).
Regional analysis reveals Asia-Pacific as the dominant manufacturing hub, with China, South Korea, and Japan collectively controlling 78% of advanced battery production capacity. However, significant investments in North America and Europe aim to reduce dependency on Asian supply chains, with over $25 billion committed to new battery manufacturing facilities incorporating advanced cathode technologies.
Key market drivers for nano-confined sulfur cathode technology include the theoretical energy density of sulfur (1,675 mAh/g) - approximately five times higher than conventional cathode materials - and the abundance and low cost of sulfur as a raw material. These advantages position Li-S batteries as potentially achieving costs below $80/kWh at scale, compared to current lithium-ion costs of $132-187/kWh.
Market barriers include technical challenges related to the "shuttle effect" and volume expansion during cycling, though nano-confinement strategies are specifically addressing these limitations. Additionally, manufacturing scalability remains a concern, with current production methods for nano-confined materials requiring significant optimization for mass production.
Consumer willingness to pay premiums for extended range in EVs (surveys indicate 73% of potential EV buyers would pay more for vehicles exceeding 400-mile range) suggests strong market pull for the technology once commercialized. Early adopter segments likely include premium EVs, aerospace applications, and specialized military applications where performance advantages outweigh initial cost considerations.
Market projections indicate that the advanced battery market will reach approximately $240 billion by 2027, with high-energy density solutions like Li-S batteries potentially capturing a significant portion of this growth. The compound annual growth rate (CAGR) for specialized high-performance batteries is estimated at 18.7% through 2030, substantially outpacing traditional lithium-ion technologies growing at 12.3%.
Demand signals from automotive manufacturers are particularly strong, with major players including Tesla, Volkswagen Group, and Toyota actively investing in next-generation battery technologies. The automotive sector represents the largest potential market for nano-confined sulfur cathode technology, accounting for nearly 60% of projected demand. This is followed by grid-scale energy storage (22%) and consumer electronics (15%).
Regional analysis reveals Asia-Pacific as the dominant manufacturing hub, with China, South Korea, and Japan collectively controlling 78% of advanced battery production capacity. However, significant investments in North America and Europe aim to reduce dependency on Asian supply chains, with over $25 billion committed to new battery manufacturing facilities incorporating advanced cathode technologies.
Key market drivers for nano-confined sulfur cathode technology include the theoretical energy density of sulfur (1,675 mAh/g) - approximately five times higher than conventional cathode materials - and the abundance and low cost of sulfur as a raw material. These advantages position Li-S batteries as potentially achieving costs below $80/kWh at scale, compared to current lithium-ion costs of $132-187/kWh.
Market barriers include technical challenges related to the "shuttle effect" and volume expansion during cycling, though nano-confinement strategies are specifically addressing these limitations. Additionally, manufacturing scalability remains a concern, with current production methods for nano-confined materials requiring significant optimization for mass production.
Consumer willingness to pay premiums for extended range in EVs (surveys indicate 73% of potential EV buyers would pay more for vehicles exceeding 400-mile range) suggests strong market pull for the technology once commercialized. Early adopter segments likely include premium EVs, aerospace applications, and specialized military applications where performance advantages outweigh initial cost considerations.
Current Challenges in Sulfur Cathode Development
Despite the promising theoretical energy density of lithium-sulfur (Li-S) batteries, several critical challenges impede their practical implementation. The most significant issue is the "shuttle effect," where soluble lithium polysulfides (Li2Sx, 4≤x≤8) dissolve in the electrolyte during cycling, shuttling between electrodes. This phenomenon leads to active material loss, parasitic reactions with the lithium anode, and rapid capacity fading.
Another major challenge is sulfur's inherently poor electrical conductivity (5×10^-30 S/cm), which necessitates conductive additives and results in lower energy density than theoretical predictions. The volume expansion during lithiation (approximately 80%) causes mechanical stress and structural degradation of the cathode, further compromising cycle life and performance stability.
The slow reaction kinetics of sulfur conversion reactions presents additional obstacles. The multi-step electrochemical process involves complex phase transitions between solid sulfur, dissolved polysulfides, and solid Li2S2/Li2S, with each step exhibiting different reaction rates and energy barriers. This kinetic limitation manifests as voltage hysteresis and reduced rate capability.
Electrolyte compatibility issues further complicate Li-S battery development. The high electrolyte-to-sulfur ratio required for polysulfide dissolution significantly reduces the practical energy density. Moreover, conventional electrolytes often decompose when in contact with polysulfides, forming an unstable solid-electrolyte interphase (SEI) that increases cell impedance over time.
Nano-confinement strategies have emerged as promising approaches to address these challenges. By encapsulating sulfur within nanoporous structures, researchers aim to physically restrict polysulfide diffusion while maintaining efficient ion transport. However, achieving uniform sulfur distribution within these nanostructures remains technically challenging, and the additional weight of host materials reduces the practical energy density advantage.
The trade-off between sulfur loading and electrochemical performance presents another significant hurdle. High sulfur loading is essential for commercial viability, but it exacerbates issues related to ion transport, electronic conductivity, and mechanical stability. Current high-loading cathodes (>5 mg/cm²) typically suffer from poor rate capability and accelerated capacity decay.
Manufacturing scalability represents a critical barrier to commercialization. Many laboratory-scale solutions involve complex synthesis procedures, expensive materials, or processes incompatible with existing battery manufacturing infrastructure. Developing cost-effective, scalable production methods for nano-confined sulfur cathodes remains a significant challenge for industrial adoption.
Another major challenge is sulfur's inherently poor electrical conductivity (5×10^-30 S/cm), which necessitates conductive additives and results in lower energy density than theoretical predictions. The volume expansion during lithiation (approximately 80%) causes mechanical stress and structural degradation of the cathode, further compromising cycle life and performance stability.
The slow reaction kinetics of sulfur conversion reactions presents additional obstacles. The multi-step electrochemical process involves complex phase transitions between solid sulfur, dissolved polysulfides, and solid Li2S2/Li2S, with each step exhibiting different reaction rates and energy barriers. This kinetic limitation manifests as voltage hysteresis and reduced rate capability.
Electrolyte compatibility issues further complicate Li-S battery development. The high electrolyte-to-sulfur ratio required for polysulfide dissolution significantly reduces the practical energy density. Moreover, conventional electrolytes often decompose when in contact with polysulfides, forming an unstable solid-electrolyte interphase (SEI) that increases cell impedance over time.
Nano-confinement strategies have emerged as promising approaches to address these challenges. By encapsulating sulfur within nanoporous structures, researchers aim to physically restrict polysulfide diffusion while maintaining efficient ion transport. However, achieving uniform sulfur distribution within these nanostructures remains technically challenging, and the additional weight of host materials reduces the practical energy density advantage.
The trade-off between sulfur loading and electrochemical performance presents another significant hurdle. High sulfur loading is essential for commercial viability, but it exacerbates issues related to ion transport, electronic conductivity, and mechanical stability. Current high-loading cathodes (>5 mg/cm²) typically suffer from poor rate capability and accelerated capacity decay.
Manufacturing scalability represents a critical barrier to commercialization. Many laboratory-scale solutions involve complex synthesis procedures, expensive materials, or processes incompatible with existing battery manufacturing infrastructure. Developing cost-effective, scalable production methods for nano-confined sulfur cathodes remains a significant challenge for industrial adoption.
Current Nano-confinement Approaches for Sulfur Cathodes
01 Sulfur cathode composition and structure
The composition and structure of sulfur cathodes significantly impact their electrochemical kinetics. Various approaches include using sulfur-carbon composites, nanostructured sulfur materials, and specific binders to enhance conductivity and stability. These structural modifications help address issues like volume expansion during cycling and improve the overall electrochemical performance by facilitating faster ion transport and reaction kinetics.- Sulfur cathode composition and structure: The composition and structure of sulfur cathodes significantly impact their electrochemical kinetics. Various approaches include using carbon-sulfur composites, polymer binders, and nanostructured materials to enhance conductivity and stability. These structural modifications help address issues like volume expansion during cycling and improve the utilization of active sulfur material, resulting in better electrochemical performance and faster reaction kinetics.
- Electrolyte formulations for sulfur cathodes: Specialized electrolyte formulations play a crucial role in enhancing the electrochemical kinetics of sulfur cathodes. These formulations often include additives that suppress the shuttle effect of polysulfides, improve ionic conductivity, and form stable solid-electrolyte interphases. By optimizing the electrolyte composition, researchers can significantly improve the reaction rates, cycling stability, and overall performance of sulfur-based battery systems.
- Catalysts and mediators for sulfur redox reactions: Incorporating catalysts and redox mediators into sulfur cathodes can dramatically enhance electrochemical kinetics. These materials facilitate the conversion between sulfur and lithium sulfides by lowering activation energy barriers and providing alternative reaction pathways. Metal oxides, metal sulfides, and transition metal compounds are commonly used as catalysts to accelerate the sluggish redox reactions of sulfur, improving rate capability and utilization of active material.
- Advanced characterization and modeling of sulfur electrochemistry: Advanced characterization techniques and computational modeling approaches are essential for understanding the complex electrochemical kinetics of sulfur cathodes. In-situ and operando methods such as X-ray diffraction, spectroscopy, and microscopy provide insights into reaction mechanisms and degradation processes. Computational models help predict performance limitations and guide the design of improved sulfur cathode materials with enhanced kinetic properties.
- Interface engineering for improved kinetics: Interface engineering strategies focus on optimizing the interactions between sulfur, conductive additives, and the electrolyte to enhance electrochemical kinetics. Approaches include surface functionalization of carbon hosts, protective coatings on sulfur particles, and interlayers that trap polysulfides while maintaining fast ion transport. These modifications reduce interfacial resistance, improve electron/ion transfer rates, and enhance the overall reaction kinetics in sulfur cathodes.
02 Electrolyte formulations for sulfur cathodes
Specialized electrolyte formulations play a crucial role in enhancing the electrochemical kinetics of sulfur cathodes. These formulations often include additives that suppress the shuttle effect, improve ionic conductivity, and form stable interfaces with the cathode material. By optimizing the electrolyte composition, the dissolution of polysulfides can be controlled, leading to improved cycling stability and faster reaction kinetics at the cathode-electrolyte interface.Expand Specific Solutions03 Catalysts and mediators for sulfur redox reactions
Incorporating catalysts and redox mediators into sulfur cathodes can significantly enhance electrochemical kinetics. These materials facilitate the conversion between sulfur and polysulfides, accelerating the redox reactions that occur during battery operation. Metal oxides, metal sulfides, and certain conductive polymers have been shown to effectively catalyze these reactions, resulting in improved rate capability, reduced polarization, and enhanced utilization of active sulfur material.Expand Specific Solutions04 Analysis and characterization techniques for sulfur cathodes
Advanced analytical and characterization techniques are essential for understanding the electrochemical kinetics of sulfur cathodes. These include electrochemical impedance spectroscopy, cyclic voltammetry, in-situ/operando spectroscopy, and computational modeling. These methods help researchers identify rate-limiting steps, understand reaction mechanisms, and quantify kinetic parameters, ultimately guiding the design of improved sulfur cathode materials with enhanced electrochemical performance.Expand Specific Solutions05 Interface engineering for improved kinetics
Engineering the interfaces within sulfur cathodes is critical for enhancing electrochemical kinetics. This includes modifying the sulfur-carbon interface, creating protective layers to prevent polysulfide dissolution, and designing structured interfaces that facilitate ion transport. Various coating materials, functional interlayers, and surface treatments have been developed to optimize these interfaces, resulting in reduced charge transfer resistance and improved reaction kinetics throughout the cathode structure.Expand Specific Solutions
Leading Research Groups and Companies in Sulfur Battery Technology
The lithium-sulfur battery market is currently in an early growth phase, characterized by intensive R&D efforts to overcome key technical challenges in nano-confined sulfur cathodes for improved electrochemical kinetics. The global market is projected to reach $1.5-2 billion by 2030, driven by demand for higher energy density solutions. Leading companies like Sion Power and Conamix are advancing commercialization with proprietary technologies such as Sion's Licerion® technology offering 500 Wh/kg energy density. Major industrial players (Robert Bosch, Toyota, China Petroleum & Chemical Corp.) are investing heavily, while research institutions (Drexel University, California Institute of Technology, Tsinghua University) are developing fundamental innovations in sulfur confinement strategies. The competitive landscape shows collaboration between academic institutions and industry partners to address challenges in cycle life, sulfur utilization, and manufacturing scalability.
Sion Power Corp.
Technical Solution: Sion Power has developed proprietary Licerion® technology that utilizes nano-confined sulfur cathodes within a protected lithium metal anode architecture. Their approach involves encapsulating sulfur particles within conductive carbon matrices at the nanoscale, creating interconnected porous networks that effectively confine polysulfides while facilitating lithium-ion transport. The company employs specialized carbon nanostructures with tailored pore sizes (typically 2-50 nm) to physically trap sulfur and its discharge products. This nano-confinement strategy is combined with their protected lithium anode technology to address both cathode and anode limitations simultaneously, resulting in batteries with energy densities exceeding 500 Wh/kg and 1000 Wh/L.
Strengths: Industry-leading energy density metrics; integrated solution addressing both cathode and anode challenges; mature manufacturing processes ready for commercialization. Weaknesses: Higher production costs compared to conventional lithium-ion batteries; potential scalability challenges for mass production; proprietary materials may limit broader adoption across the industry.
The Regents of the University of California
Technical Solution: UC researchers have pioneered a multifunctional approach to nano-confined sulfur cathodes using yolk-shell nanostructures. Their technology employs hollow carbon nanospheres with precisely controlled internal void spaces (50-200 nm) that accommodate sulfur volumetric expansion during cycling. The carbon shells are engineered with micropores (< 2 nm) that allow lithium ion transport while physically restricting polysulfide diffusion. UC's innovation extends to surface modification of these carbon hosts with polar metal compounds (primarily titanium nitride and vanadium nitride) that serve dual functions: chemically binding polysulfides and catalyzing their conversion reactions. Recent advancements include incorporating conductive polymers (PEDOT:PSS) as additional functional layers, creating a hierarchical confinement system that addresses multiple degradation mechanisms simultaneously. This integrated approach has demonstrated remarkable cycling stability with capacity retention exceeding 80% after 1000 cycles.
Strengths: Sophisticated nanostructure design addressing multiple failure mechanisms; excellent long-term cycling stability; fundamental understanding of structure-property relationships in confined sulfur systems. Weaknesses: Complex multi-step synthesis procedures may limit commercial scalability; higher manufacturing costs compared to conventional cathode production; potential challenges in achieving high sulfur loading while maintaining confinement benefits.
Key Innovations in Electrochemical Kinetics Enhancement
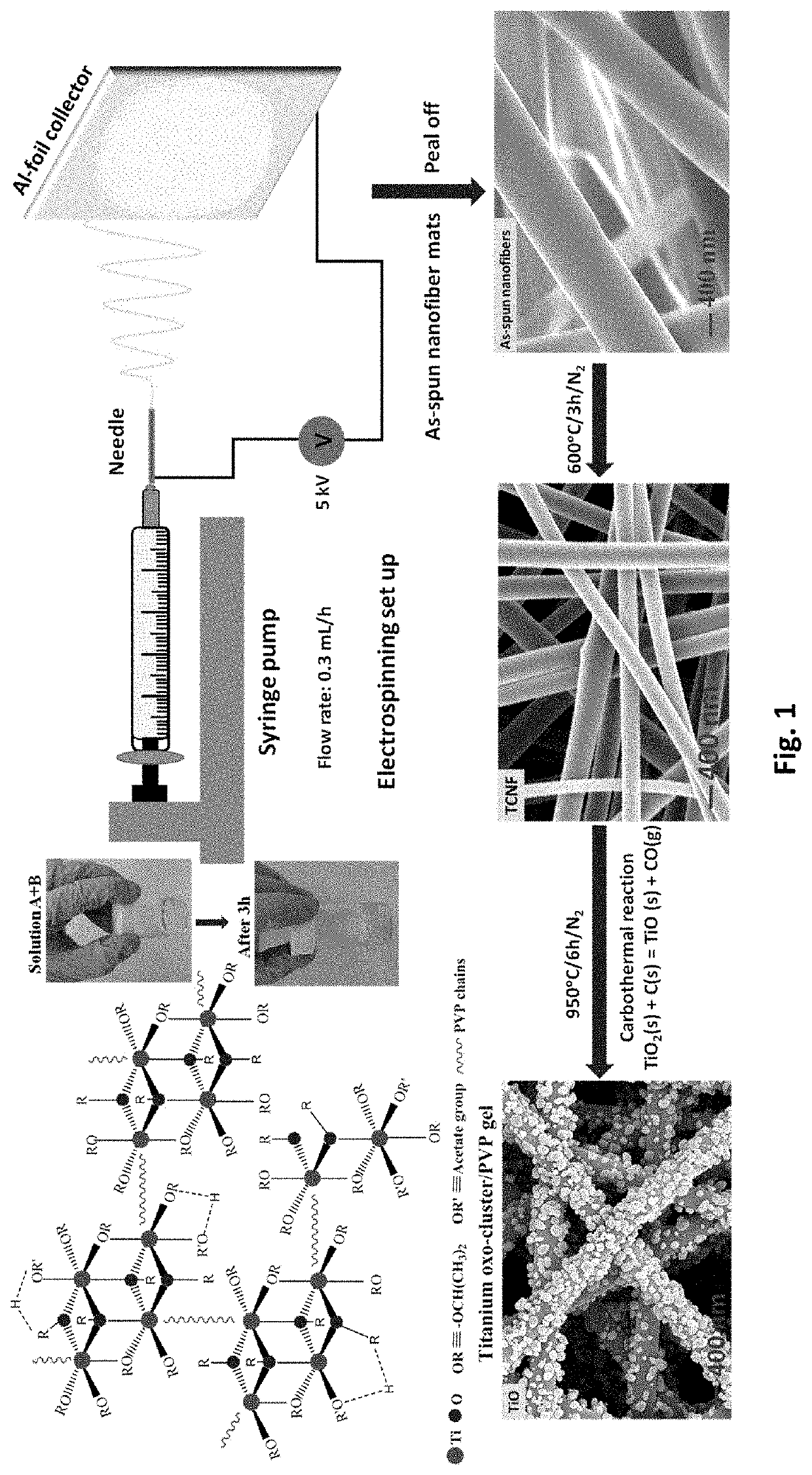
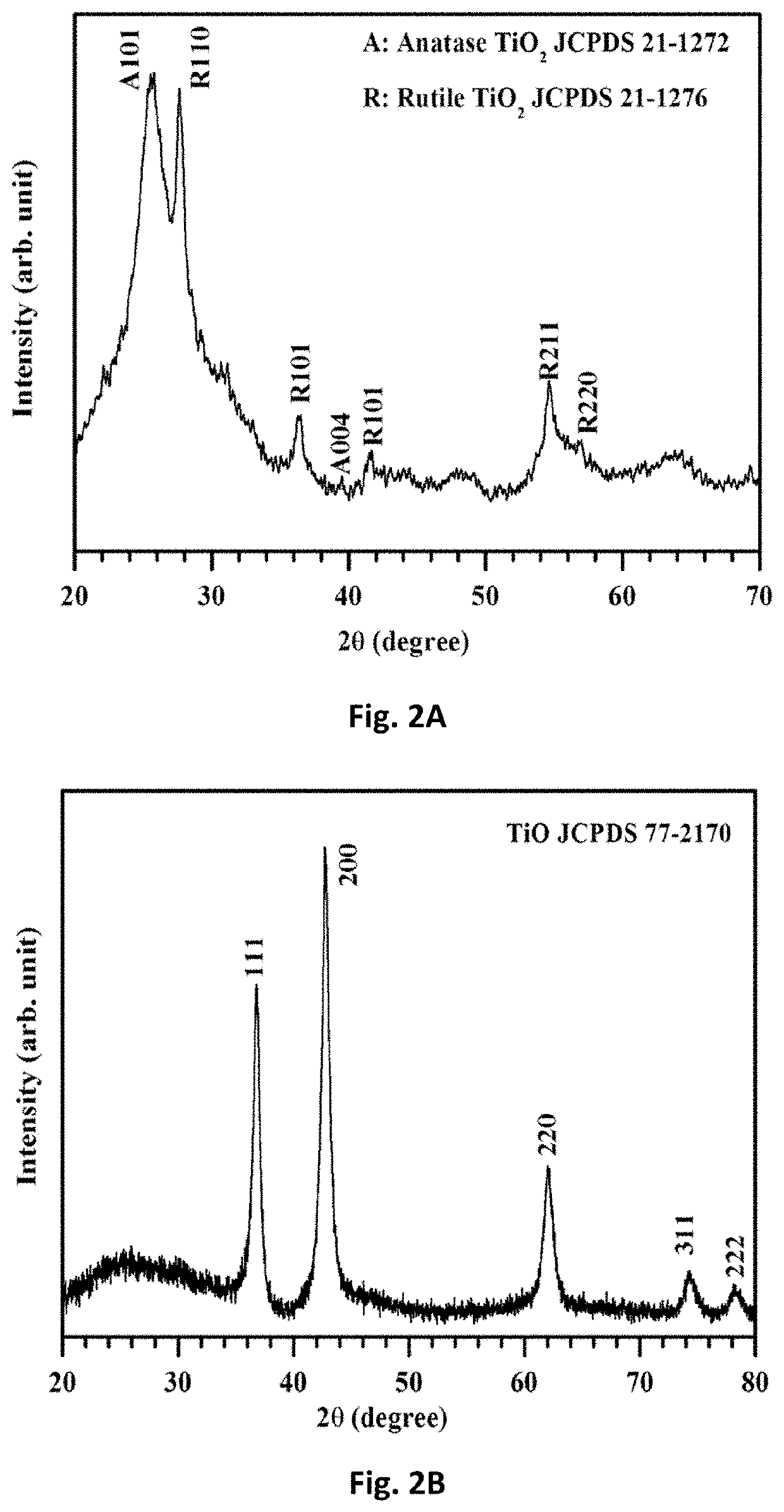
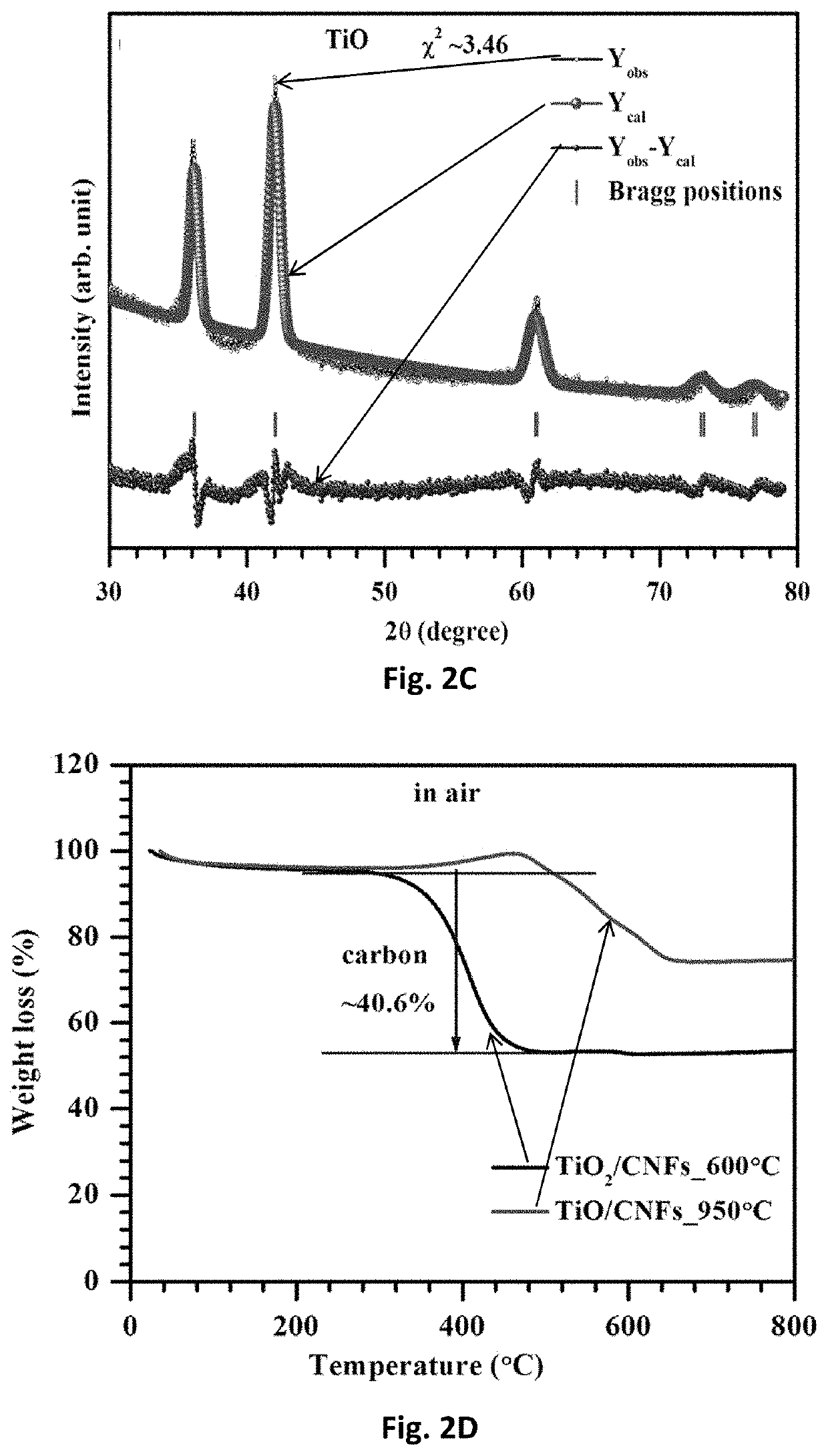
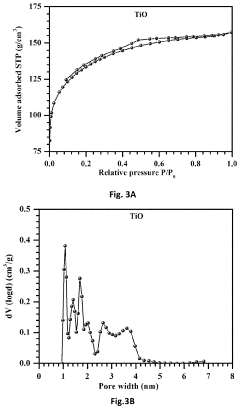
Free-standing, binder-free metal monoxide/suboxide nanofiber as cathodes or anodes for batteries
PatentActiveUS20210111390A1
Innovation
- The development of free-standing, binder-free nanofiber mats with high surface area and conductive metal oxide nanofibers, produced through electrospinning and carbothermal processes, which allow rapid sulfur diffusion and eliminate the need for harsh slurry casting, providing a robust 3D conducting network for uninterrupted electron supply and strong interactions with lithium polysulfides.
Cathodes for lithium-sulfur batteries with nanocatalysts
PatentWO2024081684A3
Innovation
- Development of lithium-sulfur battery cathodes with a graded structure that incorporates an actively electro-catalyzing and polysulfide-trapping system.
- Economic and scalable synthesis and coating methods for preparing graded structure Li-S cathodes, making them commercially viable.
- Dual-function design that simultaneously addresses sulfur utilization and capacity retention issues in lithium-sulfur batteries.
Sustainability and Resource Considerations for Sulfur Batteries
The development of sulfur batteries represents a significant advancement in sustainable energy storage technologies, primarily due to sulfur's abundance, low cost, and high theoretical capacity. Unlike traditional lithium-ion batteries that rely on critical materials such as cobalt and nickel, sulfur-based cathodes utilize an element that constitutes approximately 0.03% of Earth's crust, making it the 16th most abundant element on our planet.
From a resource perspective, sulfur offers remarkable advantages as it is widely available as a byproduct of petroleum refining and natural gas processing. The global annual production of sulfur exceeds 70 million tons, with significant reserves distributed across various regions worldwide. This abundance translates to lower material costs, potentially reducing battery production expenses by 70-80% compared to conventional lithium-ion technologies.
Environmental considerations further enhance the appeal of nano-confined sulfur cathodes. The carbon footprint associated with sulfur extraction and processing is substantially lower than that of traditional cathode materials. Life cycle assessments indicate that sulfur batteries could reduce greenhouse gas emissions by approximately 25-30% compared to conventional lithium-ion batteries, primarily due to the simplified supply chain and reduced energy requirements during material processing.
The recyclability of sulfur batteries presents both opportunities and challenges. While the theoretical recovery rate of sulfur from spent batteries could exceed 90%, current recycling technologies require further development to achieve commercial viability. The nano-confinement structures, often composed of carbon materials, can be designed with end-of-life considerations, potentially enabling more efficient separation and recovery processes.
Water usage represents another critical sustainability metric. Conventional lithium-ion battery production requires significant water resources, particularly for extraction and processing of cathode materials. Sulfur-based alternatives could reduce water consumption by approximately 35-40%, contributing to more sustainable manufacturing practices, especially in water-stressed regions.
The transition to nano-confined sulfur cathodes also addresses concerns regarding critical material dependencies. By reducing reliance on geopolitically concentrated resources like cobalt, these batteries enhance supply chain resilience and mitigate potential resource conflicts. This aspect becomes increasingly important as global demand for energy storage solutions continues to grow exponentially.
Looking forward, the sustainability profile of sulfur batteries could be further enhanced through integration with renewable energy systems for manufacturing, development of aqueous processing techniques, and implementation of design-for-recycling principles that facilitate material recovery at end-of-life.
From a resource perspective, sulfur offers remarkable advantages as it is widely available as a byproduct of petroleum refining and natural gas processing. The global annual production of sulfur exceeds 70 million tons, with significant reserves distributed across various regions worldwide. This abundance translates to lower material costs, potentially reducing battery production expenses by 70-80% compared to conventional lithium-ion technologies.
Environmental considerations further enhance the appeal of nano-confined sulfur cathodes. The carbon footprint associated with sulfur extraction and processing is substantially lower than that of traditional cathode materials. Life cycle assessments indicate that sulfur batteries could reduce greenhouse gas emissions by approximately 25-30% compared to conventional lithium-ion batteries, primarily due to the simplified supply chain and reduced energy requirements during material processing.
The recyclability of sulfur batteries presents both opportunities and challenges. While the theoretical recovery rate of sulfur from spent batteries could exceed 90%, current recycling technologies require further development to achieve commercial viability. The nano-confinement structures, often composed of carbon materials, can be designed with end-of-life considerations, potentially enabling more efficient separation and recovery processes.
Water usage represents another critical sustainability metric. Conventional lithium-ion battery production requires significant water resources, particularly for extraction and processing of cathode materials. Sulfur-based alternatives could reduce water consumption by approximately 35-40%, contributing to more sustainable manufacturing practices, especially in water-stressed regions.
The transition to nano-confined sulfur cathodes also addresses concerns regarding critical material dependencies. By reducing reliance on geopolitically concentrated resources like cobalt, these batteries enhance supply chain resilience and mitigate potential resource conflicts. This aspect becomes increasingly important as global demand for energy storage solutions continues to grow exponentially.
Looking forward, the sustainability profile of sulfur batteries could be further enhanced through integration with renewable energy systems for manufacturing, development of aqueous processing techniques, and implementation of design-for-recycling principles that facilitate material recovery at end-of-life.
Commercialization Pathways and Scale-up Challenges
The commercialization of nano-confined sulfur cathodes represents a critical transition from laboratory research to industrial application. Current market entry strategies primarily focus on niche applications where performance advantages outweigh cost considerations, such as high-end consumer electronics and specialized military applications requiring exceptional energy density.
Manufacturing scale-up presents significant challenges that must be addressed systematically. The precise control of nanostructure formation during mass production remains technically demanding, with current laboratory-scale synthesis methods often utilizing expensive precursors and complex processing steps. Transitioning to industrial-scale production requires development of continuous flow processes and adaptation of existing battery manufacturing infrastructure to accommodate these novel materials.
Cost reduction pathways must target both materials and processing. The carbon host materials, particularly advanced graphene and carbon nanotube structures, contribute substantially to overall cathode costs. Strategic approaches include developing less expensive carbon precursors, optimizing sulfur loading to maximize energy density, and reducing the complexity of confinement architectures while maintaining performance benefits.
Quality control represents another critical challenge, as nano-confined structures require more sophisticated characterization techniques than conventional battery materials. Implementation of in-line monitoring systems capable of detecting nanoscale defects and ensuring uniform sulfur distribution will be essential for consistent product quality.
Supply chain development presents both challenges and opportunities. While sulfur itself is abundant and inexpensive as a petroleum refining byproduct, specialized carbon materials and electrolyte additives required for optimal performance may face supply constraints. Vertical integration strategies and strategic partnerships with materials suppliers can help mitigate these risks.
Regulatory compliance and safety certification will require extensive testing protocols specific to nano-confined sulfur cathodes. Their unique characteristics may necessitate modified safety standards addressing potential failure modes different from conventional lithium-ion batteries.
Timeline projections suggest initial commercialization in premium market segments within 3-5 years, with broader market penetration dependent on achieving cost parity with conventional lithium-ion technologies. Strategic partnerships between research institutions, material suppliers, and battery manufacturers will be crucial to accelerate this timeline and overcome the technical and economic barriers to widespread adoption.
Manufacturing scale-up presents significant challenges that must be addressed systematically. The precise control of nanostructure formation during mass production remains technically demanding, with current laboratory-scale synthesis methods often utilizing expensive precursors and complex processing steps. Transitioning to industrial-scale production requires development of continuous flow processes and adaptation of existing battery manufacturing infrastructure to accommodate these novel materials.
Cost reduction pathways must target both materials and processing. The carbon host materials, particularly advanced graphene and carbon nanotube structures, contribute substantially to overall cathode costs. Strategic approaches include developing less expensive carbon precursors, optimizing sulfur loading to maximize energy density, and reducing the complexity of confinement architectures while maintaining performance benefits.
Quality control represents another critical challenge, as nano-confined structures require more sophisticated characterization techniques than conventional battery materials. Implementation of in-line monitoring systems capable of detecting nanoscale defects and ensuring uniform sulfur distribution will be essential for consistent product quality.
Supply chain development presents both challenges and opportunities. While sulfur itself is abundant and inexpensive as a petroleum refining byproduct, specialized carbon materials and electrolyte additives required for optimal performance may face supply constraints. Vertical integration strategies and strategic partnerships with materials suppliers can help mitigate these risks.
Regulatory compliance and safety certification will require extensive testing protocols specific to nano-confined sulfur cathodes. Their unique characteristics may necessitate modified safety standards addressing potential failure modes different from conventional lithium-ion batteries.
Timeline projections suggest initial commercialization in premium market segments within 3-5 years, with broader market penetration dependent on achieving cost parity with conventional lithium-ion technologies. Strategic partnerships between research institutions, material suppliers, and battery manufacturers will be crucial to accelerate this timeline and overcome the technical and economic barriers to widespread adoption.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!