Polysulfide shuttle suppression in lithium-sulfur batteries
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Polysulfide Shuttle Effect Background and Research Objectives
The polysulfide shuttle effect represents one of the most significant challenges in lithium-sulfur (Li-S) battery technology since its identification in the early 1990s. This phenomenon occurs during the charge-discharge cycles when soluble lithium polysulfides (Li2Sx, 4≤x≤8) formed at the cathode dissolve in the electrolyte and migrate to the anode, where they react with lithium metal to form lower-order polysulfides. These species then diffuse back to the cathode and are reoxidized, creating a parasitic redox shuttle that severely compromises battery performance.
The historical development of understanding this effect began with pioneering work by Mikhaylik and Akridge in 2004, who quantitatively described the shuttle mechanism and its impact on coulombic efficiency. Subsequent research has revealed that this shuttle effect contributes to multiple degradation pathways, including active material loss, electrolyte depletion, lithium anode corrosion, and self-discharge behavior during battery storage.
From a fundamental perspective, the polysulfide shuttle effect stems from the high solubility of intermediate lithium polysulfides in conventional ether-based electrolytes, coupled with their high mobility and reactivity. The concentration gradient-driven diffusion of these species creates a continuous redox cycle that consumes energy without contributing to external work, resulting in low coulombic efficiency, capacity fading, and shortened battery lifespan.
The technological evolution in this field has progressed from initial recognition of the problem to increasingly sophisticated mitigation strategies. Early approaches focused on electrolyte modifications, while more recent innovations have explored advanced cathode architectures, functional separators, and protective anode coatings. Despite these advances, a comprehensive solution remains elusive due to the complex interplay of electrochemical, physical, and chemical processes involved.
The primary research objectives in polysulfide shuttle suppression are multifaceted. First, to develop fundamental understanding of polysulfide formation mechanisms, dissolution kinetics, and transport behaviors across different electrolyte systems. Second, to design and synthesize novel materials that can effectively trap or catalytically convert polysulfides within the cathode structure. Third, to engineer robust interfaces that prevent polysulfide migration while maintaining efficient lithium-ion transport.
The ultimate goal is to enable Li-S batteries that deliver on their theoretical promise of high energy density (2600 Wh/kg), low cost, and environmental compatibility, positioning them as viable alternatives to conventional lithium-ion batteries for applications ranging from portable electronics to electric vehicles and grid-scale energy storage.
The historical development of understanding this effect began with pioneering work by Mikhaylik and Akridge in 2004, who quantitatively described the shuttle mechanism and its impact on coulombic efficiency. Subsequent research has revealed that this shuttle effect contributes to multiple degradation pathways, including active material loss, electrolyte depletion, lithium anode corrosion, and self-discharge behavior during battery storage.
From a fundamental perspective, the polysulfide shuttle effect stems from the high solubility of intermediate lithium polysulfides in conventional ether-based electrolytes, coupled with their high mobility and reactivity. The concentration gradient-driven diffusion of these species creates a continuous redox cycle that consumes energy without contributing to external work, resulting in low coulombic efficiency, capacity fading, and shortened battery lifespan.
The technological evolution in this field has progressed from initial recognition of the problem to increasingly sophisticated mitigation strategies. Early approaches focused on electrolyte modifications, while more recent innovations have explored advanced cathode architectures, functional separators, and protective anode coatings. Despite these advances, a comprehensive solution remains elusive due to the complex interplay of electrochemical, physical, and chemical processes involved.
The primary research objectives in polysulfide shuttle suppression are multifaceted. First, to develop fundamental understanding of polysulfide formation mechanisms, dissolution kinetics, and transport behaviors across different electrolyte systems. Second, to design and synthesize novel materials that can effectively trap or catalytically convert polysulfides within the cathode structure. Third, to engineer robust interfaces that prevent polysulfide migration while maintaining efficient lithium-ion transport.
The ultimate goal is to enable Li-S batteries that deliver on their theoretical promise of high energy density (2600 Wh/kg), low cost, and environmental compatibility, positioning them as viable alternatives to conventional lithium-ion batteries for applications ranging from portable electronics to electric vehicles and grid-scale energy storage.
Market Analysis of Li-S Battery Demand and Applications
The lithium-sulfur (Li-S) battery market is experiencing significant growth potential due to the technology's theoretical energy density of 2600 Wh/kg, which far exceeds the capabilities of conventional lithium-ion batteries (typically 250-300 Wh/kg). This substantial energy density advantage positions Li-S batteries as a promising solution for applications requiring high energy storage capacity with minimal weight constraints.
The electric vehicle (EV) sector represents the largest potential market for Li-S batteries, with demand projected to grow substantially as automotive manufacturers seek lighter, higher-capacity energy storage solutions. The aviation and aerospace industries also demonstrate strong interest in Li-S technology, particularly for electric aircraft and satellite applications where weight reduction directly translates to operational efficiency and extended mission capabilities.
Consumer electronics manufacturers are exploring Li-S batteries for next-generation portable devices, anticipating that the technology could enable significantly longer operating times between charges. The military and defense sectors have identified Li-S batteries as strategically important for powering unmanned aerial vehicles, portable soldier equipment, and remote sensing devices.
Grid-scale energy storage represents another emerging application area, where the lower material costs of sulfur compared to cobalt and nickel in conventional lithium-ion batteries could provide economic advantages at scale. This cost advantage becomes particularly relevant as renewable energy integration increases the demand for large-capacity storage solutions.
Market analysis indicates regional variations in Li-S battery demand, with North America and Europe leading in research investment and early adoption plans, while Asia-Pacific countries, particularly China, South Korea, and Japan, dominate in manufacturing capacity development and commercialization efforts.
The market growth trajectory for Li-S batteries is closely tied to overcoming technical challenges, with polysulfide shuttle effect suppression being the most critical barrier to widespread commercial adoption. Industry forecasts suggest that successful resolution of this technical challenge could accelerate market penetration, potentially enabling Li-S batteries to capture 5-10% of the premium battery market within the next decade.
Current market limitations stem from performance inconsistencies, with cycle life limitations directly attributable to the polysulfide shuttle effect. Market research indicates that consumers and industrial buyers require a minimum of 1000 stable cycles before considering widespread adoption, highlighting the commercial importance of shuttle suppression technologies.
The electric vehicle (EV) sector represents the largest potential market for Li-S batteries, with demand projected to grow substantially as automotive manufacturers seek lighter, higher-capacity energy storage solutions. The aviation and aerospace industries also demonstrate strong interest in Li-S technology, particularly for electric aircraft and satellite applications where weight reduction directly translates to operational efficiency and extended mission capabilities.
Consumer electronics manufacturers are exploring Li-S batteries for next-generation portable devices, anticipating that the technology could enable significantly longer operating times between charges. The military and defense sectors have identified Li-S batteries as strategically important for powering unmanned aerial vehicles, portable soldier equipment, and remote sensing devices.
Grid-scale energy storage represents another emerging application area, where the lower material costs of sulfur compared to cobalt and nickel in conventional lithium-ion batteries could provide economic advantages at scale. This cost advantage becomes particularly relevant as renewable energy integration increases the demand for large-capacity storage solutions.
Market analysis indicates regional variations in Li-S battery demand, with North America and Europe leading in research investment and early adoption plans, while Asia-Pacific countries, particularly China, South Korea, and Japan, dominate in manufacturing capacity development and commercialization efforts.
The market growth trajectory for Li-S batteries is closely tied to overcoming technical challenges, with polysulfide shuttle effect suppression being the most critical barrier to widespread commercial adoption. Industry forecasts suggest that successful resolution of this technical challenge could accelerate market penetration, potentially enabling Li-S batteries to capture 5-10% of the premium battery market within the next decade.
Current market limitations stem from performance inconsistencies, with cycle life limitations directly attributable to the polysulfide shuttle effect. Market research indicates that consumers and industrial buyers require a minimum of 1000 stable cycles before considering widespread adoption, highlighting the commercial importance of shuttle suppression technologies.
Current Challenges in Polysulfide Shuttle Suppression
The polysulfide shuttle effect represents one of the most significant challenges in lithium-sulfur (Li-S) battery technology, severely limiting its commercial viability despite its theoretical advantages. This phenomenon occurs when soluble lithium polysulfides (Li₂Sₙ, 4≤n≤8) formed during discharge dissolve in the electrolyte and migrate between electrodes, causing active material loss, self-discharge, and rapid capacity fading.
Current suppression strategies face several critical limitations. Physical barriers such as separators and interlayers often suffer from trade-offs between polysulfide blocking efficiency and lithium-ion transport. While these approaches demonstrate initial effectiveness, long-term cycling reveals degradation of these physical barriers, allowing polysulfide migration to resume after extended operation.
Chemical approaches utilizing functional materials to adsorb polysulfides show promising results in laboratory settings but face scalability challenges. The adsorption capacity of these materials becomes saturated during extended cycling, diminishing their effectiveness over time. Additionally, many high-performance adsorbent materials rely on precious metals or complex synthesis procedures, making them economically unfeasible for mass production.
Electrolyte engineering represents another significant challenge area. While solid-state and gel polymer electrolytes theoretically eliminate polysulfide dissolution, they introduce new problems including poor interfacial contact and reduced ionic conductivity. Localized concentration gradients within these alternative electrolytes can still permit limited polysulfide migration, particularly at elevated temperatures or during fast charging conditions.
Cathode structure optimization approaches face difficulties in balancing sulfur loading, electrolyte accessibility, and polysulfide confinement. High sulfur loading designs often require complex nanostructured hosts that are difficult to manufacture at scale. The volumetric expansion (up to 80%) during lithiation creates mechanical stress that degrades carefully engineered structures over multiple cycles.
From a systems perspective, current battery management systems lack sophisticated algorithms to mitigate the shuttle effect through operational controls. The complex electrochemical processes involved in polysulfide formation and migration are highly sensitive to charging protocols, temperature fluctuations, and aging effects, making them difficult to manage through external control systems.
Analytical limitations further complicate research progress, as in-situ characterization of polysulfide species during battery operation remains challenging. The dynamic nature of polysulfide formation and migration requires advanced techniques that can provide real-time, spatially resolved information without disturbing the electrochemical environment.
Current suppression strategies face several critical limitations. Physical barriers such as separators and interlayers often suffer from trade-offs between polysulfide blocking efficiency and lithium-ion transport. While these approaches demonstrate initial effectiveness, long-term cycling reveals degradation of these physical barriers, allowing polysulfide migration to resume after extended operation.
Chemical approaches utilizing functional materials to adsorb polysulfides show promising results in laboratory settings but face scalability challenges. The adsorption capacity of these materials becomes saturated during extended cycling, diminishing their effectiveness over time. Additionally, many high-performance adsorbent materials rely on precious metals or complex synthesis procedures, making them economically unfeasible for mass production.
Electrolyte engineering represents another significant challenge area. While solid-state and gel polymer electrolytes theoretically eliminate polysulfide dissolution, they introduce new problems including poor interfacial contact and reduced ionic conductivity. Localized concentration gradients within these alternative electrolytes can still permit limited polysulfide migration, particularly at elevated temperatures or during fast charging conditions.
Cathode structure optimization approaches face difficulties in balancing sulfur loading, electrolyte accessibility, and polysulfide confinement. High sulfur loading designs often require complex nanostructured hosts that are difficult to manufacture at scale. The volumetric expansion (up to 80%) during lithiation creates mechanical stress that degrades carefully engineered structures over multiple cycles.
From a systems perspective, current battery management systems lack sophisticated algorithms to mitigate the shuttle effect through operational controls. The complex electrochemical processes involved in polysulfide formation and migration are highly sensitive to charging protocols, temperature fluctuations, and aging effects, making them difficult to manage through external control systems.
Analytical limitations further complicate research progress, as in-situ characterization of polysulfide species during battery operation remains challenging. The dynamic nature of polysulfide formation and migration requires advanced techniques that can provide real-time, spatially resolved information without disturbing the electrochemical environment.
State-of-the-Art Polysulfide Suppression Strategies
01 Separator modifications to prevent polysulfide shuttle
Modified separators can effectively block polysulfide migration in lithium-sulfur batteries. These separators incorporate functional materials such as carbon coatings, polymer layers, or inorganic particles that physically or chemically interact with polysulfides to prevent their shuttling between electrodes. Such modifications improve cycle stability and coulombic efficiency by maintaining active sulfur material within the cathode compartment.- Separator modifications to inhibit polysulfide shuttle: Modified separators can effectively block polysulfide migration between electrodes in lithium-sulfur batteries. These modifications include coating separators with functional materials that physically obstruct polysulfide diffusion or chemically interact with polysulfides to prevent their movement. Such separator designs create barriers that maintain polysulfides within the cathode region, significantly reducing the shuttle effect and improving battery cycling stability and capacity retention.
- Cathode structure engineering for polysulfide confinement: Advanced cathode designs can effectively trap polysulfides within the cathode structure, preventing their dissolution into the electrolyte. These designs incorporate porous carbon frameworks, conductive polymers, or metal oxide hosts that physically confine or chemically bind polysulfides. By creating strong interactions between the host material and sulfur species, these engineered cathodes minimize polysulfide shuttling, leading to improved coulombic efficiency and extended cycle life.
- Electrolyte additives and formulations: Specialized electrolyte additives can suppress the polysulfide shuttle effect by forming protective films on electrodes or by directly interacting with dissolved polysulfides. These additives include lithium nitrate, fluorinated compounds, and various salts that modify the solubility and mobility of polysulfides. Modified electrolyte formulations with optimized solvent compositions can also reduce polysulfide solubility while maintaining good ionic conductivity, thereby enhancing battery performance and longevity.
- Interlayer and functional coating strategies: Introducing functional interlayers between the cathode and separator provides an additional barrier against polysulfide migration. These interlayers can be composed of carbon materials, polymers, or metal compounds with strong polysulfide adsorption capabilities. Similarly, functional coatings applied to electrode surfaces can trap polysulfides through physical confinement or chemical bonding. These strategies effectively reduce the shuttle effect while maintaining efficient electron and ion transport within the battery.
- Novel cell designs and architectures: Innovative cell configurations and architectures can fundamentally address the polysulfide shuttle issue. These designs include integrated electrode structures, flow-based systems, or compartmentalized cells that physically separate the sulfur reaction zone from the lithium anode. Some approaches incorporate special membranes or utilize concentration gradient management to control polysulfide movement. These novel cell designs represent structural solutions to the shuttle effect, potentially enabling high-energy lithium-sulfur batteries with extended cycle life.
02 Cathode material engineering to trap polysulfides
Advanced cathode materials can be designed to chemically or physically trap polysulfides within the cathode structure. These materials include porous carbon hosts, metal oxides, sulfides, or nitrides with strong polysulfide adsorption capabilities. By incorporating these materials into the cathode, the dissolution and diffusion of polysulfides into the electrolyte can be significantly reduced, mitigating the shuttle effect and improving battery performance.Expand Specific Solutions03 Electrolyte additives and formulations
Specialized electrolyte additives and formulations can suppress the polysulfide shuttle effect in lithium-sulfur batteries. These include lithium nitrate, fluorinated compounds, ionic liquids, and various salts that can form protective films on the lithium anode or directly interact with polysulfides. Such electrolyte modifications reduce polysulfide solubility and mobility, thereby enhancing battery cycling performance and efficiency.Expand Specific Solutions04 Protective coatings for lithium anodes
Protective coatings applied to lithium metal anodes can significantly reduce the polysulfide shuttle effect. These coatings create physical barriers that prevent direct contact between the lithium metal and dissolved polysulfides, reducing unwanted side reactions. Materials used include artificial solid electrolyte interphases, polymers, ceramics, and composite layers that are both ionically conductive and chemically stable against polysulfides.Expand Specific Solutions05 Interlayers and functional interfaces
Introducing functional interlayers between the cathode and separator can effectively mitigate the polysulfide shuttle effect. These interlayers serve as additional barriers that selectively allow lithium ions to pass while blocking polysulfide migration. They can be made from conductive polymers, carbon materials, metal compounds, or composites that have strong interactions with polysulfides, thereby confining them within the cathode region and improving battery cycling stability.Expand Specific Solutions
Leading Companies and Research Institutions in Li-S Battery Development
The lithium-sulfur battery market is currently in an early growth phase, characterized by intensive R&D efforts to overcome the polysulfide shuttle effect—a key technical barrier limiting commercialization. The global market is projected to reach $1.5-2 billion by 2030, with a CAGR exceeding 30%. Major players include established battery manufacturers like LG Energy Solution, LG Chem, and Sony Group, alongside chemical companies such as BASF and Robert Bosch developing specialized materials. Academic-industrial partnerships are prominent, with institutions like Central South University, KAIST, and Huazhong University collaborating with companies like Tianmu Lake Institute and Guangdong Bangpu. The technology remains at TRL 4-6, with recent breakthroughs in functional separators, cathode design, and electrolyte additives advancing toward commercial viability.
LG Chem Ltd.
Technical Solution: LG Chem has developed a multi-functional interlayer approach to suppress polysulfide shuttling in lithium-sulfur batteries. Their technology incorporates carbon-based materials with polar metal oxides (primarily titanium dioxide and aluminum oxide) to create a physical barrier between the sulfur cathode and lithium anode. This interlayer functions through both physical obstruction and chemical adsorption mechanisms. The company has pioneered the use of nitrogen and oxygen co-doped carbon frameworks that provide strong chemical anchoring sites for polysulfides. Additionally, LG Chem has implemented conductive polymer coatings on separators that effectively trap dissolved polysulfides while maintaining ionic conductivity. Their recent advancements include the development of lithiated Nafion-based separators that utilize electrostatic repulsion to prevent polysulfide migration.
Strengths: Excellent balance between physical and chemical polysulfide trapping mechanisms; solutions maintain high ionic conductivity while blocking polysulfides; technologies are scalable for mass production. Weaknesses: Some approaches add weight and volume to the battery system; certain materials increase production costs; long-term stability of functional coatings remains a challenge in commercial applications.
BASF Corp.
Technical Solution: BASF has developed an innovative approach to polysulfide shuttle suppression through their Metal-Organic Framework (MOF) technology. Their solution incorporates specially designed MOFs with tailored pore structures and functionalized surfaces that effectively trap polysulfides through both physical confinement and chemical bonding. BASF's proprietary MOF materials feature high surface areas (>1000 m²/g) with precisely engineered metal nodes (primarily utilizing Ti, Zr, and Fe centers) that form strong coordination bonds with polysulfide species. The company has also developed composite separator coatings combining these MOFs with conductive polymers to create a functional barrier that allows lithium ion transport while restricting polysulfide migration. Additionally, BASF has pioneered electrolyte additives containing fluorinated compounds that form protective films on lithium surfaces, further preventing side reactions with dissolved polysulfides and enhancing overall battery performance.
Strengths: Highly customizable MOF structures allow precise engineering of polysulfide trapping properties; solutions integrate well with existing battery manufacturing processes; comprehensive approach addressing multiple aspects of the shuttle effect. Weaknesses: MOF synthesis can be complex and costly at scale; some solutions may reduce energy density; long-term stability of MOF structures in battery environments requires further optimization.
Key Patents and Scientific Breakthroughs in Shuttle Effect Mitigation
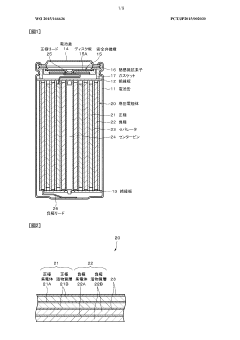
Lithium-sulfur battery having high energy density
PatentWO2022071722A1
Innovation
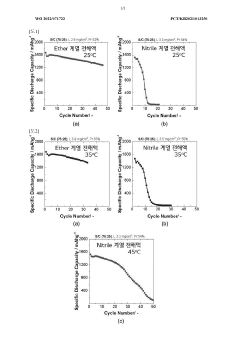
- A lithium-sulfur battery employing a sparing solvating electrolyte system with a fluorine-based ether compound and a glyme-based solvent, combined with a lithium salt, and a high specific surface area carbon material in the positive electrode, which suppresses polysulfide dissolution and maintains stability without nitrile-based solvents, enhancing sulfur utilization and battery performance.
Electrolyte solution, battery, battery pack, electronic device, electric vehicle, electricity storage device and electric power system
PatentWO2015166636A1
Innovation
- The use of an electrolytic solution containing a liquid complex or liquid salt where polysulfides are insoluble or almost insoluble, combined with a solvent where polysulfides are soluble, maintaining a saturated sulfur concentration of Li2S8 within the range of 10 mM to 400 mM, to suppress sulfur elution and enhance battery stability.
Environmental Impact and Sustainability of Li-S Battery Materials
The environmental impact of lithium-sulfur (Li-S) batteries represents a critical consideration in their development, particularly in the context of polysulfide shuttle suppression strategies. Unlike conventional lithium-ion batteries that rely on cobalt and nickel, Li-S batteries utilize sulfur as a cathode material, which is abundantly available as a byproduct of petroleum refining processes. This natural abundance significantly reduces the environmental footprint associated with resource extraction compared to traditional battery technologies.
The sustainability advantages of Li-S batteries extend beyond raw material availability. The sulfur cathode material requires substantially less energy to process than transition metal oxides used in conventional batteries, resulting in lower carbon emissions during manufacturing. Additionally, the theoretical energy density of Li-S batteries (2600 Wh/kg) far exceeds that of lithium-ion batteries, potentially enabling more efficient energy storage solutions with reduced material requirements per unit of stored energy.
However, the environmental implications of polysulfide shuttle suppression strategies warrant careful examination. Many current approaches involve introducing additional materials such as carbon-based additives, metal oxides, or polymeric materials to physically or chemically trap polysulfides. The production of these materials may introduce new environmental concerns, including increased energy consumption and potential toxic emissions during synthesis processes.
Particularly concerning are approaches utilizing nanomaterials for polysulfide suppression, as their production often involves energy-intensive processes and potentially hazardous chemicals. The long-term environmental fate and ecotoxicological impacts of these nanomaterials remain inadequately understood, raising questions about their sustainability despite their technical effectiveness.
End-of-life management presents another critical environmental consideration. The recyclability of Li-S batteries incorporating various polysulfide suppression technologies remains largely unexplored. While sulfur itself is highly recyclable, the complex composite structures created by advanced suppression strategies may complicate recycling processes and reduce material recovery rates.
Recent life cycle assessment studies suggest that the environmental benefits of Li-S batteries strongly depend on effective recycling infrastructure development. Without proper recycling pathways, the sustainability advantages of these batteries may be significantly diminished, particularly as deployment scales increase. Developing environmentally benign polysulfide suppression approaches that maintain compatibility with recycling processes represents a crucial research direction for maximizing the sustainability potential of Li-S technology.
The sustainability advantages of Li-S batteries extend beyond raw material availability. The sulfur cathode material requires substantially less energy to process than transition metal oxides used in conventional batteries, resulting in lower carbon emissions during manufacturing. Additionally, the theoretical energy density of Li-S batteries (2600 Wh/kg) far exceeds that of lithium-ion batteries, potentially enabling more efficient energy storage solutions with reduced material requirements per unit of stored energy.
However, the environmental implications of polysulfide shuttle suppression strategies warrant careful examination. Many current approaches involve introducing additional materials such as carbon-based additives, metal oxides, or polymeric materials to physically or chemically trap polysulfides. The production of these materials may introduce new environmental concerns, including increased energy consumption and potential toxic emissions during synthesis processes.
Particularly concerning are approaches utilizing nanomaterials for polysulfide suppression, as their production often involves energy-intensive processes and potentially hazardous chemicals. The long-term environmental fate and ecotoxicological impacts of these nanomaterials remain inadequately understood, raising questions about their sustainability despite their technical effectiveness.
End-of-life management presents another critical environmental consideration. The recyclability of Li-S batteries incorporating various polysulfide suppression technologies remains largely unexplored. While sulfur itself is highly recyclable, the complex composite structures created by advanced suppression strategies may complicate recycling processes and reduce material recovery rates.
Recent life cycle assessment studies suggest that the environmental benefits of Li-S batteries strongly depend on effective recycling infrastructure development. Without proper recycling pathways, the sustainability advantages of these batteries may be significantly diminished, particularly as deployment scales increase. Developing environmentally benign polysulfide suppression approaches that maintain compatibility with recycling processes represents a crucial research direction for maximizing the sustainability potential of Li-S technology.
Scalability and Manufacturing Challenges for Commercial Implementation
The transition from laboratory-scale research to commercial production of lithium-sulfur batteries faces significant manufacturing challenges, particularly regarding polysulfide shuttle suppression technologies. Current laboratory methods for creating specialized separators, interlayers, and modified cathodes often involve complex, multi-step processes that are difficult to scale. For instance, the atomic layer deposition techniques used to create metal oxide coatings on separators require specialized equipment and precise control, making them prohibitively expensive for mass production.
Material consistency presents another major hurdle. The uniform application of functional materials across large-area electrodes and separators is technically demanding. Variations in coating thickness, porosity, and composition can lead to inconsistent battery performance and accelerated degradation. This challenge becomes particularly acute when manufacturing batteries with dimensions suitable for electric vehicles or grid storage applications.
Cost considerations further complicate commercialization efforts. Many effective polysulfide suppression strategies rely on precious metals, advanced carbon materials, or complex metal-organic frameworks that significantly increase production costs. For example, platinum-based catalysts and graphene-based interlayers, while effective in laboratory settings, remain economically unfeasible for widespread commercial implementation.
Process integration represents a critical challenge when incorporating novel materials into existing battery manufacturing lines. The introduction of additional processing steps for polysulfide suppression technologies disrupts established workflows and requires substantial capital investment. Manufacturers must balance performance improvements against increased production complexity and cost.
Environmental and safety concerns also impact scalability. Some polysulfide suppression approaches involve toxic solvents or hazardous materials that require specialized handling and waste management protocols. Developing greener alternatives that maintain effectiveness while meeting regulatory requirements remains an ongoing challenge for the industry.
Quality control and testing methodologies need significant adaptation for commercial-scale production. Current laboratory techniques for evaluating polysulfide shuttle suppression are time-consuming and often destructive, making them impractical for high-volume manufacturing environments. The development of rapid, non-destructive testing methods is essential for ensuring consistent product quality.
Addressing these challenges requires collaborative efforts between materials scientists, chemical engineers, and manufacturing specialists. Recent industry trends show increasing focus on developing simplified approaches that can be integrated into existing production lines with minimal modification, such as water-based electrode slurries containing polysulfide-trapping additives and pre-functionalized separator materials that can be handled with conventional equipment.
Material consistency presents another major hurdle. The uniform application of functional materials across large-area electrodes and separators is technically demanding. Variations in coating thickness, porosity, and composition can lead to inconsistent battery performance and accelerated degradation. This challenge becomes particularly acute when manufacturing batteries with dimensions suitable for electric vehicles or grid storage applications.
Cost considerations further complicate commercialization efforts. Many effective polysulfide suppression strategies rely on precious metals, advanced carbon materials, or complex metal-organic frameworks that significantly increase production costs. For example, platinum-based catalysts and graphene-based interlayers, while effective in laboratory settings, remain economically unfeasible for widespread commercial implementation.
Process integration represents a critical challenge when incorporating novel materials into existing battery manufacturing lines. The introduction of additional processing steps for polysulfide suppression technologies disrupts established workflows and requires substantial capital investment. Manufacturers must balance performance improvements against increased production complexity and cost.
Environmental and safety concerns also impact scalability. Some polysulfide suppression approaches involve toxic solvents or hazardous materials that require specialized handling and waste management protocols. Developing greener alternatives that maintain effectiveness while meeting regulatory requirements remains an ongoing challenge for the industry.
Quality control and testing methodologies need significant adaptation for commercial-scale production. Current laboratory techniques for evaluating polysulfide shuttle suppression are time-consuming and often destructive, making them impractical for high-volume manufacturing environments. The development of rapid, non-destructive testing methods is essential for ensuring consistent product quality.
Addressing these challenges requires collaborative efforts between materials scientists, chemical engineers, and manufacturing specialists. Recent industry trends show increasing focus on developing simplified approaches that can be integrated into existing production lines with minimal modification, such as water-based electrode slurries containing polysulfide-trapping additives and pre-functionalized separator materials that can be handled with conventional equipment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!