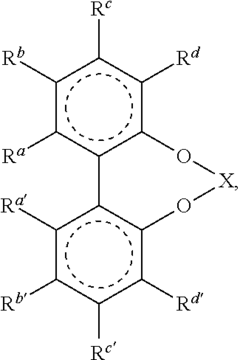
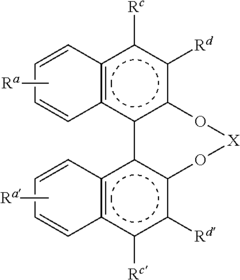
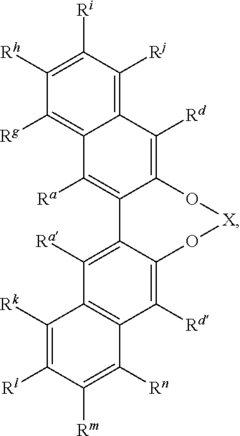
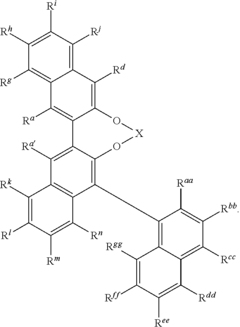
Advancing Catalytic Science Using Fluoroantimonic Acid
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid Catalysis Background and Objectives
Fluoroantimonic acid, a superacid formed by combining hydrogen fluoride and antimony pentafluoride, has emerged as a powerful catalyst in organic synthesis and industrial processes. Its exceptional acidity, surpassing that of conventional acids, has revolutionized catalytic science and opened new avenues for chemical transformations.
The development of fluoroantimonic acid catalysis can be traced back to the mid-20th century when researchers began exploring superacids for their unique properties. The field gained momentum in the 1960s and 1970s with groundbreaking work by George A. Olah, who later received the Nobel Prize in Chemistry for his contributions to carbocation chemistry.
Over the past decades, fluoroantimonic acid has demonstrated its potential in various catalytic applications, including hydrocarbon cracking, isomerization, and alkylation reactions. Its ability to protonate even weak bases and generate highly reactive carbocations has made it an invaluable tool in organic synthesis and petrochemical industries.
The evolution of fluoroantimonic acid catalysis has been driven by the increasing demand for more efficient and selective chemical processes. As environmental concerns and economic pressures have grown, researchers have sought to develop catalysts that can operate under milder conditions, reduce waste, and improve product yields.
Current research in fluoroantimonic acid catalysis aims to address several key objectives. Firstly, there is a focus on enhancing the stability and recyclability of the catalyst, as its corrosive nature and sensitivity to moisture pose challenges in practical applications. Secondly, efforts are being made to fine-tune the acidity and selectivity of fluoroantimonic acid-based systems to expand their scope in organic transformations.
Another important goal is to develop heterogeneous catalysts incorporating fluoroantimonic acid, which would facilitate easier separation and recovery of the catalyst from reaction mixtures. This approach could significantly improve the efficiency and sustainability of catalytic processes in industrial settings.
Furthermore, researchers are exploring the potential of fluoroantimonic acid in emerging fields such as biomass conversion and the synthesis of advanced materials. The unique properties of this superacid may offer novel solutions to challenges in these areas, potentially leading to breakthroughs in renewable energy and materials science.
As we look to the future, the advancement of fluoroantimonic acid catalysis is expected to play a crucial role in addressing global challenges related to energy production, environmental sustainability, and the development of new materials. By continuing to push the boundaries of catalytic science, researchers aim to unlock new possibilities for chemical synthesis and industrial processes, ultimately contributing to a more sustainable and efficient chemical industry.
The development of fluoroantimonic acid catalysis can be traced back to the mid-20th century when researchers began exploring superacids for their unique properties. The field gained momentum in the 1960s and 1970s with groundbreaking work by George A. Olah, who later received the Nobel Prize in Chemistry for his contributions to carbocation chemistry.
Over the past decades, fluoroantimonic acid has demonstrated its potential in various catalytic applications, including hydrocarbon cracking, isomerization, and alkylation reactions. Its ability to protonate even weak bases and generate highly reactive carbocations has made it an invaluable tool in organic synthesis and petrochemical industries.
The evolution of fluoroantimonic acid catalysis has been driven by the increasing demand for more efficient and selective chemical processes. As environmental concerns and economic pressures have grown, researchers have sought to develop catalysts that can operate under milder conditions, reduce waste, and improve product yields.
Current research in fluoroantimonic acid catalysis aims to address several key objectives. Firstly, there is a focus on enhancing the stability and recyclability of the catalyst, as its corrosive nature and sensitivity to moisture pose challenges in practical applications. Secondly, efforts are being made to fine-tune the acidity and selectivity of fluoroantimonic acid-based systems to expand their scope in organic transformations.
Another important goal is to develop heterogeneous catalysts incorporating fluoroantimonic acid, which would facilitate easier separation and recovery of the catalyst from reaction mixtures. This approach could significantly improve the efficiency and sustainability of catalytic processes in industrial settings.
Furthermore, researchers are exploring the potential of fluoroantimonic acid in emerging fields such as biomass conversion and the synthesis of advanced materials. The unique properties of this superacid may offer novel solutions to challenges in these areas, potentially leading to breakthroughs in renewable energy and materials science.
As we look to the future, the advancement of fluoroantimonic acid catalysis is expected to play a crucial role in addressing global challenges related to energy production, environmental sustainability, and the development of new materials. By continuing to push the boundaries of catalytic science, researchers aim to unlock new possibilities for chemical synthesis and industrial processes, ultimately contributing to a more sustainable and efficient chemical industry.
Industrial Applications and Market Demand
Fluoroantimonic acid, known as the strongest superacid, has garnered significant attention in the field of catalytic science due to its exceptional proton-donating ability. This powerful compound has found diverse applications across various industrial sectors, driving market demand and technological advancements.
In the petrochemical industry, fluoroantimonic acid has revolutionized hydrocarbon processing. Its superior catalytic properties enable more efficient cracking and isomerization reactions, leading to improved yields and product quality in fuel production. Refineries have shown increasing interest in incorporating this superacid into their processes to enhance overall efficiency and reduce energy consumption.
The pharmaceutical sector has also recognized the potential of fluoroantimonic acid in synthesizing complex organic compounds. Its ability to catalyze challenging reactions has opened new avenues for drug discovery and development. Pharmaceutical companies are investing in research to exploit this superacid's capabilities in creating novel active pharmaceutical ingredients and intermediates.
In the polymer industry, fluoroantimonic acid has demonstrated promise in catalyzing polymerization reactions. Its use has led to the development of high-performance materials with enhanced properties, such as improved thermal stability and chemical resistance. This has created a growing demand for fluoroantimonic acid in the production of specialty polymers for aerospace, automotive, and electronics applications.
The electronics industry has shown interest in fluoroantimonic acid for its potential in semiconductor manufacturing. Its strong acidic properties make it suitable for etching and cleaning processes in microchip production. As the demand for smaller and more powerful electronic devices continues to rise, the market for advanced etching agents like fluoroantimonic acid is expected to grow.
Environmental applications of fluoroantimonic acid are emerging, particularly in waste treatment and pollution control. Its strong oxidizing properties make it effective in breaking down persistent organic pollutants and treating industrial effluents. This has led to increased demand from environmental service companies and municipal waste treatment facilities.
The global market for superacids, including fluoroantimonic acid, is experiencing steady growth driven by these diverse applications. While exact market size figures are not readily available due to the specialized nature of the product, industry reports indicate a positive growth trajectory. The Asia-Pacific region, particularly China and India, is expected to be a major growth driver due to rapid industrialization and increasing investments in research and development.
However, the market demand for fluoroantimonic acid is tempered by challenges related to its handling and storage. Its extremely corrosive nature necessitates specialized equipment and safety measures, which can increase operational costs. This has led to ongoing research into developing safer formulations and handling techniques to broaden its industrial applicability and market acceptance.
In the petrochemical industry, fluoroantimonic acid has revolutionized hydrocarbon processing. Its superior catalytic properties enable more efficient cracking and isomerization reactions, leading to improved yields and product quality in fuel production. Refineries have shown increasing interest in incorporating this superacid into their processes to enhance overall efficiency and reduce energy consumption.
The pharmaceutical sector has also recognized the potential of fluoroantimonic acid in synthesizing complex organic compounds. Its ability to catalyze challenging reactions has opened new avenues for drug discovery and development. Pharmaceutical companies are investing in research to exploit this superacid's capabilities in creating novel active pharmaceutical ingredients and intermediates.
In the polymer industry, fluoroantimonic acid has demonstrated promise in catalyzing polymerization reactions. Its use has led to the development of high-performance materials with enhanced properties, such as improved thermal stability and chemical resistance. This has created a growing demand for fluoroantimonic acid in the production of specialty polymers for aerospace, automotive, and electronics applications.
The electronics industry has shown interest in fluoroantimonic acid for its potential in semiconductor manufacturing. Its strong acidic properties make it suitable for etching and cleaning processes in microchip production. As the demand for smaller and more powerful electronic devices continues to rise, the market for advanced etching agents like fluoroantimonic acid is expected to grow.
Environmental applications of fluoroantimonic acid are emerging, particularly in waste treatment and pollution control. Its strong oxidizing properties make it effective in breaking down persistent organic pollutants and treating industrial effluents. This has led to increased demand from environmental service companies and municipal waste treatment facilities.
The global market for superacids, including fluoroantimonic acid, is experiencing steady growth driven by these diverse applications. While exact market size figures are not readily available due to the specialized nature of the product, industry reports indicate a positive growth trajectory. The Asia-Pacific region, particularly China and India, is expected to be a major growth driver due to rapid industrialization and increasing investments in research and development.
However, the market demand for fluoroantimonic acid is tempered by challenges related to its handling and storage. Its extremely corrosive nature necessitates specialized equipment and safety measures, which can increase operational costs. This has led to ongoing research into developing safer formulations and handling techniques to broaden its industrial applicability and market acceptance.
Current State and Challenges in Superacid Catalysis
Superacid catalysis, particularly using fluoroantimonic acid, represents a cutting-edge field in catalytic science. The current state of this technology is characterized by significant advancements in reaction efficiency and selectivity, yet it faces several critical challenges that hinder its widespread industrial application.
Fluoroantimonic acid, recognized as the strongest known superacid, has demonstrated remarkable catalytic properties in various organic transformations. Its extreme acidity enables the activation of typically unreactive C-H bonds, facilitating novel synthetic pathways. Recent research has shown its efficacy in isomerization reactions, alkylations, and the cracking of hydrocarbons, offering potential for more efficient petrochemical processes.
Despite these promising developments, the corrosive nature of fluoroantimonic acid poses substantial challenges for reactor design and material compatibility. Conventional materials rapidly degrade under its extreme acidity, necessitating the development of specialized containment systems. This limitation has largely confined its use to laboratory-scale experiments, impeding industrial-scale applications.
Another significant challenge lies in the controlled handling and disposal of fluoroantimonic acid. Its extreme reactivity with water and most organic compounds presents safety concerns and environmental risks. Researchers are actively exploring methods to mitigate these issues, including the development of supported catalysts and ionic liquid systems that can harness the superacid's catalytic power while minimizing its hazardous properties.
The scalability of superacid catalysis processes remains a major hurdle. While small-scale reactions have shown promising results, translating these to industrial-scale operations presents numerous engineering challenges. Issues such as heat management, product separation, and catalyst recovery need to be addressed to make these processes economically viable and environmentally sustainable.
Furthermore, the mechanistic understanding of superacid-catalyzed reactions is still evolving. The extreme acidity of these systems often leads to complex reaction pathways that are difficult to predict and control. Ongoing research focuses on elucidating these mechanisms through advanced spectroscopic techniques and computational modeling, aiming to enhance reaction predictability and selectivity.
The high cost and limited availability of fluoroantimonic acid also present significant barriers to its widespread adoption. Current production methods are expensive and energy-intensive, prompting research into more cost-effective synthesis routes and alternative superacid systems that could offer similar catalytic performance with reduced economic and environmental impact.
Fluoroantimonic acid, recognized as the strongest known superacid, has demonstrated remarkable catalytic properties in various organic transformations. Its extreme acidity enables the activation of typically unreactive C-H bonds, facilitating novel synthetic pathways. Recent research has shown its efficacy in isomerization reactions, alkylations, and the cracking of hydrocarbons, offering potential for more efficient petrochemical processes.
Despite these promising developments, the corrosive nature of fluoroantimonic acid poses substantial challenges for reactor design and material compatibility. Conventional materials rapidly degrade under its extreme acidity, necessitating the development of specialized containment systems. This limitation has largely confined its use to laboratory-scale experiments, impeding industrial-scale applications.
Another significant challenge lies in the controlled handling and disposal of fluoroantimonic acid. Its extreme reactivity with water and most organic compounds presents safety concerns and environmental risks. Researchers are actively exploring methods to mitigate these issues, including the development of supported catalysts and ionic liquid systems that can harness the superacid's catalytic power while minimizing its hazardous properties.
The scalability of superacid catalysis processes remains a major hurdle. While small-scale reactions have shown promising results, translating these to industrial-scale operations presents numerous engineering challenges. Issues such as heat management, product separation, and catalyst recovery need to be addressed to make these processes economically viable and environmentally sustainable.
Furthermore, the mechanistic understanding of superacid-catalyzed reactions is still evolving. The extreme acidity of these systems often leads to complex reaction pathways that are difficult to predict and control. Ongoing research focuses on elucidating these mechanisms through advanced spectroscopic techniques and computational modeling, aiming to enhance reaction predictability and selectivity.
The high cost and limited availability of fluoroantimonic acid also present significant barriers to its widespread adoption. Current production methods are expensive and energy-intensive, prompting research into more cost-effective synthesis routes and alternative superacid systems that could offer similar catalytic performance with reduced economic and environmental impact.
Existing Fluoroantimonic Acid Catalytic Solutions
01 Catalytic activity in organic reactions
Fluoroantimonic acid exhibits strong catalytic activity in various organic reactions, particularly in hydrocarbon transformations. Its super-acidic properties make it effective for isomerization, alkylation, and cracking processes. The acid's ability to generate carbocations facilitates these reactions, making it valuable in petrochemical industries.- Catalytic activity in organic reactions: Fluoroantimonic acid exhibits strong catalytic activity in various organic reactions, particularly in hydrocarbon transformations. Its super-acidic properties make it effective for isomerization, alkylation, and cracking processes. The acid's ability to generate carbocations facilitates these reactions, making it valuable in petrochemical industries.
- Application in polymerization processes: Fluoroantimonic acid serves as a potent catalyst in polymerization reactions. It can initiate cationic polymerization of olefins and other monomers, leading to the formation of high molecular weight polymers. The acid's strong electron-withdrawing properties contribute to its effectiveness in these processes.
- Use in electrochemical applications: The catalytic activity of fluoroantimonic acid extends to electrochemical processes. It can be employed in electrolyte solutions for batteries and fuel cells, enhancing their performance. The acid's unique properties allow for improved electron transfer and ionic conductivity in these systems.
- Catalysis in inorganic synthesis: Fluoroantimonic acid demonstrates catalytic activity in the synthesis of various inorganic compounds. It can facilitate reactions involving metal complexes, oxide formations, and other inorganic transformations. The acid's strong protonating ability plays a crucial role in these processes.
- Environmental and safety considerations: While fluoroantimonic acid shows remarkable catalytic activity, its use comes with significant environmental and safety concerns. Research focuses on developing safer handling methods, containment strategies, and potential substitutes that can provide similar catalytic performance with reduced risks.
02 Application in polymerization processes
Fluoroantimonic acid serves as a potent catalyst in polymerization reactions. It initiates cationic polymerization of olefins and other monomers, leading to the formation of high molecular weight polymers. The acid's strong electron-withdrawing properties contribute to its effectiveness in controlling polymer chain growth and structure.Expand Specific Solutions03 Use in electrochemical applications
The unique properties of fluoroantimonic acid make it suitable for various electrochemical applications. It can be used in the development of high-performance batteries, fuel cells, and other energy storage devices. The acid's ability to facilitate ion transport and electron transfer processes enhances the efficiency of these electrochemical systems.Expand Specific Solutions04 Role in materials synthesis and modification
Fluoroantimonic acid plays a crucial role in the synthesis and modification of advanced materials. It can be used to create novel nanostructures, modify surface properties of materials, and facilitate the formation of complex compounds. The acid's strong protonating ability enables unique chemical transformations in material science applications.Expand Specific Solutions05 Environmental and safety considerations
Due to its extremely corrosive and reactive nature, the use of fluoroantimonic acid requires stringent safety measures and environmental considerations. Research focuses on developing safer handling methods, containment strategies, and alternative catalysts with similar activity but reduced hazards. Efforts are also directed towards minimizing environmental impact and improving disposal techniques.Expand Specific Solutions
Key Players in Superacid Catalysis Research
The field of catalytic science using fluoroantimonic acid is in a nascent stage of development, with significant potential for growth. The market size is currently limited but expected to expand as research progresses. Technologically, it remains in the early stages of maturity, with ongoing research and development efforts. Key players like 3M Innovative Properties Co., BASF Corp., and Novartis AG are actively contributing to advancements in this area. Universities such as Yale University and The Regents of the University of California are also conducting crucial research. The involvement of diverse entities, including chemical companies, pharmaceutical firms, and academic institutions, indicates a growing interest in exploiting the unique properties of fluoroantimonic acid for catalytic applications.
The Regents of the University of California
Technical Solution: The University of California has made significant contributions to advancing catalytic science using fluoroantimonic acid through a multidisciplinary research approach. Their team has developed a novel method for in-situ generation of fluoroantimonic acid within zeolite frameworks, creating a powerful solid acid catalyst. This approach combines the extreme acidity of fluoroantimonic acid with the shape-selectivity of zeolites, enabling highly efficient and selective catalytic transformations. The university has also pioneered the use of advanced spectroscopic techniques to study the behavior of the superacid in confined spaces, providing unprecedented insights into its catalytic mechanisms at the molecular level.
Strengths: Innovative combination of superacid chemistry and zeolite technology, potential for highly selective catalysis, and advanced characterization capabilities. Weaknesses: Challenges in large-scale synthesis of specialized zeolite materials, potential limitations in the types of reactions that can be catalyzed effectively.
BASF Corp.
Technical Solution: BASF has developed a novel approach to utilizing fluoroantimonic acid in catalytic processes. Their method involves stabilizing the superacid through the use of specially designed ionic liquids, which act as a reaction medium. This allows for controlled catalysis in a more manageable environment, reducing the corrosive effects typically associated with fluoroantimonic acid. The company has also engineered a unique reactor design that incorporates materials resistant to the acid's extreme reactivity, enabling longer catalyst lifetimes and more efficient chemical transformations.
Strengths: Enhanced stability and control of the superacid, improved safety in handling, and potential for wider industrial applications. Weaknesses: High costs associated with specialized equipment and materials, limited scalability for certain processes.
Core Innovations in Superacid Catalysis
Asymmetric electrophilic fluorination using an anionic chiral phase-transfer catalyst
PatentWO2013096971A1
Innovation
- Development of chiral anionic phase-transfer catalysts that facilitate enantioselective electrophilic addition reactions by forming soluble ion pairs with insoluble cationic electrophilic reagents, enabling efficient and enantiocontrolled C-F bond formation through electrophilic addition reactions.
Asymmetric electrophilic fluorination using an anionic chiral phase-transfer catalyst
PatentActiveUS20140350253A1
Innovation
- Development of chiral anion phase-transfer catalysts that facilitate electrophilic addition reactions, allowing for enantioselective fluorination using stable and inexpensive reagents, and enabling the formation of fluorinated compounds that can be further functionalized.
Environmental Impact and Safety Considerations
Fluoroantimonic acid, known as the world's strongest superacid, presents significant environmental and safety challenges that must be carefully considered in its application to catalytic science. The extreme corrosiveness and reactivity of this compound necessitate stringent handling protocols and specialized containment systems to prevent environmental contamination and ensure worker safety.
From an environmental perspective, the release of fluoroantimonic acid could have devastating consequences on ecosystems. Its ability to react violently with water and organic matter poses a severe threat to aquatic environments and soil ecosystems. Even minute quantities could lead to long-lasting ecological damage, affecting pH levels and potentially introducing toxic fluoride and antimony compounds into the food chain.
Air quality is another critical concern, as the acid can generate highly toxic and corrosive fumes. These emissions could contribute to air pollution and pose respiratory hazards to both humans and wildlife in the vicinity of research or industrial facilities utilizing this superacid. Proper ventilation systems and air filtration technologies are essential to mitigate these risks.
Worker safety is paramount when handling fluoroantimonic acid. The compound's extreme corrosiveness necessitates the use of specialized personal protective equipment (PPE), including chemical-resistant suits, gloves, and respiratory protection. Emergency response protocols must be meticulously designed and regularly practiced to address potential spills or exposures.
The storage and transportation of fluoroantimonic acid present additional challenges. Specialized containers made of materials resistant to superacids, such as polytetrafluoroethylene (PTFE), are required. These containers must be regularly inspected and maintained to prevent leaks or degradation over time. Transportation regulations for such hazardous materials are stringent and require careful adherence to ensure public safety.
Waste management is a critical aspect of environmental stewardship when working with fluoroantimonic acid. Neutralization processes must be carefully designed to handle the extreme acidity, and disposal methods must comply with strict environmental regulations. Recycling and recovery techniques for the acid and its byproducts should be explored to minimize environmental impact and resource consumption.
Research institutions and industrial facilities working with fluoroantimonic acid must implement comprehensive safety management systems. This includes regular risk assessments, employee training programs, and the establishment of clear standard operating procedures (SOPs) for all activities involving the superacid. Continuous monitoring of environmental parameters and health surveillance of workers are essential components of a robust safety program.
From an environmental perspective, the release of fluoroantimonic acid could have devastating consequences on ecosystems. Its ability to react violently with water and organic matter poses a severe threat to aquatic environments and soil ecosystems. Even minute quantities could lead to long-lasting ecological damage, affecting pH levels and potentially introducing toxic fluoride and antimony compounds into the food chain.
Air quality is another critical concern, as the acid can generate highly toxic and corrosive fumes. These emissions could contribute to air pollution and pose respiratory hazards to both humans and wildlife in the vicinity of research or industrial facilities utilizing this superacid. Proper ventilation systems and air filtration technologies are essential to mitigate these risks.
Worker safety is paramount when handling fluoroantimonic acid. The compound's extreme corrosiveness necessitates the use of specialized personal protective equipment (PPE), including chemical-resistant suits, gloves, and respiratory protection. Emergency response protocols must be meticulously designed and regularly practiced to address potential spills or exposures.
The storage and transportation of fluoroantimonic acid present additional challenges. Specialized containers made of materials resistant to superacids, such as polytetrafluoroethylene (PTFE), are required. These containers must be regularly inspected and maintained to prevent leaks or degradation over time. Transportation regulations for such hazardous materials are stringent and require careful adherence to ensure public safety.
Waste management is a critical aspect of environmental stewardship when working with fluoroantimonic acid. Neutralization processes must be carefully designed to handle the extreme acidity, and disposal methods must comply with strict environmental regulations. Recycling and recovery techniques for the acid and its byproducts should be explored to minimize environmental impact and resource consumption.
Research institutions and industrial facilities working with fluoroantimonic acid must implement comprehensive safety management systems. This includes regular risk assessments, employee training programs, and the establishment of clear standard operating procedures (SOPs) for all activities involving the superacid. Continuous monitoring of environmental parameters and health surveillance of workers are essential components of a robust safety program.
Regulatory Framework for Superacid Usage
The regulatory framework for superacid usage, particularly fluoroantimonic acid, is a critical aspect of advancing catalytic science. Given the extreme reactivity and potential hazards associated with superacids, stringent regulations have been established to ensure safe handling, storage, and application in research and industrial settings.
At the international level, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a foundation for the classification and communication of chemical hazards. Under this system, fluoroantimonic acid is categorized as a highly corrosive and toxic substance, requiring specific labeling and safety data sheets.
In the United States, the Occupational Safety and Health Administration (OSHA) has set strict guidelines for the use of superacids in laboratory and industrial environments. These regulations mandate the implementation of engineering controls, such as fume hoods and closed systems, to minimize exposure risks. Personal protective equipment (PPE) requirements are also specified, including chemical-resistant suits, gloves, and respiratory protection.
The Environmental Protection Agency (EPA) regulates the transportation, storage, and disposal of superacids under the Resource Conservation and Recovery Act (RCRA). Facilities handling fluoroantimonic acid must obtain proper permits and adhere to strict waste management protocols to prevent environmental contamination.
In the European Union, the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation governs the use of superacids. Manufacturers and importers must register substances like fluoroantimonic acid with the European Chemicals Agency (ECHA) and provide comprehensive safety data. The Classification, Labelling and Packaging (CLP) Regulation further ensures that hazards are clearly communicated to workers and consumers.
Japan's Chemical Substances Control Law (CSCL) and Industrial Safety and Health Law (ISHL) provide regulatory oversight for superacid usage in research and industrial applications. These laws require thorough risk assessments and the implementation of appropriate safety measures.
Research institutions and industrial facilities working with fluoroantimonic acid must establish comprehensive safety management systems. These typically include standard operating procedures (SOPs), regular safety training programs, and emergency response plans. Periodic audits and inspections are conducted to ensure compliance with regulatory requirements and best practices.
As catalytic science advances, regulatory frameworks continue to evolve to address emerging challenges and incorporate new safety technologies. Collaborative efforts between regulatory agencies, research institutions, and industry stakeholders are essential to maintain a balance between scientific progress and safety considerations in the use of superacids like fluoroantimonic acid.
At the international level, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a foundation for the classification and communication of chemical hazards. Under this system, fluoroantimonic acid is categorized as a highly corrosive and toxic substance, requiring specific labeling and safety data sheets.
In the United States, the Occupational Safety and Health Administration (OSHA) has set strict guidelines for the use of superacids in laboratory and industrial environments. These regulations mandate the implementation of engineering controls, such as fume hoods and closed systems, to minimize exposure risks. Personal protective equipment (PPE) requirements are also specified, including chemical-resistant suits, gloves, and respiratory protection.
The Environmental Protection Agency (EPA) regulates the transportation, storage, and disposal of superacids under the Resource Conservation and Recovery Act (RCRA). Facilities handling fluoroantimonic acid must obtain proper permits and adhere to strict waste management protocols to prevent environmental contamination.
In the European Union, the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation governs the use of superacids. Manufacturers and importers must register substances like fluoroantimonic acid with the European Chemicals Agency (ECHA) and provide comprehensive safety data. The Classification, Labelling and Packaging (CLP) Regulation further ensures that hazards are clearly communicated to workers and consumers.
Japan's Chemical Substances Control Law (CSCL) and Industrial Safety and Health Law (ISHL) provide regulatory oversight for superacid usage in research and industrial applications. These laws require thorough risk assessments and the implementation of appropriate safety measures.
Research institutions and industrial facilities working with fluoroantimonic acid must establish comprehensive safety management systems. These typically include standard operating procedures (SOPs), regular safety training programs, and emergency response plans. Periodic audits and inspections are conducted to ensure compliance with regulatory requirements and best practices.
As catalytic science advances, regulatory frameworks continue to evolve to address emerging challenges and incorporate new safety technologies. Collaborative efforts between regulatory agencies, research institutions, and industry stakeholders are essential to maintain a balance between scientific progress and safety considerations in the use of superacids like fluoroantimonic acid.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!