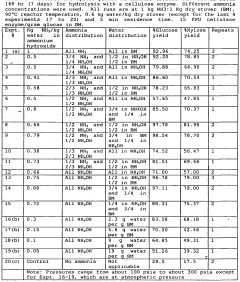
Ammonium hydroxide's role in catalytic biomass conversion
AUG 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Catalytic Biomass Conversion Background and Objectives
Catalytic biomass conversion has emerged as a pivotal technology in the quest for sustainable energy and chemical production. This process involves the transformation of renewable biomass resources into valuable fuels and chemicals through catalytic reactions. The field has witnessed significant advancements over the past few decades, driven by the urgent need to reduce dependence on fossil fuels and mitigate environmental impacts.
The evolution of catalytic biomass conversion can be traced back to the early 20th century, with initial focus on the production of biofuels. However, it was not until the late 1970s and early 1980s that research in this area gained momentum, spurred by the oil crisis and growing environmental concerns. Since then, the field has expanded to encompass a wide range of biomass feedstocks and conversion processes, including thermochemical, biochemical, and hybrid approaches.
In recent years, the role of ammonium hydroxide in catalytic biomass conversion has garnered increasing attention. This compound has shown promise in enhancing the efficiency and selectivity of various conversion processes, particularly in the pretreatment and fractionation of lignocellulosic biomass. Ammonium hydroxide's ability to disrupt the complex structure of biomass and facilitate the separation of its components has opened new avenues for more effective conversion strategies.
The primary objectives of research in this field are multifaceted. Firstly, there is a focus on improving the overall efficiency of biomass conversion processes, aiming to maximize the yield of desired products while minimizing waste and energy consumption. Secondly, researchers are working towards developing more selective catalysts and reaction conditions that can target specific biomass components and produce high-value chemicals with greater precision.
Another key goal is to enhance the economic viability of catalytic biomass conversion technologies. This involves not only optimizing the conversion processes themselves but also developing integrated biorefinery concepts that can utilize multiple biomass feedstocks and produce a diverse range of products. The role of ammonium hydroxide in these systems is being explored as a potential means to achieve these economic objectives.
Furthermore, there is a growing emphasis on understanding the fundamental mechanisms underlying the interactions between ammonium hydroxide, biomass components, and catalysts. This knowledge is crucial for designing more effective and tailored conversion processes. Researchers are also investigating the potential environmental impacts and sustainability aspects of using ammonium hydroxide in large-scale biomass conversion operations.
The evolution of catalytic biomass conversion can be traced back to the early 20th century, with initial focus on the production of biofuels. However, it was not until the late 1970s and early 1980s that research in this area gained momentum, spurred by the oil crisis and growing environmental concerns. Since then, the field has expanded to encompass a wide range of biomass feedstocks and conversion processes, including thermochemical, biochemical, and hybrid approaches.
In recent years, the role of ammonium hydroxide in catalytic biomass conversion has garnered increasing attention. This compound has shown promise in enhancing the efficiency and selectivity of various conversion processes, particularly in the pretreatment and fractionation of lignocellulosic biomass. Ammonium hydroxide's ability to disrupt the complex structure of biomass and facilitate the separation of its components has opened new avenues for more effective conversion strategies.
The primary objectives of research in this field are multifaceted. Firstly, there is a focus on improving the overall efficiency of biomass conversion processes, aiming to maximize the yield of desired products while minimizing waste and energy consumption. Secondly, researchers are working towards developing more selective catalysts and reaction conditions that can target specific biomass components and produce high-value chemicals with greater precision.
Another key goal is to enhance the economic viability of catalytic biomass conversion technologies. This involves not only optimizing the conversion processes themselves but also developing integrated biorefinery concepts that can utilize multiple biomass feedstocks and produce a diverse range of products. The role of ammonium hydroxide in these systems is being explored as a potential means to achieve these economic objectives.
Furthermore, there is a growing emphasis on understanding the fundamental mechanisms underlying the interactions between ammonium hydroxide, biomass components, and catalysts. This knowledge is crucial for designing more effective and tailored conversion processes. Researchers are also investigating the potential environmental impacts and sustainability aspects of using ammonium hydroxide in large-scale biomass conversion operations.
Market Analysis for Biomass-Derived Products
The market for biomass-derived products has been experiencing significant growth in recent years, driven by increasing environmental concerns and the push for sustainable alternatives to fossil-based materials. The global biomass conversion market is expected to expand substantially, with a compound annual growth rate (CAGR) projected to be in the double digits through 2030. This growth is primarily fueled by the rising demand for renewable energy sources and bio-based chemicals.
In the context of ammonium hydroxide's role in catalytic biomass conversion, the market analysis reveals several key trends. Firstly, there is a growing interest in efficient and cost-effective catalytic processes for biomass conversion, where ammonium hydroxide plays a crucial role. This compound has shown promise in enhancing the yield and selectivity of various biomass conversion reactions, particularly in the production of platform chemicals and biofuels.
The market for biomass-derived products is diverse, encompassing sectors such as biofuels, biochemicals, and biomaterials. Among these, the biofuels segment currently dominates the market share, with bioethanol and biodiesel being the primary products. However, the biochemicals segment is expected to witness the highest growth rate in the coming years, driven by the increasing adoption of bio-based plastics and other specialty chemicals.
Geographically, North America and Europe are the leading markets for biomass-derived products, owing to favorable government policies and a strong focus on reducing carbon emissions. However, Asia-Pacific is emerging as a rapidly growing market, with countries like China and India investing heavily in biomass conversion technologies.
The market analysis also highlights the challenges faced by the biomass-derived products industry. These include the high cost of production compared to conventional fossil-based alternatives, technological limitations in large-scale biomass processing, and the need for more efficient catalytic systems. Ammonium hydroxide's potential in addressing some of these challenges, particularly in improving conversion efficiencies and reducing production costs, is garnering increased attention from industry stakeholders.
Consumer awareness and demand for sustainable products are also driving market growth. There is a noticeable shift in consumer preferences towards eco-friendly and bio-based products across various industries, including packaging, textiles, and automotive. This trend is expected to further boost the demand for biomass-derived products in the coming years.
In conclusion, the market for biomass-derived products shows promising growth potential, with ammonium hydroxide's role in catalytic biomass conversion becoming increasingly important. As research and development efforts continue to improve conversion technologies and reduce production costs, the market is poised for significant expansion across various sectors and geographical regions.
In the context of ammonium hydroxide's role in catalytic biomass conversion, the market analysis reveals several key trends. Firstly, there is a growing interest in efficient and cost-effective catalytic processes for biomass conversion, where ammonium hydroxide plays a crucial role. This compound has shown promise in enhancing the yield and selectivity of various biomass conversion reactions, particularly in the production of platform chemicals and biofuels.
The market for biomass-derived products is diverse, encompassing sectors such as biofuels, biochemicals, and biomaterials. Among these, the biofuels segment currently dominates the market share, with bioethanol and biodiesel being the primary products. However, the biochemicals segment is expected to witness the highest growth rate in the coming years, driven by the increasing adoption of bio-based plastics and other specialty chemicals.
Geographically, North America and Europe are the leading markets for biomass-derived products, owing to favorable government policies and a strong focus on reducing carbon emissions. However, Asia-Pacific is emerging as a rapidly growing market, with countries like China and India investing heavily in biomass conversion technologies.
The market analysis also highlights the challenges faced by the biomass-derived products industry. These include the high cost of production compared to conventional fossil-based alternatives, technological limitations in large-scale biomass processing, and the need for more efficient catalytic systems. Ammonium hydroxide's potential in addressing some of these challenges, particularly in improving conversion efficiencies and reducing production costs, is garnering increased attention from industry stakeholders.
Consumer awareness and demand for sustainable products are also driving market growth. There is a noticeable shift in consumer preferences towards eco-friendly and bio-based products across various industries, including packaging, textiles, and automotive. This trend is expected to further boost the demand for biomass-derived products in the coming years.
In conclusion, the market for biomass-derived products shows promising growth potential, with ammonium hydroxide's role in catalytic biomass conversion becoming increasingly important. As research and development efforts continue to improve conversion technologies and reduce production costs, the market is poised for significant expansion across various sectors and geographical regions.
Ammonium Hydroxide in Biomass Conversion: Current Status
Ammonium hydroxide has emerged as a significant player in catalytic biomass conversion, offering a promising avenue for sustainable energy and chemical production. The current status of this technology reflects a growing interest in utilizing this versatile compound to enhance the efficiency and selectivity of biomass transformation processes.
In recent years, researchers have made substantial progress in understanding the role of ammonium hydroxide in various biomass conversion pathways. One of the key areas of focus has been its application in pretreatment processes, where it has shown remarkable ability to disrupt the complex structure of lignocellulosic biomass. This pretreatment step is crucial for increasing the accessibility of cellulose and hemicellulose to enzymatic hydrolysis, thereby improving the overall conversion efficiency.
The use of ammonium hydroxide in catalytic systems has also gained traction, particularly in the production of platform chemicals from biomass. Its basic nature and unique chemical properties make it an effective catalyst or co-catalyst in numerous reactions. For instance, it has been successfully employed in the conversion of glucose to valuable products such as 5-hydroxymethylfurfural (HMF) and levulinic acid, demonstrating improved yields and selectivity compared to traditional acid-catalyzed processes.
Another significant development in the current landscape is the integration of ammonium hydroxide in hybrid catalytic systems. These systems combine the benefits of homogeneous and heterogeneous catalysis, leveraging the high reactivity of ammonium hydroxide in solution while addressing challenges related to catalyst recovery and reuse. Such hybrid approaches have shown promise in enhancing the economic viability of biomass conversion processes at larger scales.
The environmental implications of using ammonium hydroxide in biomass conversion have also been a subject of recent investigations. Studies have indicated that, when properly managed, ammonium hydroxide-based processes can offer a more environmentally friendly alternative to conventional acid-based methods. This is particularly important given the growing emphasis on developing sustainable and green chemistry approaches in the biorefinery sector.
Despite these advancements, challenges remain in fully harnessing the potential of ammonium hydroxide in catalytic biomass conversion. Current research efforts are focused on optimizing reaction conditions, improving catalyst design, and developing more efficient separation and purification techniques. Additionally, there is ongoing work to better understand the reaction mechanisms and kinetics involved in ammonium hydroxide-mediated biomass transformations, which is crucial for process optimization and scale-up.
In recent years, researchers have made substantial progress in understanding the role of ammonium hydroxide in various biomass conversion pathways. One of the key areas of focus has been its application in pretreatment processes, where it has shown remarkable ability to disrupt the complex structure of lignocellulosic biomass. This pretreatment step is crucial for increasing the accessibility of cellulose and hemicellulose to enzymatic hydrolysis, thereby improving the overall conversion efficiency.
The use of ammonium hydroxide in catalytic systems has also gained traction, particularly in the production of platform chemicals from biomass. Its basic nature and unique chemical properties make it an effective catalyst or co-catalyst in numerous reactions. For instance, it has been successfully employed in the conversion of glucose to valuable products such as 5-hydroxymethylfurfural (HMF) and levulinic acid, demonstrating improved yields and selectivity compared to traditional acid-catalyzed processes.
Another significant development in the current landscape is the integration of ammonium hydroxide in hybrid catalytic systems. These systems combine the benefits of homogeneous and heterogeneous catalysis, leveraging the high reactivity of ammonium hydroxide in solution while addressing challenges related to catalyst recovery and reuse. Such hybrid approaches have shown promise in enhancing the economic viability of biomass conversion processes at larger scales.
The environmental implications of using ammonium hydroxide in biomass conversion have also been a subject of recent investigations. Studies have indicated that, when properly managed, ammonium hydroxide-based processes can offer a more environmentally friendly alternative to conventional acid-based methods. This is particularly important given the growing emphasis on developing sustainable and green chemistry approaches in the biorefinery sector.
Despite these advancements, challenges remain in fully harnessing the potential of ammonium hydroxide in catalytic biomass conversion. Current research efforts are focused on optimizing reaction conditions, improving catalyst design, and developing more efficient separation and purification techniques. Additionally, there is ongoing work to better understand the reaction mechanisms and kinetics involved in ammonium hydroxide-mediated biomass transformations, which is crucial for process optimization and scale-up.
Ammonium Hydroxide-Based Catalytic Solutions
01 Use in chemical processes
Ammonium hydroxide is widely used in various chemical processes as a reactant, catalyst, or pH regulator. It plays a crucial role in the synthesis of organic compounds, production of fertilizers, and treatment of industrial waste. Its alkaline properties make it suitable for neutralizing acidic solutions and controlling pH levels in different applications.- Use of ammonium hydroxide in chemical processes: Ammonium hydroxide is widely used in various chemical processes as a reactant, catalyst, or pH adjuster. It plays a crucial role in the synthesis of organic compounds, production of fertilizers, and treatment of industrial waste. Its alkaline properties make it suitable for neutralizing acidic solutions and controlling pH levels in different applications.
- Application in hair coloring and treatment: Ammonium hydroxide is commonly used in hair dyes and coloring products. It helps to open the hair cuticle, allowing the dye to penetrate the hair shaft more effectively. Additionally, it can be used in hair straightening treatments to alter the hair's structure. The alkaline nature of ammonium hydroxide contributes to its effectiveness in these cosmetic applications.
- Role in cleaning and household products: Ammonium hydroxide is a key ingredient in many cleaning products due to its ability to dissolve grease and grime. It is used in glass cleaners, floor cleaners, and all-purpose household cleaners. The compound's strong basic properties make it effective in removing tough stains and disinfecting surfaces.
- Use in textile processing: In the textile industry, ammonium hydroxide is used for various purposes, including fabric treatment, dyeing, and printing. It can help in the mercerization process of cotton fabrics, improving their luster and dye affinity. Ammonium hydroxide also plays a role in the production of certain synthetic fibers and in the treatment of wool.
- Environmental and agricultural applications: Ammonium hydroxide has applications in environmental remediation and agriculture. It can be used to neutralize acidic soils and adjust the pH of water bodies. In agriculture, it serves as a source of nitrogen for plants when used as a fertilizer. Additionally, it plays a role in the treatment of agricultural waste and the production of certain pesticides.
02 Application in cleaning and surface treatment
Ammonium hydroxide is utilized in cleaning formulations and surface treatment processes. It is effective in removing grease, oils, and other contaminants from various surfaces. In the semiconductor industry, it is used for etching and cleaning silicon wafers. Additionally, it finds applications in the textile industry for fabric treatment and in the leather industry for dehairing hides.Expand Specific Solutions03 Role in environmental remediation
Ammonium hydroxide is employed in environmental remediation processes, particularly in air pollution control. It is used in flue gas treatment systems to neutralize acidic components and remove sulfur dioxide. In water treatment, it helps in adjusting pH levels and removing heavy metals. Its ability to form complexes with certain pollutants makes it valuable in soil remediation techniques.Expand Specific Solutions04 Use in personal care and cosmetic products
Ammonium hydroxide finds applications in personal care and cosmetic products. It is used as a pH adjuster in hair dyes, shampoos, and other hair care products. In some cosmetic formulations, it acts as a buffering agent or helps in the emulsification process. Its alkaline nature assists in opening hair cuticles, allowing for better penetration of coloring agents in hair dye products.Expand Specific Solutions05 Application in food processing
Ammonium hydroxide has limited use in food processing as a leavening agent and pH control agent. It is used in the production of certain types of caramel coloring and in some baking processes. However, its use in food applications is strictly regulated and limited due to safety concerns. In some countries, it is approved as a food additive in specific applications under controlled conditions.Expand Specific Solutions
Key Players in Catalytic Biomass Conversion Industry
The catalytic biomass conversion using ammonium hydroxide is in an early development stage, with a growing market potential as the renewable energy sector expands. The technology's maturity is still evolving, with various research institutions and companies exploring its applications. Key players like Michigan State University, DuPont, and Shell are actively involved in research and development. The competitive landscape is diverse, including academic institutions (e.g., Dartmouth College, Texas A&M University), established chemical companies (e.g., ExxonMobil, Bayer AG), and specialized biotechnology firms (e.g., Inaeris Technologies). This mix of players indicates a dynamic field with opportunities for innovation and collaboration across different sectors.
Board of Trustees of Michigan State University
Technical Solution: Michigan State University has developed a novel approach for catalytic biomass conversion using ammonium hydroxide. Their method involves a two-step process: first, pretreating lignocellulosic biomass with ammonium hydroxide to enhance its accessibility, followed by catalytic conversion. This pretreatment significantly improves the efficiency of subsequent enzymatic hydrolysis and fermentation processes[1]. The university's researchers have demonstrated that ammonium hydroxide pretreatment can increase glucose yields by up to 90% compared to untreated biomass[2]. Additionally, they have explored the use of ammonia recycling to make the process more economically viable and environmentally friendly[3].
Strengths: High efficiency in biomass conversion, improved accessibility of cellulose, potential for ammonia recycling. Weaknesses: Energy-intensive pretreatment process, potential ammonia emissions if not properly controlled.
Research Triangle Institute
Technical Solution: Research Triangle Institute (RTI) has developed an innovative approach to catalytic biomass conversion utilizing ammonium hydroxide. Their process involves a low-temperature, aqueous-phase catalytic system where ammonium hydroxide serves as both a reactant and a catalyst promoter. RTI's method focuses on the conversion of biomass-derived sugars into valuable platform chemicals, such as 5-hydroxymethylfurfural (HMF) and levulinic acid[1]. The use of ammonium hydroxide in their catalytic system has been shown to enhance selectivity towards desired products while minimizing unwanted side reactions[2]. RTI has also explored the integration of this process with in situ product separation techniques to improve overall efficiency and reduce downstream processing costs[3].
Strengths: High selectivity towards valuable platform chemicals, low-temperature operation, potential for integrated product separation. Weaknesses: Limited to certain types of biomass feedstocks, potential challenges in scaling up the process.
Innovative Ammonium Hydroxide Catalytic Mechanisms
Process for the treatment of lignocellulosic biomass
PatentWO2007130337A1
Innovation
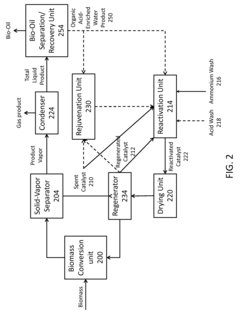
- A process involving the use of concentrated ammonium hydroxide under pressure to treat lignocellulosic biomass, followed by rapid pressure release and ammonia recycling, enhances the digestibility of structural carbohydrates, allowing for high-yield conversion to monosaccharides through enzymatic hydrolysis, while minimizing biomass degradation and chemical usage.
Process of reactivating a metal contaminated biomass conversion catalyst
PatentInactiveUS20150202615A1
Innovation
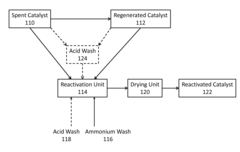
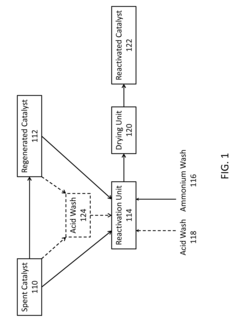
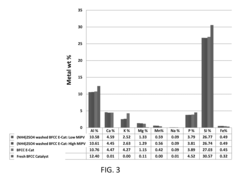
- A process involving an ammonium wash to remove potassium from metal-contaminated spent or regenerated catalysts, restoring acidity and physical properties, which can be conducted under milder conditions and potentially in-situ, using ammonium sulfate, nitrate, hydroxide, or acetate solutions, with optional acid washes to disperse contaminants.
Environmental Impact of Ammonium Hydroxide Use
The use of ammonium hydroxide in catalytic biomass conversion processes has significant environmental implications that warrant careful consideration. While this compound plays a crucial role in enhancing the efficiency of biomass conversion, its environmental impact must be thoroughly assessed and mitigated.
One of the primary environmental concerns associated with ammonium hydroxide use is its potential for air pollution. When released into the atmosphere, ammonia can contribute to the formation of particulate matter, specifically PM2.5, which has adverse effects on air quality and human health. Additionally, ammonia emissions can lead to the formation of secondary aerosols, further exacerbating air pollution issues in surrounding areas.
Water pollution is another critical environmental aspect to consider. Ammonium hydroxide, if not properly managed, can contaminate water sources through runoff or accidental spills. Elevated levels of ammonia in aquatic ecosystems can be toxic to fish and other aquatic organisms, disrupting the ecological balance. Furthermore, the introduction of excess nitrogen into water bodies can contribute to eutrophication, leading to algal blooms and oxygen depletion.
The production and transportation of ammonium hydroxide also carry environmental risks. The manufacturing process typically involves the Haber-Bosch process, which is energy-intensive and relies heavily on fossil fuels, contributing to greenhouse gas emissions. Transportation of this hazardous material poses risks of accidental spills or releases, potentially impacting soil and water quality along distribution routes.
However, it is important to note that the use of ammonium hydroxide in catalytic biomass conversion can also have positive environmental impacts. By improving the efficiency of biomass conversion processes, it can contribute to the reduction of fossil fuel dependence and associated carbon emissions. This aligns with broader sustainability goals and the transition towards renewable energy sources.
To mitigate the environmental risks associated with ammonium hydroxide use, several strategies can be employed. Implementing robust emission control technologies in production facilities can significantly reduce air pollution. Proper handling, storage, and transportation protocols are essential to prevent accidental releases. Additionally, exploring alternatives or developing more environmentally friendly catalysts could help minimize the reliance on ammonium hydroxide in biomass conversion processes.
In conclusion, while ammonium hydroxide plays a vital role in catalytic biomass conversion, its environmental impact must be carefully managed. Balancing the benefits of improved conversion efficiency with the potential environmental risks requires a comprehensive approach that includes stringent safety measures, emission controls, and ongoing research into more sustainable alternatives.
One of the primary environmental concerns associated with ammonium hydroxide use is its potential for air pollution. When released into the atmosphere, ammonia can contribute to the formation of particulate matter, specifically PM2.5, which has adverse effects on air quality and human health. Additionally, ammonia emissions can lead to the formation of secondary aerosols, further exacerbating air pollution issues in surrounding areas.
Water pollution is another critical environmental aspect to consider. Ammonium hydroxide, if not properly managed, can contaminate water sources through runoff or accidental spills. Elevated levels of ammonia in aquatic ecosystems can be toxic to fish and other aquatic organisms, disrupting the ecological balance. Furthermore, the introduction of excess nitrogen into water bodies can contribute to eutrophication, leading to algal blooms and oxygen depletion.
The production and transportation of ammonium hydroxide also carry environmental risks. The manufacturing process typically involves the Haber-Bosch process, which is energy-intensive and relies heavily on fossil fuels, contributing to greenhouse gas emissions. Transportation of this hazardous material poses risks of accidental spills or releases, potentially impacting soil and water quality along distribution routes.
However, it is important to note that the use of ammonium hydroxide in catalytic biomass conversion can also have positive environmental impacts. By improving the efficiency of biomass conversion processes, it can contribute to the reduction of fossil fuel dependence and associated carbon emissions. This aligns with broader sustainability goals and the transition towards renewable energy sources.
To mitigate the environmental risks associated with ammonium hydroxide use, several strategies can be employed. Implementing robust emission control technologies in production facilities can significantly reduce air pollution. Proper handling, storage, and transportation protocols are essential to prevent accidental releases. Additionally, exploring alternatives or developing more environmentally friendly catalysts could help minimize the reliance on ammonium hydroxide in biomass conversion processes.
In conclusion, while ammonium hydroxide plays a vital role in catalytic biomass conversion, its environmental impact must be carefully managed. Balancing the benefits of improved conversion efficiency with the potential environmental risks requires a comprehensive approach that includes stringent safety measures, emission controls, and ongoing research into more sustainable alternatives.
Techno-Economic Assessment of Catalytic Processes
The techno-economic assessment of catalytic processes for ammonium hydroxide's role in biomass conversion is crucial for evaluating the feasibility and potential of this technology. This assessment involves analyzing the economic viability, technical performance, and environmental impact of using ammonium hydroxide as a catalyst in biomass conversion processes.
From an economic perspective, the use of ammonium hydroxide as a catalyst offers several advantages. It is a relatively inexpensive and readily available compound, which can potentially reduce the overall production costs compared to more expensive metal-based catalysts. The assessment should consider the capital expenditure (CAPEX) for equipment and infrastructure, as well as the operational expenditure (OPEX) associated with the use of ammonium hydroxide, including procurement, storage, and handling costs.
The technical performance of ammonium hydroxide in catalytic biomass conversion processes is another critical aspect of the assessment. Ammonium hydroxide has shown promising results in enhancing the efficiency of biomass conversion, particularly in the pretreatment and hydrolysis stages. The assessment should evaluate the conversion rates, product yields, and selectivity achieved with ammonium hydroxide catalysis compared to other catalytic systems. Additionally, the scalability of the process and its compatibility with existing biomass conversion technologies should be considered.
Environmental considerations play a significant role in the techno-economic assessment. Ammonium hydroxide is generally considered more environmentally friendly compared to some metal-based catalysts, as it does not introduce heavy metals into the process. However, the assessment should include a comprehensive life cycle analysis to evaluate the overall environmental impact, including energy consumption, greenhouse gas emissions, and potential for catalyst recovery and recycling.
The economic viability of the process should be evaluated through a detailed cost-benefit analysis. This analysis should consider factors such as feedstock costs, utility expenses, labor requirements, and potential revenue streams from the produced biofuels or biochemicals. Sensitivity analyses can be conducted to assess the impact of varying market conditions, such as fluctuations in biomass prices or changes in product demand, on the overall economic performance.
Furthermore, the assessment should explore the potential for process integration and optimization. This may include investigating opportunities for heat integration, waste minimization, and co-product valorization to enhance the overall efficiency and economic viability of the catalytic biomass conversion process using ammonium hydroxide.
Lastly, the techno-economic assessment should consider the regulatory landscape and potential policy incentives that may impact the adoption of ammonium hydroxide-based catalytic processes for biomass conversion. This includes evaluating compliance with environmental regulations, safety standards, and potential government support for renewable energy technologies.
From an economic perspective, the use of ammonium hydroxide as a catalyst offers several advantages. It is a relatively inexpensive and readily available compound, which can potentially reduce the overall production costs compared to more expensive metal-based catalysts. The assessment should consider the capital expenditure (CAPEX) for equipment and infrastructure, as well as the operational expenditure (OPEX) associated with the use of ammonium hydroxide, including procurement, storage, and handling costs.
The technical performance of ammonium hydroxide in catalytic biomass conversion processes is another critical aspect of the assessment. Ammonium hydroxide has shown promising results in enhancing the efficiency of biomass conversion, particularly in the pretreatment and hydrolysis stages. The assessment should evaluate the conversion rates, product yields, and selectivity achieved with ammonium hydroxide catalysis compared to other catalytic systems. Additionally, the scalability of the process and its compatibility with existing biomass conversion technologies should be considered.
Environmental considerations play a significant role in the techno-economic assessment. Ammonium hydroxide is generally considered more environmentally friendly compared to some metal-based catalysts, as it does not introduce heavy metals into the process. However, the assessment should include a comprehensive life cycle analysis to evaluate the overall environmental impact, including energy consumption, greenhouse gas emissions, and potential for catalyst recovery and recycling.
The economic viability of the process should be evaluated through a detailed cost-benefit analysis. This analysis should consider factors such as feedstock costs, utility expenses, labor requirements, and potential revenue streams from the produced biofuels or biochemicals. Sensitivity analyses can be conducted to assess the impact of varying market conditions, such as fluctuations in biomass prices or changes in product demand, on the overall economic performance.
Furthermore, the assessment should explore the potential for process integration and optimization. This may include investigating opportunities for heat integration, waste minimization, and co-product valorization to enhance the overall efficiency and economic viability of the catalytic biomass conversion process using ammonium hydroxide.
Lastly, the techno-economic assessment should consider the regulatory landscape and potential policy incentives that may impact the adoption of ammonium hydroxide-based catalytic processes for biomass conversion. This includes evaluating compliance with environmental regulations, safety standards, and potential government support for renewable energy technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!