Analysis of Hydrogen storage materials for electrode kinetics and adsorption performance
SEP 24, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogen Storage Materials Evolution and Research Objectives
Hydrogen storage materials have evolved significantly over the past several decades, transitioning from conventional metal hydrides to advanced nanomaterials with enhanced properties. The journey began in the 1970s with the discovery of intermetallic compounds like LaNi5, which demonstrated reversible hydrogen storage capabilities. These early materials, while groundbreaking, suffered from limited gravimetric capacity and slow kinetics, restricting their practical applications.
The 1990s marked a pivotal shift with the exploration of lightweight metal hydrides, particularly those based on magnesium and aluminum. These materials offered improved storage capacities but continued to face challenges related to thermodynamic stability and reaction kinetics. The turn of the millennium witnessed the emergence of complex hydrides and chemical hydrogen storage materials, which promised higher theoretical capacities but introduced new challenges in reversibility and system integration.
Recent advancements have focused on nanostructured materials, including metal-organic frameworks (MOFs), covalent organic frameworks (COFs), and carbon-based materials. These structures leverage increased surface area and tunable pore sizes to enhance hydrogen adsorption performance. Particularly noteworthy is the development of hybrid materials that combine physisorption and chemisorption mechanisms to optimize both storage capacity and release kinetics.
The electrode kinetics of hydrogen storage materials has become increasingly important as these materials find applications in electrochemical systems. Research has revealed that surface modifications, catalyst incorporation, and defect engineering can significantly improve charge transfer processes and reaction rates at electrode interfaces. Understanding the relationship between material structure and electrode performance has emerged as a critical factor in developing next-generation energy storage solutions.
Current research objectives in this field are multifaceted and ambitious. Primary goals include achieving the U.S. Department of Energy's targets for onboard hydrogen storage systems: 6.5 wt% gravimetric capacity and 50 g/L volumetric capacity under moderate temperature and pressure conditions. Additionally, researchers aim to develop materials with enhanced cycling stability, improved thermal management characteristics, and reduced production costs to enable commercial viability.
Another crucial objective is understanding the fundamental mechanisms governing hydrogen adsorption and desorption processes at the molecular level. This includes investigating the role of surface chemistry, pore architecture, and electronic structure in determining storage performance. Advanced characterization techniques, including in-situ spectroscopy and neutron scattering, are being employed to provide real-time insights into these processes.
The integration of computational methods with experimental approaches represents another significant research direction. Density functional theory calculations, molecular dynamics simulations, and machine learning algorithms are increasingly being utilized to predict material properties and guide experimental design, accelerating the discovery of novel hydrogen storage materials with optimized electrode kinetics and adsorption performance.
The 1990s marked a pivotal shift with the exploration of lightweight metal hydrides, particularly those based on magnesium and aluminum. These materials offered improved storage capacities but continued to face challenges related to thermodynamic stability and reaction kinetics. The turn of the millennium witnessed the emergence of complex hydrides and chemical hydrogen storage materials, which promised higher theoretical capacities but introduced new challenges in reversibility and system integration.
Recent advancements have focused on nanostructured materials, including metal-organic frameworks (MOFs), covalent organic frameworks (COFs), and carbon-based materials. These structures leverage increased surface area and tunable pore sizes to enhance hydrogen adsorption performance. Particularly noteworthy is the development of hybrid materials that combine physisorption and chemisorption mechanisms to optimize both storage capacity and release kinetics.
The electrode kinetics of hydrogen storage materials has become increasingly important as these materials find applications in electrochemical systems. Research has revealed that surface modifications, catalyst incorporation, and defect engineering can significantly improve charge transfer processes and reaction rates at electrode interfaces. Understanding the relationship between material structure and electrode performance has emerged as a critical factor in developing next-generation energy storage solutions.
Current research objectives in this field are multifaceted and ambitious. Primary goals include achieving the U.S. Department of Energy's targets for onboard hydrogen storage systems: 6.5 wt% gravimetric capacity and 50 g/L volumetric capacity under moderate temperature and pressure conditions. Additionally, researchers aim to develop materials with enhanced cycling stability, improved thermal management characteristics, and reduced production costs to enable commercial viability.
Another crucial objective is understanding the fundamental mechanisms governing hydrogen adsorption and desorption processes at the molecular level. This includes investigating the role of surface chemistry, pore architecture, and electronic structure in determining storage performance. Advanced characterization techniques, including in-situ spectroscopy and neutron scattering, are being employed to provide real-time insights into these processes.
The integration of computational methods with experimental approaches represents another significant research direction. Density functional theory calculations, molecular dynamics simulations, and machine learning algorithms are increasingly being utilized to predict material properties and guide experimental design, accelerating the discovery of novel hydrogen storage materials with optimized electrode kinetics and adsorption performance.
Market Analysis for Hydrogen Storage Technologies
The global hydrogen storage materials market is experiencing significant growth, driven primarily by the increasing adoption of hydrogen as a clean energy carrier. As of 2023, the market is valued at approximately 5.2 billion USD, with projections indicating a compound annual growth rate (CAGR) of 8.3% through 2030. This growth trajectory is largely attributed to the expanding hydrogen economy and the critical role storage materials play in enabling efficient hydrogen utilization across various sectors.
The demand for advanced hydrogen storage materials is particularly pronounced in the automotive industry, where hydrogen fuel cell vehicles require materials with superior electrode kinetics and adsorption performance. Currently, this segment accounts for roughly 35% of the total market share, with expectations of further expansion as major automotive manufacturers increase their investments in hydrogen-powered transportation solutions.
Industrial applications represent another substantial market segment, comprising approximately 28% of the current demand. Here, materials that offer enhanced adsorption capacity and improved kinetics for rapid hydrogen release are highly sought after for applications ranging from power generation to chemical processing.
Regionally, Asia-Pacific dominates the market with approximately 42% share, led by significant investments in hydrogen infrastructure in Japan, South Korea, and increasingly China. Europe follows with 31% market share, driven by ambitious decarbonization policies and substantial funding for hydrogen technologies. North America accounts for 22% of the market, with growing interest in hydrogen as part of broader clean energy strategies.
From an investment perspective, venture capital funding for startups focused on novel hydrogen storage materials has seen a remarkable 65% increase over the past three years. This influx of capital is primarily directed toward materials that demonstrate superior electrode kinetics and adsorption characteristics, reflecting market recognition of these properties as critical performance differentiators.
Customer requirements are increasingly focused on materials that can achieve higher gravimetric and volumetric storage capacities while maintaining favorable kinetics. End-users across sectors are demanding storage solutions that can operate effectively under moderate temperature and pressure conditions, with particular emphasis on materials that enable rapid hydrogen uptake and release with minimal energy penalties.
The competitive landscape features both established materials science companies and innovative startups, with significant market consolidation observed through strategic partnerships and acquisitions aimed at accelerating commercialization of advanced storage materials with optimized electrode kinetics and adsorption properties.
The demand for advanced hydrogen storage materials is particularly pronounced in the automotive industry, where hydrogen fuel cell vehicles require materials with superior electrode kinetics and adsorption performance. Currently, this segment accounts for roughly 35% of the total market share, with expectations of further expansion as major automotive manufacturers increase their investments in hydrogen-powered transportation solutions.
Industrial applications represent another substantial market segment, comprising approximately 28% of the current demand. Here, materials that offer enhanced adsorption capacity and improved kinetics for rapid hydrogen release are highly sought after for applications ranging from power generation to chemical processing.
Regionally, Asia-Pacific dominates the market with approximately 42% share, led by significant investments in hydrogen infrastructure in Japan, South Korea, and increasingly China. Europe follows with 31% market share, driven by ambitious decarbonization policies and substantial funding for hydrogen technologies. North America accounts for 22% of the market, with growing interest in hydrogen as part of broader clean energy strategies.
From an investment perspective, venture capital funding for startups focused on novel hydrogen storage materials has seen a remarkable 65% increase over the past three years. This influx of capital is primarily directed toward materials that demonstrate superior electrode kinetics and adsorption characteristics, reflecting market recognition of these properties as critical performance differentiators.
Customer requirements are increasingly focused on materials that can achieve higher gravimetric and volumetric storage capacities while maintaining favorable kinetics. End-users across sectors are demanding storage solutions that can operate effectively under moderate temperature and pressure conditions, with particular emphasis on materials that enable rapid hydrogen uptake and release with minimal energy penalties.
The competitive landscape features both established materials science companies and innovative startups, with significant market consolidation observed through strategic partnerships and acquisitions aimed at accelerating commercialization of advanced storage materials with optimized electrode kinetics and adsorption properties.
Current Challenges in Electrode Kinetics and Adsorption
Despite significant advancements in hydrogen storage technologies, electrode kinetics and adsorption performance remain critical bottlenecks in the widespread implementation of hydrogen-based energy systems. Current hydrogen storage materials face substantial challenges in achieving optimal electrode kinetics, particularly in terms of hydrogen dissociation and recombination rates at material interfaces. The sluggish kinetics observed in many promising materials significantly impairs their practical utility in real-world applications.
Metal hydrides, while offering high volumetric storage capacity, frequently exhibit poor reaction kinetics at ambient temperatures, requiring elevated temperatures (often exceeding 300°C) to achieve reasonable hydrogen absorption/desorption rates. This thermal requirement creates substantial energy penalties and system complexity that undermine overall efficiency. Additionally, surface passivation and oxidation of metal hydride particles further deteriorate kinetic performance over multiple cycling processes.
Complex hydrides present another category of materials with promising theoretical capacity but severe kinetic limitations. The multi-step reaction pathways in these materials create significant energy barriers that hinder rapid hydrogen exchange. Current research indicates that even with advanced catalysts, the reaction rates remain insufficient for applications requiring rapid response, such as transportation or load-following power generation.
Regarding adsorption performance, physisorption-based materials like metal-organic frameworks (MOFs) and carbon nanostructures demonstrate inadequate binding energies at ambient conditions. The typical adsorption enthalpies (4-10 kJ/mol) are too low to enable significant hydrogen uptake at room temperature and moderate pressures. Conversely, chemisorption materials often bind hydrogen too strongly, making release energetically costly and kinetically challenging.
Surface heterogeneity presents another significant challenge, as real-world materials rarely exhibit the uniform adsorption sites assumed in theoretical models. This heterogeneity creates variable binding energies across the material surface, resulting in unpredictable adsorption/desorption profiles and hysteresis effects that complicate system engineering and control strategies.
The trade-off between kinetics and capacity remains unresolved, with materials demonstrating fast kinetics typically showing limited storage capacity and vice versa. This fundamental challenge is exacerbated by the degradation of kinetic performance over multiple cycles, particularly in complex hydrides and nanostructured materials where structural reorganization and particle agglomeration progressively reduce active surface area.
Computational modeling efforts have struggled to accurately predict kinetic behavior in complex storage systems, with significant discrepancies between theoretical predictions and experimental observations. This modeling gap hampers rational material design and optimization, forcing researchers to rely heavily on empirical approaches that are both time-consuming and resource-intensive.
Metal hydrides, while offering high volumetric storage capacity, frequently exhibit poor reaction kinetics at ambient temperatures, requiring elevated temperatures (often exceeding 300°C) to achieve reasonable hydrogen absorption/desorption rates. This thermal requirement creates substantial energy penalties and system complexity that undermine overall efficiency. Additionally, surface passivation and oxidation of metal hydride particles further deteriorate kinetic performance over multiple cycling processes.
Complex hydrides present another category of materials with promising theoretical capacity but severe kinetic limitations. The multi-step reaction pathways in these materials create significant energy barriers that hinder rapid hydrogen exchange. Current research indicates that even with advanced catalysts, the reaction rates remain insufficient for applications requiring rapid response, such as transportation or load-following power generation.
Regarding adsorption performance, physisorption-based materials like metal-organic frameworks (MOFs) and carbon nanostructures demonstrate inadequate binding energies at ambient conditions. The typical adsorption enthalpies (4-10 kJ/mol) are too low to enable significant hydrogen uptake at room temperature and moderate pressures. Conversely, chemisorption materials often bind hydrogen too strongly, making release energetically costly and kinetically challenging.
Surface heterogeneity presents another significant challenge, as real-world materials rarely exhibit the uniform adsorption sites assumed in theoretical models. This heterogeneity creates variable binding energies across the material surface, resulting in unpredictable adsorption/desorption profiles and hysteresis effects that complicate system engineering and control strategies.
The trade-off between kinetics and capacity remains unresolved, with materials demonstrating fast kinetics typically showing limited storage capacity and vice versa. This fundamental challenge is exacerbated by the degradation of kinetic performance over multiple cycles, particularly in complex hydrides and nanostructured materials where structural reorganization and particle agglomeration progressively reduce active surface area.
Computational modeling efforts have struggled to accurately predict kinetic behavior in complex storage systems, with significant discrepancies between theoretical predictions and experimental observations. This modeling gap hampers rational material design and optimization, forcing researchers to rely heavily on empirical approaches that are both time-consuming and resource-intensive.
State-of-the-Art Solutions for Enhanced Kinetics and Adsorption
01 Metal hydride materials for hydrogen storage
Metal hydrides are promising materials for hydrogen storage due to their high volumetric capacity and safety. These materials store hydrogen through chemical bonding, forming metal-hydrogen compounds. The electrode kinetics of metal hydrides are crucial for their performance in hydrogen storage applications, affecting the rate of hydrogen absorption and desorption. Various metal hydride compositions have been developed to optimize hydrogen storage capacity, adsorption/desorption kinetics, and cycling stability.- Metal hydride materials for hydrogen storage: Metal hydrides are promising materials for hydrogen storage due to their high volumetric capacity and safety. These materials form chemical bonds with hydrogen, allowing for reversible storage through absorption and desorption processes. The electrode kinetics of metal hydrides can be enhanced through alloying, nanostructuring, and catalyst addition, which improves hydrogen uptake and release rates. Various metal hydride compositions have been developed to optimize storage capacity, operating conditions, and cycling stability.
- Carbon-based materials for hydrogen adsorption: Carbon-based materials such as activated carbon, carbon nanotubes, and graphene demonstrate significant potential for hydrogen storage through physical adsorption mechanisms. These materials offer advantages including lightweight properties, tunable porosity, and large specific surface areas that enhance hydrogen adsorption performance. Surface functionalization and doping with heteroatoms can further improve the adsorption capacity and binding energy with hydrogen molecules, making them suitable for both storage applications and electrode materials in energy systems.
- Metal-organic frameworks for hydrogen storage: Metal-organic frameworks (MOFs) represent an advanced class of porous materials with exceptional hydrogen storage capabilities. Their crystalline structure consists of metal nodes connected by organic linkers, creating highly ordered frameworks with tunable pore sizes and exceptionally high surface areas. The adsorption performance of MOFs can be optimized through metal center selection, linker design, and post-synthetic modifications. Their well-defined structure allows for precise control over adsorption sites, binding energies, and diffusion pathways, making them promising candidates for next-generation hydrogen storage applications.
- Electrode materials with enhanced kinetics for hydrogen evolution: Advanced electrode materials have been developed specifically to enhance the kinetics of hydrogen evolution and adsorption processes. These materials incorporate catalytic components that lower activation barriers for hydrogen reactions at the electrode surface. Nanostructured designs increase active surface area and create abundant reaction sites, while composite structures combine the benefits of different materials to achieve synergistic effects. The electrode kinetics can be further improved through interface engineering and electronic structure modification, resulting in more efficient hydrogen production and storage systems.
- Composite and hybrid hydrogen storage systems: Composite and hybrid hydrogen storage systems combine multiple storage mechanisms or materials to overcome limitations of individual approaches. These systems often integrate physical adsorption materials with chemical storage components to achieve improved performance across various operating conditions. Advanced composites may incorporate catalysts, heat management materials, and structural supports to enhance kinetics, thermal behavior, and mechanical stability. The synergistic effects in these hybrid systems can lead to enhanced hydrogen capacity, improved adsorption-desorption kinetics, and better cycling performance compared to single-material approaches.
02 Carbon-based materials for hydrogen storage
Carbon-based materials, including carbon nanotubes, graphene, and activated carbon, have been investigated for hydrogen storage applications. These materials store hydrogen primarily through physical adsorption mechanisms. The adsorption performance depends on the surface area, pore structure, and surface functionalization. Research focuses on enhancing the hydrogen adsorption capacity and kinetics by modifying the structure and composition of carbon-based materials, as well as operating under optimized temperature and pressure conditions.Expand Specific Solutions03 Metal-organic frameworks for hydrogen storage
Metal-organic frameworks (MOFs) are crystalline porous materials composed of metal ions coordinated to organic ligands. MOFs have emerged as promising hydrogen storage materials due to their exceptionally high surface areas, tunable pore sizes, and versatile chemistry. The hydrogen adsorption performance of MOFs can be enhanced by incorporating open metal sites, optimizing pore geometry, and introducing functional groups. The electrode kinetics in MOF-based systems are influenced by the framework structure and the presence of catalytic sites.Expand Specific Solutions04 Composite hydrogen storage materials
Composite hydrogen storage materials combine different types of materials to achieve enhanced performance. These composites often integrate metal hydrides with carbon materials, polymers, or catalysts to improve hydrogen adsorption capacity, kinetics, and cycling stability. The synergistic effects between components can lead to improved electrode kinetics and adsorption performance. Design strategies include core-shell structures, nanoconfinement, and catalyst doping to optimize hydrogen storage properties.Expand Specific Solutions05 Electrode design and kinetics enhancement for hydrogen storage systems
The design of electrodes plays a crucial role in the performance of hydrogen storage systems. Factors such as electrode architecture, particle size, catalyst distribution, and binding materials significantly affect the electrode kinetics and hydrogen adsorption/desorption rates. Various approaches have been developed to enhance kinetics, including nanostructuring, catalyst incorporation, surface modification, and optimization of operating conditions. Advanced characterization techniques are employed to understand the kinetic mechanisms and identify rate-limiting steps in hydrogen storage processes.Expand Specific Solutions
Leading Research Institutions and Industrial Players
The hydrogen storage materials market for electrode kinetics and adsorption performance is in a growth phase, driven by increasing demand for clean energy solutions. The market is expanding rapidly with an estimated value of several billion dollars by 2030. Technologically, the field shows varying maturity levels across applications. Leading players include BASF SE and Toyota Motor Corp., who have established strong patent portfolios, while specialized companies like Santoku Corp. and GS Yuasa focus on hydrogen-absorbing alloys and battery technologies. Research institutions such as Advanced Industrial Science & Technology and Case Western Reserve University contribute significant innovations. Japanese companies (SANYO, Toshiba, Panasonic) demonstrate advanced technological capabilities, particularly in battery-related hydrogen storage applications, positioning them as key market influencers.
Advanced Industrial Science & Technology
Technical Solution: AIST has pioneered innovative hydrogen storage materials based on carbon nanostructures and metal-doped frameworks. Their research focuses on enhancing electrode kinetics through precise control of material morphology and surface chemistry. AIST's carbon-based materials feature hierarchical pore structures with tailored micropores (<2 nm) for hydrogen adsorption and mesopores (2-50 nm) for rapid diffusion pathways. These materials demonstrate hydrogen uptake of 4-6 wt% under moderate conditions. AIST has developed novel synthesis methods for producing nanostructured metal hydrides with dramatically improved kinetics, reducing activation energies for hydrogen absorption/desorption by 30-40% compared to conventional materials. Their electrode materials incorporate specialized catalysts at strategic sites to facilitate hydrogen dissociation and recombination, addressing the rate-limiting steps in hydrogen storage processes. AIST's materials also feature enhanced thermal conductivity to manage heat evolution during hydrogen cycling.
Strengths: Excellent balance between storage capacity and kinetics; superior thermal management properties; innovative hierarchical structures that optimize both adsorption and transport. Weaknesses: Complex synthesis procedures limit large-scale production; performance degradation under repeated cycling; sensitivity to environmental contaminants.
BASF SE
Technical Solution: BASF has developed a comprehensive portfolio of hydrogen storage materials focused on metal-organic frameworks (MOFs) with exceptional surface areas exceeding 3000 m²/g. Their proprietary MOF structures feature precisely engineered pore sizes (0.6-1.2 nm) and functionalized organic linkers that enhance hydrogen binding energies to 15-20 kJ/mol, significantly above traditional physisorption materials. BASF's materials demonstrate hydrogen uptake of 5-7 wt% at moderate pressures (50-100 bar) and cryogenic temperatures, with ongoing research to improve room temperature performance. Their electrode materials incorporate conductive additives and specialized binders to maintain electrical connectivity throughout charge-discharge cycles. BASF has also pioneered composite systems combining MOFs with metal hydrides to create synergistic materials that benefit from both physisorption and chemisorption mechanisms, addressing the traditional kinetic limitations of pure metal hydrides.
Strengths: Exceptional surface area and porosity for maximum hydrogen adsorption; tunable pore architecture for optimized binding energies; scalable manufacturing processes. Weaknesses: Performance still limited at ambient temperatures; requires higher pressures for practical storage densities; more complex synthesis compared to conventional materials.
Critical Patents and Breakthroughs in Hydrogen Storage
Hydrogen storage materials having excellent kinetics, capacity, and cycle stability
PatentInactiveUS7344676B2
Innovation
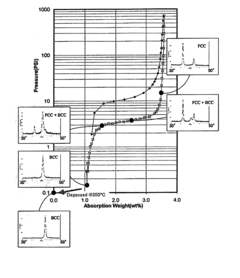
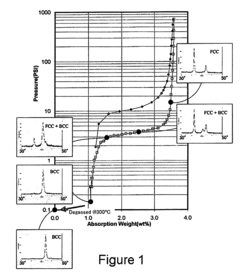
- A hydrogen storage alloy with a single-phase body-centered cubic (BCC) structure, composed of titanium, vanadium, and chromium, along with modifier elements, that absorbs and desorbs hydrogen rapidly and reversibly, maintaining high capacity and stability across multiple cycles, with a lattice constant range of 3.015 to 3.045 angstroms, and a quenching process to inhibit oxide formation.
Hydrogen storage material, electrode and nickel hydride storage battery
PatentActiveJP2016069692A
Innovation
- A hydrogen storage alloy with a specific composition (RE 1-a-b SM a Mg b )(Ni 1-c-d Al c M d ) x, where 0.1 ≤ a ≤ 0.25, 0.1 < b < 0.2, 0.02 < cx < 0.2, 0. ≤ dx ≤ 0.1, 3.6 ≤ x ≤ 3.7, and containing a high proportion of Pr 5 Co 19 and Ce 5 Co 19 phases, enhances corrosion resistance and durability.
Safety and Durability Considerations
Safety and durability considerations are paramount in the development and implementation of hydrogen storage materials for electrode applications. The inherent reactivity of hydrogen presents significant safety challenges that must be addressed through comprehensive risk assessment and mitigation strategies. Materials used for hydrogen storage must be designed to prevent leakage, which could lead to explosive conditions when hydrogen concentrations reach 4-75% in air. This necessitates rigorous testing protocols and safety standards specific to hydrogen storage systems.
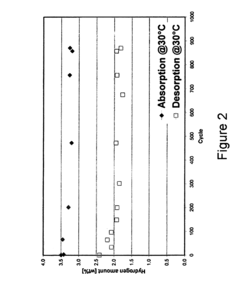
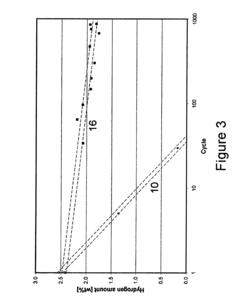
Material degradation represents a critical durability concern for hydrogen storage electrodes. Repeated hydrogen absorption and desorption cycles can lead to structural changes, including lattice expansion and contraction, which may result in material fatigue and eventual failure. For metal hydrides, this cycling can cause pulverization, reducing effective surface area and diminishing storage capacity over time. Advanced materials engineering approaches, such as nanostructuring and composite formation, are being explored to enhance mechanical stability during cycling.
Chemical stability presents another significant challenge, particularly in electrochemical environments. Electrode materials must withstand not only hydrogen-induced effects but also potential corrosion from electrolytes and oxidation at varying potentials. Surface passivation layers often form during operation, which can initially protect the material but may eventually impede hydrogen diffusion and reaction kinetics. Research into protective coatings and surface modifications shows promise for extending operational lifetimes without compromising performance.
Temperature management is essential for both safety and durability. Many hydrogen storage materials exhibit exothermic absorption and endothermic desorption characteristics, creating thermal management challenges. Inadequate heat dissipation during rapid charging can lead to hotspots, accelerated degradation, or even thermal runaway in extreme cases. Conversely, insufficient heat supply during discharge may limit hydrogen release rates. Integrated thermal management systems are being developed to address these issues.
Long-term stability under various environmental conditions remains an ongoing research focus. Humidity, atmospheric contaminants, and temperature fluctuations can all impact material performance and safety over time. Accelerated aging tests are being standardized to better predict real-world durability, while in-situ monitoring technologies are advancing to provide early warning of potential degradation or safety issues. These developments are crucial for establishing confidence in hydrogen storage technologies for widespread commercial adoption.
Material degradation represents a critical durability concern for hydrogen storage electrodes. Repeated hydrogen absorption and desorption cycles can lead to structural changes, including lattice expansion and contraction, which may result in material fatigue and eventual failure. For metal hydrides, this cycling can cause pulverization, reducing effective surface area and diminishing storage capacity over time. Advanced materials engineering approaches, such as nanostructuring and composite formation, are being explored to enhance mechanical stability during cycling.
Chemical stability presents another significant challenge, particularly in electrochemical environments. Electrode materials must withstand not only hydrogen-induced effects but also potential corrosion from electrolytes and oxidation at varying potentials. Surface passivation layers often form during operation, which can initially protect the material but may eventually impede hydrogen diffusion and reaction kinetics. Research into protective coatings and surface modifications shows promise for extending operational lifetimes without compromising performance.
Temperature management is essential for both safety and durability. Many hydrogen storage materials exhibit exothermic absorption and endothermic desorption characteristics, creating thermal management challenges. Inadequate heat dissipation during rapid charging can lead to hotspots, accelerated degradation, or even thermal runaway in extreme cases. Conversely, insufficient heat supply during discharge may limit hydrogen release rates. Integrated thermal management systems are being developed to address these issues.
Long-term stability under various environmental conditions remains an ongoing research focus. Humidity, atmospheric contaminants, and temperature fluctuations can all impact material performance and safety over time. Accelerated aging tests are being standardized to better predict real-world durability, while in-situ monitoring technologies are advancing to provide early warning of potential degradation or safety issues. These developments are crucial for establishing confidence in hydrogen storage technologies for widespread commercial adoption.
Environmental Impact and Sustainability Assessment
The environmental impact of hydrogen storage materials extends beyond their technical performance, encompassing their entire lifecycle from production to disposal. Materials such as metal hydrides, carbon-based structures, and metal-organic frameworks (MOFs) each present distinct environmental considerations. The extraction and processing of rare earth elements for certain metal hydrides can lead to significant land degradation, water pollution, and energy consumption. Conversely, carbon-based materials often require less environmentally damaging production processes, though their synthesis may still involve energy-intensive conditions and potentially harmful chemicals.
The sustainability of hydrogen storage systems is intrinsically linked to their energy efficiency throughout the hydrogen cycle. Materials with superior adsorption performance but requiring high temperatures or pressures for hydrogen release may diminish the overall energy efficiency of the system. This energy balance is critical when evaluating the true environmental benefit of hydrogen as an alternative energy carrier. Materials demonstrating optimal electrode kinetics at ambient conditions offer substantial advantages in this regard, reducing the operational energy footprint.
Lifecycle assessment (LCA) studies indicate that the environmental impact of hydrogen storage materials varies significantly based on production methods and operational parameters. For instance, palladium-based materials exhibit excellent hydrogen absorption properties but present sustainability challenges due to the scarcity and energy-intensive mining of palladium. In contrast, nickel-based alloys may offer a more sustainable alternative despite potentially lower performance metrics.
Water consumption represents another critical environmental consideration, particularly for materials requiring extensive washing or purification steps during synthesis. Some MOFs, while promising for hydrogen storage, demand substantial water resources during their production, raising concerns in water-stressed regions. Additionally, the potential for material degradation over multiple adsorption-desorption cycles necessitates consideration of longevity in environmental assessments.
The recyclability of hydrogen storage materials significantly influences their long-term environmental impact. Materials designed with end-of-life considerations, allowing for component separation and reuse, demonstrate superior sustainability profiles. Research indicates that certain metal hydrides can be effectively recycled, recovering valuable metals while minimizing waste. However, composite materials with intricate structures often present recycling challenges, potentially leading to increased waste generation.
Carbon footprint analysis reveals that the environmental benefits of hydrogen storage systems are maximized when the hydrogen itself is produced through renewable energy sources. The integration of sustainable hydrogen production with environmentally conscious storage materials creates a synergistic effect, substantially reducing the overall environmental impact of hydrogen as an energy vector.
The sustainability of hydrogen storage systems is intrinsically linked to their energy efficiency throughout the hydrogen cycle. Materials with superior adsorption performance but requiring high temperatures or pressures for hydrogen release may diminish the overall energy efficiency of the system. This energy balance is critical when evaluating the true environmental benefit of hydrogen as an alternative energy carrier. Materials demonstrating optimal electrode kinetics at ambient conditions offer substantial advantages in this regard, reducing the operational energy footprint.
Lifecycle assessment (LCA) studies indicate that the environmental impact of hydrogen storage materials varies significantly based on production methods and operational parameters. For instance, palladium-based materials exhibit excellent hydrogen absorption properties but present sustainability challenges due to the scarcity and energy-intensive mining of palladium. In contrast, nickel-based alloys may offer a more sustainable alternative despite potentially lower performance metrics.
Water consumption represents another critical environmental consideration, particularly for materials requiring extensive washing or purification steps during synthesis. Some MOFs, while promising for hydrogen storage, demand substantial water resources during their production, raising concerns in water-stressed regions. Additionally, the potential for material degradation over multiple adsorption-desorption cycles necessitates consideration of longevity in environmental assessments.
The recyclability of hydrogen storage materials significantly influences their long-term environmental impact. Materials designed with end-of-life considerations, allowing for component separation and reuse, demonstrate superior sustainability profiles. Research indicates that certain metal hydrides can be effectively recycled, recovering valuable metals while minimizing waste. However, composite materials with intricate structures often present recycling challenges, potentially leading to increased waste generation.
Carbon footprint analysis reveals that the environmental benefits of hydrogen storage systems are maximized when the hydrogen itself is produced through renewable energy sources. The integration of sustainable hydrogen production with environmentally conscious storage materials creates a synergistic effect, substantially reducing the overall environmental impact of hydrogen as an energy vector.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!