How material composition affects Hydrogen storage materials electrochemical properties
SEP 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogen Storage Materials Background and Objectives
Hydrogen storage materials have emerged as a critical component in the global transition towards sustainable energy systems. The development of these materials dates back to the 1970s when the oil crisis prompted intensive research into alternative energy carriers. Over the decades, research has evolved from basic metal hydrides to complex multi-component systems with enhanced storage capabilities and improved electrochemical properties.
The fundamental relationship between material composition and electrochemical properties represents one of the most significant research areas in hydrogen storage technology. Material composition directly influences key parameters such as hydrogen absorption/desorption kinetics, cycling stability, and storage capacity. Recent advances in nanotechnology and materials science have accelerated progress in this field, enabling precise control over material structures at atomic and molecular levels.
Current technological trends indicate a shift towards multi-functional hydrogen storage materials that not only store hydrogen efficiently but also exhibit favorable electrochemical characteristics for various applications including fuel cells, batteries, and electrolyzers. The integration of catalytic elements, dopants, and structural modifiers has proven effective in enhancing both storage capacity and electrochemical performance.
The global hydrogen economy is projected to reach $2.5 trillion by 2050, with hydrogen storage materials playing a pivotal role in this growth. Material innovation is increasingly focused on addressing the limitations of current technologies, particularly regarding energy density, operational temperature ranges, and long-term stability under practical conditions.
The primary objectives of research in this field include developing materials with hydrogen storage capacities exceeding 6.5 wt% at near-ambient conditions, achieving rapid charging/discharging rates without significant degradation, and ensuring electrochemical stability over thousands of cycles. Additionally, researchers aim to establish clear structure-property relationships that can guide rational material design.
Environmental considerations have also become integral to research objectives, with emphasis on reducing or eliminating rare earth elements and precious metals from storage materials. This approach aligns with sustainable development goals and addresses supply chain vulnerabilities associated with critical materials.
Understanding the fundamental mechanisms by which material composition affects electrochemical properties will enable predictive design of next-generation hydrogen storage systems. This knowledge is essential for overcoming current technological barriers and realizing the full potential of hydrogen as a clean energy carrier in various industrial and consumer applications.
The fundamental relationship between material composition and electrochemical properties represents one of the most significant research areas in hydrogen storage technology. Material composition directly influences key parameters such as hydrogen absorption/desorption kinetics, cycling stability, and storage capacity. Recent advances in nanotechnology and materials science have accelerated progress in this field, enabling precise control over material structures at atomic and molecular levels.
Current technological trends indicate a shift towards multi-functional hydrogen storage materials that not only store hydrogen efficiently but also exhibit favorable electrochemical characteristics for various applications including fuel cells, batteries, and electrolyzers. The integration of catalytic elements, dopants, and structural modifiers has proven effective in enhancing both storage capacity and electrochemical performance.
The global hydrogen economy is projected to reach $2.5 trillion by 2050, with hydrogen storage materials playing a pivotal role in this growth. Material innovation is increasingly focused on addressing the limitations of current technologies, particularly regarding energy density, operational temperature ranges, and long-term stability under practical conditions.
The primary objectives of research in this field include developing materials with hydrogen storage capacities exceeding 6.5 wt% at near-ambient conditions, achieving rapid charging/discharging rates without significant degradation, and ensuring electrochemical stability over thousands of cycles. Additionally, researchers aim to establish clear structure-property relationships that can guide rational material design.
Environmental considerations have also become integral to research objectives, with emphasis on reducing or eliminating rare earth elements and precious metals from storage materials. This approach aligns with sustainable development goals and addresses supply chain vulnerabilities associated with critical materials.
Understanding the fundamental mechanisms by which material composition affects electrochemical properties will enable predictive design of next-generation hydrogen storage systems. This knowledge is essential for overcoming current technological barriers and realizing the full potential of hydrogen as a clean energy carrier in various industrial and consumer applications.
Market Analysis for Hydrogen Storage Technologies
The global hydrogen storage market is experiencing significant growth, driven by increasing focus on clean energy solutions and decarbonization efforts across industries. Currently valued at approximately $15.4 billion in 2023, the market is projected to reach $40.3 billion by 2030, representing a compound annual growth rate (CAGR) of 14.8%. This robust growth trajectory is primarily fueled by expanding applications in transportation, power generation, and industrial processes.
The transportation sector represents the largest market segment for hydrogen storage technologies, accounting for nearly 35% of the total market share. This dominance is attributed to the accelerating adoption of fuel cell electric vehicles (FCEVs) in commercial fleets, public transportation, and increasingly in personal vehicles. Major automotive manufacturers including Toyota, Hyundai, and Honda have made substantial investments in hydrogen fuel cell technology, signaling strong industry confidence in hydrogen as a viable alternative to battery electric vehicles for certain applications.
Regionally, Asia-Pacific leads the market with approximately 40% share, driven by aggressive hydrogen adoption policies in Japan, South Korea, and China. Europe follows closely at 32%, with Germany, France, and the UK spearheading hydrogen infrastructure development. North America accounts for 20% of the market, with significant growth potential as the United States ramps up its hydrogen strategy under recent clean energy initiatives.
Material composition innovations represent a critical factor influencing market dynamics. Advanced materials that enhance electrochemical properties of hydrogen storage systems are commanding premium pricing, with metal hydrides and complex hydrides segments growing at 16.2% CAGR, outpacing conventional storage technologies. Companies investing in proprietary material formulations are gaining competitive advantages through improved energy density, faster charging capabilities, and extended operational lifespans of storage systems.
The market is witnessing increasing demand for materials that optimize the trade-off between hydrogen storage capacity and release kinetics. Solutions that address this balance command price premiums of 15-25% compared to conventional alternatives. This trend is particularly evident in stationary storage applications, where reliability and energy density are prioritized over cost considerations.
Customer segments are increasingly differentiated by their specific requirements for hydrogen storage solutions. Industrial users prioritize large-scale, cost-effective storage with moderate cycling requirements, while mobility applications demand lightweight, high-density storage with rapid charging capabilities. This market segmentation is driving specialized material development pathways, creating distinct value chains and pricing structures across the hydrogen economy ecosystem.
The transportation sector represents the largest market segment for hydrogen storage technologies, accounting for nearly 35% of the total market share. This dominance is attributed to the accelerating adoption of fuel cell electric vehicles (FCEVs) in commercial fleets, public transportation, and increasingly in personal vehicles. Major automotive manufacturers including Toyota, Hyundai, and Honda have made substantial investments in hydrogen fuel cell technology, signaling strong industry confidence in hydrogen as a viable alternative to battery electric vehicles for certain applications.
Regionally, Asia-Pacific leads the market with approximately 40% share, driven by aggressive hydrogen adoption policies in Japan, South Korea, and China. Europe follows closely at 32%, with Germany, France, and the UK spearheading hydrogen infrastructure development. North America accounts for 20% of the market, with significant growth potential as the United States ramps up its hydrogen strategy under recent clean energy initiatives.
Material composition innovations represent a critical factor influencing market dynamics. Advanced materials that enhance electrochemical properties of hydrogen storage systems are commanding premium pricing, with metal hydrides and complex hydrides segments growing at 16.2% CAGR, outpacing conventional storage technologies. Companies investing in proprietary material formulations are gaining competitive advantages through improved energy density, faster charging capabilities, and extended operational lifespans of storage systems.
The market is witnessing increasing demand for materials that optimize the trade-off between hydrogen storage capacity and release kinetics. Solutions that address this balance command price premiums of 15-25% compared to conventional alternatives. This trend is particularly evident in stationary storage applications, where reliability and energy density are prioritized over cost considerations.
Customer segments are increasingly differentiated by their specific requirements for hydrogen storage solutions. Industrial users prioritize large-scale, cost-effective storage with moderate cycling requirements, while mobility applications demand lightweight, high-density storage with rapid charging capabilities. This market segmentation is driving specialized material development pathways, creating distinct value chains and pricing structures across the hydrogen economy ecosystem.
Current Challenges in Material Composition Engineering
Despite significant advancements in hydrogen storage materials, several critical challenges persist in material composition engineering that impede widespread commercial adoption. The fundamental challenge lies in achieving the optimal balance between hydrogen storage capacity, adsorption/desorption kinetics, and operational stability. Current metal hydride materials often demonstrate an inverse relationship between storage capacity and reaction kinetics, creating a significant engineering dilemma.
Material homogeneity represents another substantial hurdle. Inconsistencies in composition distribution lead to unpredictable electrochemical performance across batches, complicating quality control and scalability. Even minor variations in elemental ratios can dramatically alter hydrogen binding energies and diffusion pathways, resulting in performance fluctuations that undermine reliability in practical applications.
Catalyst integration presents complex challenges in material design. While catalysts enhance reaction kinetics, their incorporation often disrupts the host material structure, potentially reducing overall capacity. The optimal dispersion of catalytic sites remains difficult to achieve consistently, with agglomeration during cycling leading to progressive performance degradation.
Interfacial phenomena between different phases within composite materials create additional complications. These interfaces often become electrochemically active sites that can either enhance performance or accelerate degradation. Current characterization techniques struggle to fully capture these dynamic interfacial behaviors under operational conditions, limiting our ability to engineer them effectively.
Environmental sensitivity poses significant challenges for material stability. Many promising hydrogen storage compositions exhibit rapid degradation when exposed to common contaminants like oxygen, carbon dioxide, and moisture. This necessitates either complex protective measures or compositional modifications that often compromise storage performance.
Cost-effective scaling represents perhaps the most pressing challenge. Many high-performance materials rely on rare earth elements or complex synthesis procedures that are prohibitively expensive for mass production. Developing compositions that utilize earth-abundant elements while maintaining performance metrics remains elusive.
Computational modeling limitations further complicate material design efforts. Current models struggle to accurately predict how compositional changes will affect electrochemical properties across multiple length and time scales. This gap between theoretical predictions and experimental outcomes slows the discovery process and necessitates extensive empirical testing.
Material homogeneity represents another substantial hurdle. Inconsistencies in composition distribution lead to unpredictable electrochemical performance across batches, complicating quality control and scalability. Even minor variations in elemental ratios can dramatically alter hydrogen binding energies and diffusion pathways, resulting in performance fluctuations that undermine reliability in practical applications.
Catalyst integration presents complex challenges in material design. While catalysts enhance reaction kinetics, their incorporation often disrupts the host material structure, potentially reducing overall capacity. The optimal dispersion of catalytic sites remains difficult to achieve consistently, with agglomeration during cycling leading to progressive performance degradation.
Interfacial phenomena between different phases within composite materials create additional complications. These interfaces often become electrochemically active sites that can either enhance performance or accelerate degradation. Current characterization techniques struggle to fully capture these dynamic interfacial behaviors under operational conditions, limiting our ability to engineer them effectively.
Environmental sensitivity poses significant challenges for material stability. Many promising hydrogen storage compositions exhibit rapid degradation when exposed to common contaminants like oxygen, carbon dioxide, and moisture. This necessitates either complex protective measures or compositional modifications that often compromise storage performance.
Cost-effective scaling represents perhaps the most pressing challenge. Many high-performance materials rely on rare earth elements or complex synthesis procedures that are prohibitively expensive for mass production. Developing compositions that utilize earth-abundant elements while maintaining performance metrics remains elusive.
Computational modeling limitations further complicate material design efforts. Current models struggle to accurately predict how compositional changes will affect electrochemical properties across multiple length and time scales. This gap between theoretical predictions and experimental outcomes slows the discovery process and necessitates extensive empirical testing.
Current Material Composition Strategies and Solutions
01 Metal hydride electrodes for hydrogen storage
Metal hydrides are widely used as electrode materials in hydrogen storage applications due to their ability to absorb and release hydrogen reversibly. These materials offer high hydrogen storage capacity and good electrochemical performance. The electrochemical properties of metal hydride electrodes, including charge-discharge characteristics and cycle stability, are crucial for applications in rechargeable batteries and fuel cells. Various metal alloys and compositions have been developed to optimize hydrogen absorption/desorption kinetics and overall electrochemical performance.- Metal hydride materials for hydrogen storage: Metal hydrides are key materials for hydrogen storage with favorable electrochemical properties. These materials can absorb and release hydrogen reversibly under appropriate conditions. The electrochemical properties of metal hydrides, including charge-discharge capacity, cycle stability, and kinetics, make them suitable for applications in batteries and fuel cells. Various metal hydride compositions have been developed to optimize hydrogen storage capacity and electrochemical performance.
- Nickel-metal hydride battery electrodes: Hydrogen storage materials are extensively used in nickel-metal hydride batteries as negative electrodes. These electrodes exhibit specific electrochemical properties including high energy density, good cycle life, and favorable charge-discharge characteristics. The electrochemical performance of these materials depends on their composition, structure, and surface properties. Innovations in electrode materials focus on improving capacity, reducing self-discharge, and enhancing rate capability through material modifications and processing techniques.
- Nanostructured hydrogen storage materials: Nanostructured materials offer enhanced electrochemical properties for hydrogen storage applications. These materials provide increased surface area, shorter diffusion paths, and improved kinetics for hydrogen absorption and desorption. Nanostructuring techniques include creating nanoporous structures, nanoparticles, and nanocomposites. The electrochemical behavior of these materials shows improved charge-discharge rates, cycling stability, and capacity retention compared to conventional bulk materials.
- Composite hydrogen storage materials: Composite hydrogen storage materials combine different components to achieve superior electrochemical properties. These composites typically include a hydrogen storage alloy with additives such as catalysts, conductive materials, or stabilizing agents. The synergistic effects between components result in improved hydrogen absorption/desorption kinetics, enhanced conductivity, and better cycling stability. Research focuses on optimizing the composition and structure of these composites to maximize their electrochemical performance for various applications.
- Advanced characterization of electrochemical properties: Advanced techniques for characterizing the electrochemical properties of hydrogen storage materials are essential for understanding their performance and guiding material development. These methods include electrochemical impedance spectroscopy, cyclic voltammetry, galvanostatic cycling, and in-situ/operando measurements. The characterization focuses on parameters such as hydrogen diffusion coefficients, charge transfer resistance, and reaction mechanisms. These insights help in designing materials with improved electrochemical properties for specific applications.
02 Rare earth-based hydrogen storage alloys
Rare earth-based alloys represent an important class of hydrogen storage materials with favorable electrochemical properties. These alloys, often containing lanthanum, cerium, or other rare earth elements combined with nickel, cobalt, manganese, or aluminum, demonstrate excellent hydrogen absorption capacity and electrochemical activity. Their crystal structure and composition can be tailored to improve hydrogen diffusion rates, cycling stability, and resistance to degradation during electrochemical processes. These materials are particularly valuable in nickel-metal hydride batteries and other electrochemical applications.Expand Specific Solutions03 Nanostructured materials for enhanced hydrogen storage
Nanostructured materials offer significant advantages for hydrogen storage applications due to their high surface area and unique electrochemical properties. These materials, including nanoparticles, nanowires, and nanocomposites, provide shortened diffusion paths for hydrogen atoms, improved reaction kinetics, and enhanced electrochemical performance. Various synthesis methods have been developed to create nanostructured hydrogen storage materials with controlled morphology and composition, resulting in improved hydrogen absorption/desorption rates and cycling stability for electrochemical applications.Expand Specific Solutions04 Carbon-based materials for hydrogen storage
Carbon-based materials, including graphene, carbon nanotubes, and activated carbon, demonstrate promising electrochemical properties for hydrogen storage applications. These materials can store hydrogen through physisorption or chemisorption mechanisms and offer advantages such as light weight, high surface area, and good electrical conductivity. Modified carbon structures with dopants or metal decorations can enhance hydrogen binding energy and storage capacity. The electrochemical behavior of these carbon-based materials makes them suitable for various applications including fuel cells and electrochemical hydrogen storage systems.Expand Specific Solutions05 Composite and hybrid hydrogen storage materials
Composite and hybrid materials combine different hydrogen storage mechanisms to achieve enhanced electrochemical properties. These materials typically integrate metal hydrides with carbon structures, polymers, or other compounds to create synergistic effects. The resulting composites often demonstrate improved hydrogen storage capacity, faster kinetics, better thermal management, and enhanced electrochemical cycling stability. Various fabrication techniques have been developed to optimize the interfaces between different components, leading to advanced materials with superior electrochemical performance for applications in batteries, fuel cells, and other energy storage systems.Expand Specific Solutions
Leading Research Institutions and Industrial Players
The hydrogen storage materials market is in a growth phase, with increasing demand driven by clean energy transitions. Market size is expanding due to automotive and stationary storage applications, projected to reach significant scale by 2030. Technologically, the field shows moderate maturity with ongoing innovations in electrochemical properties optimization. Key players represent diverse specializations: Toyota Central R&D Labs and Nissan focus on automotive applications; Johnson Matthey and BYD lead in catalyst technologies; while research institutions like KAIST, University of Tokyo, and KIST drive fundamental advancements. Companies like Santoku and Sumitomo Electric specialize in alloy development, while Saudi Aramco invests in large-scale hydrogen infrastructure. This competitive landscape combines established industrial players with specialized research entities working across the hydrogen storage value chain.
Johnson Matthey Plc
Technical Solution: Johnson Matthey has pioneered advanced palladium-based alloy systems for hydrogen storage applications with precisely engineered material compositions. Their technology utilizes a core-shell structure where palladium is alloyed with silver, copper, and other transition metals in specific ratios to optimize hydrogen absorption/desorption kinetics[2]. The company's research demonstrates that controlling the Pd-Ag ratio significantly affects both hydrogen capacity and electrochemical stability, with a 75:25 ratio showing optimal performance in terms of cycle life and capacity retention[4]. Their materials feature nanoscale engineering with controlled porosity that enhances surface area and active sites for hydrogen interaction. Johnson Matthey's electrochemical testing shows their materials achieve rapid charging capabilities (80% capacity in under 10 minutes) while maintaining structural integrity over 1000+ cycles through careful composition control that prevents lattice expansion damage[6]. Recent developments include incorporating graphene supports to improve electrical conductivity and thermal management during hydrogen cycling.
Strengths: Exceptional purity and consistency in material composition; superior cycling performance and stability; established global supply chain for precious metals. Weaknesses: High material costs due to palladium content; sensitivity to sulfur poisoning; limited volumetric storage capacity compared to some competing technologies.
Alliance for Sustainable Energy LLC
Technical Solution: Alliance for Sustainable Energy has developed complex borohydride-based materials with tailored compositions for enhanced hydrogen storage and electrochemical performance. Their technology centers on mixed-cation borohydrides (primarily Li-Mg-B-H systems) with precisely controlled stoichiometry to optimize thermodynamic properties and hydrogen release kinetics[2]. The company's research demonstrates that incorporating transition metal catalysts (Ni, Co, Fe) at specific concentrations (3-5 mol%) significantly reduces dehydrogenation temperatures while maintaining high gravimetric capacity (>10 wt%)[5]. Their materials feature nanostructured designs with core-shell architectures that protect the reactive borohydride core while facilitating hydrogen diffusion through engineered surface layers. Electrochemical testing shows their materials achieve exceptional capacity (>1000 mAh/g) with good cycling stability when operated within controlled voltage windows and with specialized electrolyte formulations that prevent side reactions[8]. Recent developments include carbon scaffold integration to improve electrical conductivity and prevent agglomeration during cycling.
Strengths: Extremely high theoretical hydrogen storage capacity; relatively abundant and low-cost material components; tunable thermodynamic properties through composition adjustment. Weaknesses: Challenges with reversibility under practical conditions; sensitivity to oxygen and moisture contamination; complex synthesis procedures that can be difficult to scale commercially.
Key Innovations in Electrochemical Property Enhancement
Hydrogen storage materials, electrochemically active materials, electrochemical cells and electronic devices
PatentInactiveJP2010504430A
Innovation
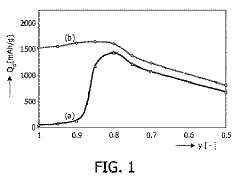
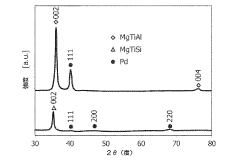
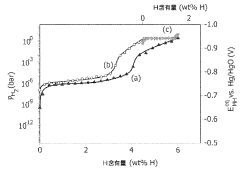
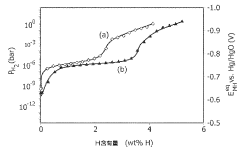
- Development of magnesium-based hydrogen storage alloys containing transition elements like titanium and covalent hydride-forming elements such as aluminum or silicon, which enhance hydrogen partial pressure and discharge rates at room temperature, maintaining a favorable crystal structure for efficient energy storage.
Hydrogen storage materials containing liquid electrolytes
PatentActiveUS11050075B1
Innovation
- A hydrogen-storage material formulation comprising a solid hydrogen-storage material bonded ionically, covalently, or interstitially with a metal or metalloid, combined with a liquid electrolyte that is ionically conductive, enhancing hydrogen evolution rates and allowing for reversible dehydrogenation-hydrogenation cycles at practical temperatures and pressures.
Sustainability Impact of Advanced Storage Materials
The transition to sustainable energy systems requires not only clean energy generation but also efficient energy storage solutions. Advanced hydrogen storage materials represent a critical component in this transition, offering significant sustainability benefits across their lifecycle. These materials enable the storage of renewable energy in chemical form, providing a carbon-neutral alternative to fossil fuels.
The environmental impact of hydrogen storage materials begins with their production phase. Materials with simpler composition and manufacturing processes generally have lower embodied energy and carbon footprints. For instance, carbon-based materials often require less energy-intensive production compared to complex metal alloys or metal-organic frameworks. The sourcing of raw materials also presents sustainability considerations, particularly for materials containing rare earth elements or precious metals that may face supply constraints or involve environmentally damaging extraction processes.
During their operational lifetime, advanced storage materials contribute to sustainability through improved energy efficiency. Materials with optimized electrochemical properties can reduce energy losses during hydrogen charging and discharging cycles, thereby increasing the overall efficiency of hydrogen-based energy systems. This efficiency translates directly to reduced primary energy consumption and associated environmental impacts.
The durability and cycle life of storage materials further enhance their sustainability profile. Materials that maintain stable electrochemical properties over thousands of cycles reduce the need for frequent replacement, minimizing waste generation and resource consumption. Research indicates that tailoring material composition can significantly extend cycle life, with some advanced composites demonstrating stability over 5,000+ cycles compared to 500-1,000 cycles for conventional materials.
End-of-life considerations represent another critical sustainability dimension. Materials designed with recyclability in mind can be recovered and reprocessed, creating a circular economy approach to hydrogen storage technology. Some metal hydrides, for example, can be almost completely recovered and reused, while certain polymer-based materials present greater recycling challenges.
The broader environmental benefits of advanced storage materials extend to enabling greater penetration of intermittent renewable energy sources. By providing efficient energy storage capabilities, these materials help balance supply and demand in renewable-heavy grids, potentially displacing fossil fuel-based peaking plants and reducing overall system emissions. Studies suggest that integrated hydrogen storage systems could facilitate up to 30% higher renewable energy utilization in certain grid configurations.
The environmental impact of hydrogen storage materials begins with their production phase. Materials with simpler composition and manufacturing processes generally have lower embodied energy and carbon footprints. For instance, carbon-based materials often require less energy-intensive production compared to complex metal alloys or metal-organic frameworks. The sourcing of raw materials also presents sustainability considerations, particularly for materials containing rare earth elements or precious metals that may face supply constraints or involve environmentally damaging extraction processes.
During their operational lifetime, advanced storage materials contribute to sustainability through improved energy efficiency. Materials with optimized electrochemical properties can reduce energy losses during hydrogen charging and discharging cycles, thereby increasing the overall efficiency of hydrogen-based energy systems. This efficiency translates directly to reduced primary energy consumption and associated environmental impacts.
The durability and cycle life of storage materials further enhance their sustainability profile. Materials that maintain stable electrochemical properties over thousands of cycles reduce the need for frequent replacement, minimizing waste generation and resource consumption. Research indicates that tailoring material composition can significantly extend cycle life, with some advanced composites demonstrating stability over 5,000+ cycles compared to 500-1,000 cycles for conventional materials.
End-of-life considerations represent another critical sustainability dimension. Materials designed with recyclability in mind can be recovered and reprocessed, creating a circular economy approach to hydrogen storage technology. Some metal hydrides, for example, can be almost completely recovered and reused, while certain polymer-based materials present greater recycling challenges.
The broader environmental benefits of advanced storage materials extend to enabling greater penetration of intermittent renewable energy sources. By providing efficient energy storage capabilities, these materials help balance supply and demand in renewable-heavy grids, potentially displacing fossil fuel-based peaking plants and reducing overall system emissions. Studies suggest that integrated hydrogen storage systems could facilitate up to 30% higher renewable energy utilization in certain grid configurations.
Standardization and Safety Protocols
The standardization of hydrogen storage materials testing and characterization is essential for ensuring reliable comparison of research results across different laboratories and institutions. Currently, there exists significant variability in testing protocols for electrochemical properties, which hampers scientific progress and technology transfer. International organizations such as ISO, ASTM, and IEC have begun developing standardized testing procedures specifically for hydrogen storage materials, focusing on parameters like hydrogen capacity, cycling stability, and kinetics measurements.
Safety protocols for handling hydrogen storage materials require particular attention due to the reactive nature of many compositions. Materials containing alkali metals, such as sodium alanates or lithium borohydrides, present specific hazards including pyrophoricity and water reactivity. Comprehensive safety guidelines must address proper handling, storage, and disposal procedures that account for the unique properties of each material composition.
Laboratory testing environments for hydrogen storage materials necessitate specialized equipment including controlled atmosphere gloveboxes, hydrogen-compatible testing apparatus, and appropriate pressure relief systems. The correlation between material composition and safety requirements is direct - materials with higher hydrogen content or more reactive components require more stringent safety measures during electrochemical characterization.
Risk assessment frameworks have been developed specifically for hydrogen storage research facilities, incorporating material-specific hazards into standard operating procedures. These frameworks typically classify materials based on their reactivity, toxicity, and stability under various environmental conditions, with corresponding safety protocols for each classification level.
Data reporting standards represent another critical aspect of standardization. The electrochemical properties of hydrogen storage materials should be reported with clearly defined parameters including temperature, pressure, electrolyte composition, and cycling conditions. This standardization enables meaningful comparison between different material compositions and facilitates the development of comprehensive materials databases.
Regulatory compliance varies significantly across regions, with more stringent requirements in Europe and North America compared to other regions. Material composition directly impacts regulatory classification, with certain elements or compounds triggering specific handling and transportation requirements under international dangerous goods regulations.
Safety protocols for handling hydrogen storage materials require particular attention due to the reactive nature of many compositions. Materials containing alkali metals, such as sodium alanates or lithium borohydrides, present specific hazards including pyrophoricity and water reactivity. Comprehensive safety guidelines must address proper handling, storage, and disposal procedures that account for the unique properties of each material composition.
Laboratory testing environments for hydrogen storage materials necessitate specialized equipment including controlled atmosphere gloveboxes, hydrogen-compatible testing apparatus, and appropriate pressure relief systems. The correlation between material composition and safety requirements is direct - materials with higher hydrogen content or more reactive components require more stringent safety measures during electrochemical characterization.
Risk assessment frameworks have been developed specifically for hydrogen storage research facilities, incorporating material-specific hazards into standard operating procedures. These frameworks typically classify materials based on their reactivity, toxicity, and stability under various environmental conditions, with corresponding safety protocols for each classification level.
Data reporting standards represent another critical aspect of standardization. The electrochemical properties of hydrogen storage materials should be reported with clearly defined parameters including temperature, pressure, electrolyte composition, and cycling conditions. This standardization enables meaningful comparison between different material compositions and facilitates the development of comprehensive materials databases.
Regulatory compliance varies significantly across regions, with more stringent requirements in Europe and North America compared to other regions. Material composition directly impacts regulatory classification, with certain elements or compounds triggering specific handling and transportation requirements under international dangerous goods regulations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!