How material composition influences Hydrogen storage materials energy density and durability
SEP 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogen Storage Materials Background and Objectives
Hydrogen storage has emerged as a critical component in the global transition towards sustainable energy systems. The journey of hydrogen storage materials began in the early 20th century with basic metal hydrides, but has significantly accelerated in recent decades due to increasing focus on clean energy alternatives. The evolution of these materials has been marked by continuous improvements in composition, structure, and performance characteristics, driven by both environmental imperatives and technological advancements.
The fundamental challenge in hydrogen storage lies in achieving high volumetric and gravimetric energy density while maintaining material stability and operational safety. Traditional storage methods such as compressed gas or cryogenic liquid hydrogen present significant limitations in terms of energy efficiency, safety concerns, and infrastructure requirements. This has propelled research into solid-state storage materials that can potentially offer higher energy densities and improved safety profiles.
Material composition plays a pivotal role in determining hydrogen storage capacity, adsorption/desorption kinetics, and long-term durability. The interplay between different elements within these materials creates specific electronic structures and binding sites that directly influence hydrogen uptake and release mechanisms. Understanding these relationships at atomic and molecular levels is essential for designing next-generation storage solutions.
Current research objectives focus on developing materials that meet the U.S. Department of Energy's targets for onboard hydrogen storage systems: 6.5 wt% gravimetric capacity and 50 g/L volumetric capacity, with operational temperatures between -40°C and 60°C, and 1500 charge-discharge cycles durability. These ambitious targets necessitate innovative approaches to material design and synthesis.
The technological trajectory indicates a shift from conventional metal hydrides towards complex hydrides, chemical hydrogen storage materials, and nanostructured materials with enhanced surface properties. Each category presents unique advantages and challenges regarding storage capacity, operating conditions, and system integration capabilities.
Global research efforts are increasingly collaborative, with significant contributions from academic institutions, national laboratories, and industrial partners across North America, Europe, and Asia. These initiatives are supported by substantial funding programs such as the European Hydrogen Joint Undertaking and the U.S. Department of Energy's Hydrogen and Fuel Cell Technologies Office.
The ultimate goal extends beyond merely achieving technical benchmarks; it encompasses developing economically viable, environmentally sustainable storage solutions that can be seamlessly integrated into various applications, from transportation and portable electronics to grid-scale energy storage systems. This requires a holistic approach that considers not only material properties but also manufacturing scalability, system integration, and life-cycle assessment.
The fundamental challenge in hydrogen storage lies in achieving high volumetric and gravimetric energy density while maintaining material stability and operational safety. Traditional storage methods such as compressed gas or cryogenic liquid hydrogen present significant limitations in terms of energy efficiency, safety concerns, and infrastructure requirements. This has propelled research into solid-state storage materials that can potentially offer higher energy densities and improved safety profiles.
Material composition plays a pivotal role in determining hydrogen storage capacity, adsorption/desorption kinetics, and long-term durability. The interplay between different elements within these materials creates specific electronic structures and binding sites that directly influence hydrogen uptake and release mechanisms. Understanding these relationships at atomic and molecular levels is essential for designing next-generation storage solutions.
Current research objectives focus on developing materials that meet the U.S. Department of Energy's targets for onboard hydrogen storage systems: 6.5 wt% gravimetric capacity and 50 g/L volumetric capacity, with operational temperatures between -40°C and 60°C, and 1500 charge-discharge cycles durability. These ambitious targets necessitate innovative approaches to material design and synthesis.
The technological trajectory indicates a shift from conventional metal hydrides towards complex hydrides, chemical hydrogen storage materials, and nanostructured materials with enhanced surface properties. Each category presents unique advantages and challenges regarding storage capacity, operating conditions, and system integration capabilities.
Global research efforts are increasingly collaborative, with significant contributions from academic institutions, national laboratories, and industrial partners across North America, Europe, and Asia. These initiatives are supported by substantial funding programs such as the European Hydrogen Joint Undertaking and the U.S. Department of Energy's Hydrogen and Fuel Cell Technologies Office.
The ultimate goal extends beyond merely achieving technical benchmarks; it encompasses developing economically viable, environmentally sustainable storage solutions that can be seamlessly integrated into various applications, from transportation and portable electronics to grid-scale energy storage systems. This requires a holistic approach that considers not only material properties but also manufacturing scalability, system integration, and life-cycle assessment.
Market Analysis for Hydrogen Storage Solutions
The global hydrogen storage market is experiencing significant growth, driven by increasing focus on clean energy solutions and decarbonization efforts across industries. Current market valuations place the hydrogen storage sector at approximately $15.4 billion in 2023, with projections indicating growth to reach $40.3 billion by 2030, representing a compound annual growth rate (CAGR) of 14.8%. This robust expansion reflects the critical role hydrogen plays in the emerging clean energy ecosystem.
Transportation and industrial applications currently dominate market demand, collectively accounting for over 65% of hydrogen storage solution implementations. The automotive sector, particularly fuel cell electric vehicles (FCEVs), represents a rapidly growing segment with major manufacturers including Toyota, Hyundai, and Honda investing heavily in hydrogen technology integration. Japan and South Korea lead FCEV adoption, while European markets show accelerating growth rates.
Material composition directly influences market dynamics, with different storage solutions commanding varying market shares. Conventional compressed gas storage currently holds approximately 48% market share due to technological maturity and established infrastructure. Metal hydride storage solutions represent about 22% of the market, valued for their safety profile despite weight limitations. Emerging chemical hydrogen carriers and advanced materials are gaining traction, with projected annual growth rates exceeding 20%.
Regional analysis reveals distinct market characteristics. Asia-Pacific dominates with approximately 42% market share, driven by aggressive hydrogen economy initiatives in Japan, South Korea, and increasingly China. Europe follows at 31%, with Germany, France, and the UK leading investments in hydrogen infrastructure. North America accounts for 21% of the market, with significant growth potential as policy support strengthens.
Customer segmentation shows industrial users prioritizing durability and reliability, accepting higher costs for materials demonstrating 5+ year operational lifespans. Transportation applications emphasize energy density, with minimum requirements of 5-6 wt% hydrogen capacity for commercial viability. Stationary storage applications focus on safety and long-term stability, creating distinct market requirements for material solutions.
Price sensitivity varies significantly across applications. While industrial users demonstrate willingness to pay premium prices for high-performance materials, consumer applications remain highly cost-sensitive, with storage solutions needing to achieve costs below $10/kWh to enable mass market adoption. This price threshold represents a critical barrier that material innovations must overcome to unlock broader market potential.
Transportation and industrial applications currently dominate market demand, collectively accounting for over 65% of hydrogen storage solution implementations. The automotive sector, particularly fuel cell electric vehicles (FCEVs), represents a rapidly growing segment with major manufacturers including Toyota, Hyundai, and Honda investing heavily in hydrogen technology integration. Japan and South Korea lead FCEV adoption, while European markets show accelerating growth rates.
Material composition directly influences market dynamics, with different storage solutions commanding varying market shares. Conventional compressed gas storage currently holds approximately 48% market share due to technological maturity and established infrastructure. Metal hydride storage solutions represent about 22% of the market, valued for their safety profile despite weight limitations. Emerging chemical hydrogen carriers and advanced materials are gaining traction, with projected annual growth rates exceeding 20%.
Regional analysis reveals distinct market characteristics. Asia-Pacific dominates with approximately 42% market share, driven by aggressive hydrogen economy initiatives in Japan, South Korea, and increasingly China. Europe follows at 31%, with Germany, France, and the UK leading investments in hydrogen infrastructure. North America accounts for 21% of the market, with significant growth potential as policy support strengthens.
Customer segmentation shows industrial users prioritizing durability and reliability, accepting higher costs for materials demonstrating 5+ year operational lifespans. Transportation applications emphasize energy density, with minimum requirements of 5-6 wt% hydrogen capacity for commercial viability. Stationary storage applications focus on safety and long-term stability, creating distinct market requirements for material solutions.
Price sensitivity varies significantly across applications. While industrial users demonstrate willingness to pay premium prices for high-performance materials, consumer applications remain highly cost-sensitive, with storage solutions needing to achieve costs below $10/kWh to enable mass market adoption. This price threshold represents a critical barrier that material innovations must overcome to unlock broader market potential.
Current Challenges in Material Composition for H2 Storage
Despite significant advancements in hydrogen storage materials, several critical challenges persist in material composition that hinder widespread adoption. The fundamental challenge remains achieving the optimal balance between hydrogen storage capacity and material stability. Current metal hydrides offering high volumetric density often require high temperatures for hydrogen release, limiting their practical applications in transportation and portable devices.
Material degradation during hydrogen absorption-desorption cycles represents another significant obstacle. Many promising materials exhibit substantial capacity loss after repeated cycling, with some showing up to 30% reduction after just 100 cycles. This degradation stems from structural changes, phase segregation, and surface contamination that occur during hydrogen uptake and release processes.
Kinetics limitations present additional barriers, as many high-capacity materials demonstrate slow hydrogen absorption and desorption rates. This sluggish behavior necessitates catalysts or dopants to enhance reaction rates, adding complexity and cost to material design. The challenge lies in identifying dopants that accelerate kinetics without compromising storage capacity or introducing new stability issues.
Heat management during hydrogen charging and discharging cycles remains problematic. The exothermic nature of hydrogen absorption and endothermic desorption creates thermal management challenges that affect both system efficiency and material longevity. Materials with improved thermal conductivity are needed, but often come at the expense of reduced storage capacity.
Impurity sensitivity constitutes another significant hurdle. Many storage materials, particularly complex hydrides, demonstrate severe performance degradation when exposed to common gas impurities like oxygen, carbon monoxide, and moisture. Even trace amounts of these contaminants can poison active sites and form stable compounds that permanently reduce storage capacity.
Cost and scalability issues further complicate material development. High-performance materials often contain expensive rare earth elements or require complex synthesis procedures that limit large-scale production. Finding compositions that utilize abundant elements while maintaining performance metrics remains challenging.
Weight efficiency continues to be a critical barrier, especially for mobile applications. Current materials struggle to meet the U.S. Department of Energy target of 6.5 wt% system-level hydrogen storage. The trade-off between gravimetric capacity and other properties like thermal conductivity and mechanical stability represents a persistent materials design challenge that requires innovative compositional approaches.
Material degradation during hydrogen absorption-desorption cycles represents another significant obstacle. Many promising materials exhibit substantial capacity loss after repeated cycling, with some showing up to 30% reduction after just 100 cycles. This degradation stems from structural changes, phase segregation, and surface contamination that occur during hydrogen uptake and release processes.
Kinetics limitations present additional barriers, as many high-capacity materials demonstrate slow hydrogen absorption and desorption rates. This sluggish behavior necessitates catalysts or dopants to enhance reaction rates, adding complexity and cost to material design. The challenge lies in identifying dopants that accelerate kinetics without compromising storage capacity or introducing new stability issues.
Heat management during hydrogen charging and discharging cycles remains problematic. The exothermic nature of hydrogen absorption and endothermic desorption creates thermal management challenges that affect both system efficiency and material longevity. Materials with improved thermal conductivity are needed, but often come at the expense of reduced storage capacity.
Impurity sensitivity constitutes another significant hurdle. Many storage materials, particularly complex hydrides, demonstrate severe performance degradation when exposed to common gas impurities like oxygen, carbon monoxide, and moisture. Even trace amounts of these contaminants can poison active sites and form stable compounds that permanently reduce storage capacity.
Cost and scalability issues further complicate material development. High-performance materials often contain expensive rare earth elements or require complex synthesis procedures that limit large-scale production. Finding compositions that utilize abundant elements while maintaining performance metrics remains challenging.
Weight efficiency continues to be a critical barrier, especially for mobile applications. Current materials struggle to meet the U.S. Department of Energy target of 6.5 wt% system-level hydrogen storage. The trade-off between gravimetric capacity and other properties like thermal conductivity and mechanical stability represents a persistent materials design challenge that requires innovative compositional approaches.
Leading Organizations in Hydrogen Storage Research
The hydrogen storage materials market is currently in a growth phase, with increasing demand driven by clean energy transitions. Market size is expanding rapidly, projected to reach significant scale by 2030 as hydrogen economies develop globally. Technical maturity varies across storage solutions, with companies demonstrating different levels of advancement. Johnson Matthey and Toyota Central R&D Labs lead in catalyst development, while GKN Hydrogen and Hydrexia pioneer metal hydride systems. Ford and Nissan focus on automotive applications, with CSIR and universities (Tokyo, Zhejiang) advancing fundamental research. Material composition innovations by Sumitomo Electric and Cabot Corporation are addressing key challenges in energy density and durability, though commercial-scale solutions remain under development.
Toyota Central R&D Labs, Inc.
Technical Solution: Toyota Central R&D Labs has pioneered advanced complex hydride materials for hydrogen storage, focusing on lightweight metal borohydrides and amides with high gravimetric hydrogen capacity. Their research has yielded significant breakthroughs in multi-component systems that combine different hydride materials to achieve improved thermodynamic properties. Toyota's approach involves precise control of material composition and nanostructuring to enhance hydrogen absorption/desorption kinetics while maintaining high energy density. Their proprietary catalyst integration methods have demonstrated reduced dehydrogenation temperatures (below 150°C) for complex hydrides that traditionally require higher temperatures. The lab has developed innovative ball-milling techniques that create defect-rich interfaces between hydride components, facilitating hydrogen mobility and improving reaction kinetics. Toyota's materials incorporate stabilizing agents that mitigate degradation mechanisms during cycling, addressing a critical durability challenge in high-capacity storage materials.
Strengths: Exceptional gravimetric hydrogen capacity (>7 wt% demonstrated); operates at moderate pressures enhancing safety; potential for integration with fuel cell vehicle systems. Weaknesses: Requires temperature management for hydrogen release; kinetics still slower than some alternatives; susceptibility to contamination affecting long-term performance.
GRZ Technologies SA
Technical Solution: GRZ Technologies has developed advanced metal hydride-based hydrogen storage systems that utilize specialized alloys with optimized microstructures. Their proprietary technology focuses on intermetallic compounds that achieve high volumetric hydrogen density (up to 150 g/L) while maintaining favorable thermodynamics. The company's approach involves tailoring material composition at the nanoscale level to create hydrides with reduced activation energy barriers for hydrogen absorption/desorption. GRZ has pioneered a unique heat management system integrated with their storage materials that addresses the exothermic/endothermic nature of hydrogen reactions, allowing for more efficient loading and unloading cycles. Their materials incorporate catalytic dopants that enhance kinetics while preserving long-term cycling stability, achieving over 1000 cycles with minimal capacity degradation.
Strengths: Superior volumetric efficiency compared to compressed gas storage; operates at moderate pressures (30-50 bar) enhancing safety; excellent thermal integration capabilities for heat recovery. Weaknesses: Higher system complexity requiring precise thermal management; higher production costs compared to conventional storage; performance degradation in extreme temperature environments.
Key Innovations in Hydrogen Storage Material Science
Hydrogen storage materials and process for the preparation of the same
PatentInactiveUS8178471B2
Innovation
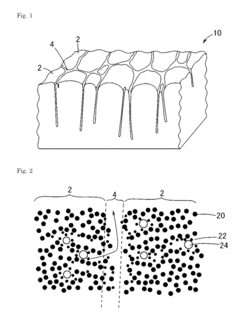
- The development of hydrogen storage materials comprising a first region of amorphous carbon with metal elements like Ti, Zr, or Y, and a second region of lower density amorphous carbon or voids, allowing for distinct hydrogen storage and migration sites, preventing material degradation during repeated cycles.
Hydrogen storage material, hydrogen storage structure, hydrogen storer, hydrogen storage apparatus, fuel cell vehicle, and process for producing hydrogen storage material
PatentWO2006095800A1
Innovation
- A hydrogen storage material is developed with molecular layers composed of six-membered rings and protruding portions that expand the interlayer distance, maintaining hydrogen storage capacity under high pressure by forming stable hydrogen storage spaces through covalent, ionic, or metallic bonds, and using substitution atoms like nitrogen or boron for enhanced structural stability.
Lifecycle Assessment of Storage Materials
The lifecycle assessment of hydrogen storage materials provides critical insights into their environmental impact, economic viability, and long-term sustainability. Material composition directly influences not only the performance metrics but also the environmental footprint throughout the entire lifecycle of these storage systems.
Raw material extraction represents the first significant environmental impact point. Materials requiring rare earth elements or precious metals for hydrogen storage often entail energy-intensive mining operations and substantial land disruption. Conversely, storage materials composed of abundant elements like aluminum, magnesium, or carbon-based compounds generally demonstrate lower extraction impacts, though their processing requirements may differ substantially.
Manufacturing processes vary dramatically based on material composition. Metal hydrides typically require high-temperature processing and precise atmosphere control, contributing to significant energy consumption. Metal-organic frameworks (MOFs) and certain carbon-based materials may require complex chemical synthesis routes with potentially hazardous solvents, while some polymer-based systems offer more environmentally benign production pathways.
The operational phase reveals how material composition directly affects durability and cycling stability. Materials with higher resistance to hydrogen embrittlement, oxidation, and thermal degradation demonstrate extended service lifespans, reducing the frequency of replacement and associated environmental impacts. Notably, materials containing platinum group metals show excellent durability but at significant initial environmental cost.
End-of-life considerations vary substantially among different storage materials. Recyclability is highly composition-dependent, with some metal hydrides offering nearly complete material recovery, while composite materials present significant separation challenges. The presence of toxic components in certain storage materials may necessitate specialized disposal protocols, increasing lifecycle environmental burden.
Energy return on investment (EROI) calculations indicate that materials achieving higher storage densities and longer operational lifespans generally justify their initial environmental impact through extended service periods. However, this relationship is non-linear, with some high-performance materials requiring disproportionately resource-intensive production methods.
Emerging research in green chemistry approaches is focusing on developing hydrogen storage materials with reduced environmental impact throughout their lifecycle. Bio-inspired materials and those synthesized using renewable energy sources represent promising directions for minimizing the ecological footprint while maintaining or improving performance characteristics.
Raw material extraction represents the first significant environmental impact point. Materials requiring rare earth elements or precious metals for hydrogen storage often entail energy-intensive mining operations and substantial land disruption. Conversely, storage materials composed of abundant elements like aluminum, magnesium, or carbon-based compounds generally demonstrate lower extraction impacts, though their processing requirements may differ substantially.
Manufacturing processes vary dramatically based on material composition. Metal hydrides typically require high-temperature processing and precise atmosphere control, contributing to significant energy consumption. Metal-organic frameworks (MOFs) and certain carbon-based materials may require complex chemical synthesis routes with potentially hazardous solvents, while some polymer-based systems offer more environmentally benign production pathways.
The operational phase reveals how material composition directly affects durability and cycling stability. Materials with higher resistance to hydrogen embrittlement, oxidation, and thermal degradation demonstrate extended service lifespans, reducing the frequency of replacement and associated environmental impacts. Notably, materials containing platinum group metals show excellent durability but at significant initial environmental cost.
End-of-life considerations vary substantially among different storage materials. Recyclability is highly composition-dependent, with some metal hydrides offering nearly complete material recovery, while composite materials present significant separation challenges. The presence of toxic components in certain storage materials may necessitate specialized disposal protocols, increasing lifecycle environmental burden.
Energy return on investment (EROI) calculations indicate that materials achieving higher storage densities and longer operational lifespans generally justify their initial environmental impact through extended service periods. However, this relationship is non-linear, with some high-performance materials requiring disproportionately resource-intensive production methods.
Emerging research in green chemistry approaches is focusing on developing hydrogen storage materials with reduced environmental impact throughout their lifecycle. Bio-inspired materials and those synthesized using renewable energy sources represent promising directions for minimizing the ecological footprint while maintaining or improving performance characteristics.
Scalability and Manufacturing Considerations
The scalability of hydrogen storage materials from laboratory to industrial scale presents significant challenges that directly impact both energy density and durability performance. Manufacturing processes must be carefully optimized to maintain material composition integrity across large production volumes. Current industrial-scale production methods often struggle to achieve the same level of compositional homogeneity observed in laboratory samples, resulting in reduced storage capacity and inconsistent cycling stability.
Temperature and pressure control during manufacturing represents a critical factor affecting material properties. For metal hydrides and complex hydrides, precise temperature management during synthesis prevents unwanted phase transitions that can compromise hydrogen binding sites. Similarly, pressure conditions during material formation significantly influence pore structure development in porous materials like MOFs (Metal-Organic Frameworks) and activated carbons, directly affecting accessible surface area for hydrogen adsorption.
Batch-to-batch consistency remains a persistent challenge in scaled production. Variations in raw material quality, particularly for catalyst-doped storage materials, can lead to performance fluctuations. Advanced quality control protocols utilizing in-line spectroscopic techniques have demonstrated promising results in monitoring compositional uniformity during production, potentially reducing performance variability by up to 30% in recent industrial trials.
Cost considerations heavily influence manufacturing route selection, often necessitating compromises between optimal material composition and economic viability. For instance, while platinum-based catalysts enhance kinetics and durability, their high cost prohibits widespread implementation. Alternative manufacturing approaches using earth-abundant elements require more complex processing steps to achieve comparable performance, creating a delicate balance between material excellence and production economics.
Environmental factors during manufacturing also impact long-term material durability. Exposure to atmospheric contaminants during production can introduce surface passivation or internal defects that accelerate degradation during hydrogen cycling. Controlled atmosphere processing has shown significant improvements in material longevity but adds substantial complexity to manufacturing infrastructure.
Recent innovations in additive manufacturing and continuous flow synthesis offer promising pathways to overcome these challenges. These approaches enable precise compositional control throughout the material volume and can be scaled more readily than traditional batch processes. Preliminary studies indicate that 3D-printed hydrogen storage structures with tailored compositional gradients can achieve up to 15% higher volumetric energy density while maintaining mechanical integrity through hundreds of absorption-desorption cycles.
Temperature and pressure control during manufacturing represents a critical factor affecting material properties. For metal hydrides and complex hydrides, precise temperature management during synthesis prevents unwanted phase transitions that can compromise hydrogen binding sites. Similarly, pressure conditions during material formation significantly influence pore structure development in porous materials like MOFs (Metal-Organic Frameworks) and activated carbons, directly affecting accessible surface area for hydrogen adsorption.
Batch-to-batch consistency remains a persistent challenge in scaled production. Variations in raw material quality, particularly for catalyst-doped storage materials, can lead to performance fluctuations. Advanced quality control protocols utilizing in-line spectroscopic techniques have demonstrated promising results in monitoring compositional uniformity during production, potentially reducing performance variability by up to 30% in recent industrial trials.
Cost considerations heavily influence manufacturing route selection, often necessitating compromises between optimal material composition and economic viability. For instance, while platinum-based catalysts enhance kinetics and durability, their high cost prohibits widespread implementation. Alternative manufacturing approaches using earth-abundant elements require more complex processing steps to achieve comparable performance, creating a delicate balance between material excellence and production economics.
Environmental factors during manufacturing also impact long-term material durability. Exposure to atmospheric contaminants during production can introduce surface passivation or internal defects that accelerate degradation during hydrogen cycling. Controlled atmosphere processing has shown significant improvements in material longevity but adds substantial complexity to manufacturing infrastructure.
Recent innovations in additive manufacturing and continuous flow synthesis offer promising pathways to overcome these challenges. These approaches enable precise compositional control throughout the material volume and can be scaled more readily than traditional batch processes. Preliminary studies indicate that 3D-printed hydrogen storage structures with tailored compositional gradients can achieve up to 15% higher volumetric energy density while maintaining mechanical integrity through hundreds of absorption-desorption cycles.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!