What material innovations improve Hydrogen storage materials efficiency and stability
SEP 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogen Storage Materials Background and Objectives
Hydrogen storage has emerged as a critical component in the global transition towards sustainable energy systems. The journey of hydrogen storage materials began in the mid-20th century with basic metal hydrides, evolving significantly through decades of research and development. Today, these materials stand at the intersection of materials science, chemistry, and energy engineering, representing a key enabler for hydrogen economy.
The evolution of hydrogen storage technologies has been driven by increasing demands for clean energy solutions and the recognition of hydrogen as a versatile energy carrier. Early research focused primarily on conventional metal hydrides, which offered moderate storage capacities but suffered from weight inefficiencies and thermal management challenges. The field has since expanded to encompass complex hydrides, chemical hydrogen storage materials, and advanced porous materials, each offering unique advantages and limitations.
Current technological trajectories point toward multi-functional materials that can simultaneously address the core challenges of hydrogen storage: capacity, kinetics, thermodynamics, and long-term stability. Recent breakthroughs in nanomaterials and composite structures have opened new pathways for enhancing storage efficiency while maintaining material integrity over numerous charge-discharge cycles.
The primary objective of hydrogen storage materials research is to develop solutions that meet the U.S. Department of Energy's targets: 6.5 wt% system gravimetric capacity and 50 g/L volumetric capacity, with operational temperatures between -40°C and 60°C, and cycling durability of 1,500 cycles. These benchmarks are essential for making hydrogen competitive with conventional fuels in transportation and stationary applications.
Beyond these technical parameters, research aims to address broader challenges including cost reduction, scalable manufacturing processes, and integration with existing energy infrastructure. The development of materials that can operate under milder conditions without sacrificing performance represents a particularly important goal, as it would significantly reduce system complexity and energy requirements.
Strategic objectives also include understanding fundamental material behaviors at the atomic and molecular levels, which can inform rational design approaches for next-generation storage materials. This includes investigating reaction mechanisms, degradation pathways, and structure-property relationships that govern hydrogen absorption and desorption processes.
The ultimate goal extends beyond technical performance to encompass sustainability considerations, including the use of earth-abundant elements, environmentally benign synthesis methods, and end-of-life recyclability. These factors will be crucial in determining the long-term viability and widespread adoption of hydrogen storage technologies in a carbon-constrained world.
The evolution of hydrogen storage technologies has been driven by increasing demands for clean energy solutions and the recognition of hydrogen as a versatile energy carrier. Early research focused primarily on conventional metal hydrides, which offered moderate storage capacities but suffered from weight inefficiencies and thermal management challenges. The field has since expanded to encompass complex hydrides, chemical hydrogen storage materials, and advanced porous materials, each offering unique advantages and limitations.
Current technological trajectories point toward multi-functional materials that can simultaneously address the core challenges of hydrogen storage: capacity, kinetics, thermodynamics, and long-term stability. Recent breakthroughs in nanomaterials and composite structures have opened new pathways for enhancing storage efficiency while maintaining material integrity over numerous charge-discharge cycles.
The primary objective of hydrogen storage materials research is to develop solutions that meet the U.S. Department of Energy's targets: 6.5 wt% system gravimetric capacity and 50 g/L volumetric capacity, with operational temperatures between -40°C and 60°C, and cycling durability of 1,500 cycles. These benchmarks are essential for making hydrogen competitive with conventional fuels in transportation and stationary applications.
Beyond these technical parameters, research aims to address broader challenges including cost reduction, scalable manufacturing processes, and integration with existing energy infrastructure. The development of materials that can operate under milder conditions without sacrificing performance represents a particularly important goal, as it would significantly reduce system complexity and energy requirements.
Strategic objectives also include understanding fundamental material behaviors at the atomic and molecular levels, which can inform rational design approaches for next-generation storage materials. This includes investigating reaction mechanisms, degradation pathways, and structure-property relationships that govern hydrogen absorption and desorption processes.
The ultimate goal extends beyond technical performance to encompass sustainability considerations, including the use of earth-abundant elements, environmentally benign synthesis methods, and end-of-life recyclability. These factors will be crucial in determining the long-term viability and widespread adoption of hydrogen storage technologies in a carbon-constrained world.
Market Analysis for Advanced Hydrogen Storage Solutions
The global hydrogen storage market is experiencing significant growth, driven by the increasing adoption of hydrogen as a clean energy carrier. As of 2023, the market was valued at approximately $15.4 billion, with projections indicating a compound annual growth rate (CAGR) of 9.7% through 2030, potentially reaching $28.1 billion by the end of the decade. This growth trajectory is primarily fueled by the global push toward decarbonization and the transition to renewable energy sources.
The demand for advanced hydrogen storage solutions spans multiple sectors, with transportation emerging as the dominant application segment. The automotive industry, particularly fuel cell electric vehicles (FCEVs), represents a significant market driver, with major manufacturers like Toyota, Hyundai, and Honda investing heavily in hydrogen technology. Additionally, industrial applications in sectors such as chemicals, refining, and metallurgy contribute substantially to market demand.
Geographically, Asia-Pacific leads the market, accounting for approximately 35% of global demand, with Japan and South Korea at the forefront of hydrogen technology adoption. Europe follows closely, driven by ambitious hydrogen strategies in countries like Germany, France, and the Netherlands. North America, particularly the United States, is showing accelerated growth with increasing government support for hydrogen infrastructure.
The market segmentation reveals distinct categories based on storage methods: physical storage (compressed gas, liquid hydrogen, and cryo-compressed) currently dominates with a 78% market share, while material-based storage (metal hydrides, chemical hydrogen storage, and adsorbent materials) is growing at a faster rate due to efficiency advantages and safety benefits.
Customer requirements are evolving rapidly, with end-users prioritizing higher gravimetric and volumetric storage densities, improved cycling stability, faster kinetics, and cost-effectiveness. The U.S. Department of Energy has established benchmarks for automotive applications, targeting 6.5 wt% gravimetric capacity and 50 g/L volumetric capacity, which are driving innovation in material-based storage solutions.
Market barriers include high costs associated with advanced materials development, technical challenges in achieving desired storage densities, and infrastructure limitations. However, these challenges are being addressed through increased R&D investments, with global funding for hydrogen storage research exceeding $2.3 billion in 2022, a 27% increase from the previous year.
The competitive landscape is characterized by a mix of established industrial gas companies (Air Liquide, Linde), specialized storage technology developers (Hexagon Purus, McPhy Energy), and materials science innovators (Hydrogenious LOHC Technologies, Hysilabs). Strategic partnerships between material developers and system integrators are becoming increasingly common, accelerating the commercialization of novel storage solutions.
The demand for advanced hydrogen storage solutions spans multiple sectors, with transportation emerging as the dominant application segment. The automotive industry, particularly fuel cell electric vehicles (FCEVs), represents a significant market driver, with major manufacturers like Toyota, Hyundai, and Honda investing heavily in hydrogen technology. Additionally, industrial applications in sectors such as chemicals, refining, and metallurgy contribute substantially to market demand.
Geographically, Asia-Pacific leads the market, accounting for approximately 35% of global demand, with Japan and South Korea at the forefront of hydrogen technology adoption. Europe follows closely, driven by ambitious hydrogen strategies in countries like Germany, France, and the Netherlands. North America, particularly the United States, is showing accelerated growth with increasing government support for hydrogen infrastructure.
The market segmentation reveals distinct categories based on storage methods: physical storage (compressed gas, liquid hydrogen, and cryo-compressed) currently dominates with a 78% market share, while material-based storage (metal hydrides, chemical hydrogen storage, and adsorbent materials) is growing at a faster rate due to efficiency advantages and safety benefits.
Customer requirements are evolving rapidly, with end-users prioritizing higher gravimetric and volumetric storage densities, improved cycling stability, faster kinetics, and cost-effectiveness. The U.S. Department of Energy has established benchmarks for automotive applications, targeting 6.5 wt% gravimetric capacity and 50 g/L volumetric capacity, which are driving innovation in material-based storage solutions.
Market barriers include high costs associated with advanced materials development, technical challenges in achieving desired storage densities, and infrastructure limitations. However, these challenges are being addressed through increased R&D investments, with global funding for hydrogen storage research exceeding $2.3 billion in 2022, a 27% increase from the previous year.
The competitive landscape is characterized by a mix of established industrial gas companies (Air Liquide, Linde), specialized storage technology developers (Hexagon Purus, McPhy Energy), and materials science innovators (Hydrogenious LOHC Technologies, Hysilabs). Strategic partnerships between material developers and system integrators are becoming increasingly common, accelerating the commercialization of novel storage solutions.
Current Challenges in Hydrogen Storage Technology
Despite significant advancements in hydrogen storage technologies, several critical challenges continue to impede widespread adoption of hydrogen as a mainstream energy carrier. The volumetric energy density of hydrogen remains a fundamental obstacle, as hydrogen gas occupies substantially more space than conventional fuels, even when compressed to 700 bar or liquefied at cryogenic temperatures. This creates significant engineering challenges for mobile applications where space constraints are paramount.
Material-based storage systems face their own set of limitations. Metal hydrides, while offering good volumetric capacity, suffer from slow kinetics, requiring high temperatures for hydrogen release and absorption. This energy penalty significantly reduces overall system efficiency. Additionally, many promising metal hydride materials contain expensive rare earth elements, limiting their commercial viability for large-scale applications.
Thermodynamic constraints present another major hurdle. The ideal hydrogen storage material requires a delicate balance - binding hydrogen strongly enough for stable storage yet weakly enough to release it under practical operating conditions. Most current materials either bind hydrogen too strongly, necessitating impractical desorption temperatures, or too weakly, resulting in inadequate storage capacity at ambient conditions.
Cycling stability represents a persistent challenge across all material-based storage systems. Repeated hydrogen absorption and desorption cycles often lead to material degradation, structural changes, and capacity loss over time. This degradation is particularly pronounced in complex hydrides and chemical hydrogen carriers, where side reactions can produce unwanted byproducts that accumulate with each cycle.
Contamination sensitivity further complicates practical implementation. Many advanced storage materials are highly reactive with atmospheric components, particularly oxygen and water vapor, requiring sophisticated handling protocols and protective measures that add complexity and cost to storage systems.
Heat management during charging and discharging cycles presents significant engineering challenges. The exothermic nature of hydrogen absorption and endothermic desorption processes necessitates efficient thermal management systems to maintain optimal operating conditions and prevent material degradation from localized overheating.
Finally, the lack of standardized testing protocols and performance metrics makes direct comparison between different storage technologies difficult, hampering systematic progress in the field. Researchers often report idealized laboratory results that fail to translate to real-world performance, creating a disconnect between fundamental research and practical applications.
Material-based storage systems face their own set of limitations. Metal hydrides, while offering good volumetric capacity, suffer from slow kinetics, requiring high temperatures for hydrogen release and absorption. This energy penalty significantly reduces overall system efficiency. Additionally, many promising metal hydride materials contain expensive rare earth elements, limiting their commercial viability for large-scale applications.
Thermodynamic constraints present another major hurdle. The ideal hydrogen storage material requires a delicate balance - binding hydrogen strongly enough for stable storage yet weakly enough to release it under practical operating conditions. Most current materials either bind hydrogen too strongly, necessitating impractical desorption temperatures, or too weakly, resulting in inadequate storage capacity at ambient conditions.
Cycling stability represents a persistent challenge across all material-based storage systems. Repeated hydrogen absorption and desorption cycles often lead to material degradation, structural changes, and capacity loss over time. This degradation is particularly pronounced in complex hydrides and chemical hydrogen carriers, where side reactions can produce unwanted byproducts that accumulate with each cycle.
Contamination sensitivity further complicates practical implementation. Many advanced storage materials are highly reactive with atmospheric components, particularly oxygen and water vapor, requiring sophisticated handling protocols and protective measures that add complexity and cost to storage systems.
Heat management during charging and discharging cycles presents significant engineering challenges. The exothermic nature of hydrogen absorption and endothermic desorption processes necessitates efficient thermal management systems to maintain optimal operating conditions and prevent material degradation from localized overheating.
Finally, the lack of standardized testing protocols and performance metrics makes direct comparison between different storage technologies difficult, hampering systematic progress in the field. Researchers often report idealized laboratory results that fail to translate to real-world performance, creating a disconnect between fundamental research and practical applications.
Current Material Solutions for Hydrogen Storage
01 Metal hydride-based hydrogen storage materials
Metal hydrides are promising materials for hydrogen storage due to their high volumetric hydrogen density. These materials form chemical bonds with hydrogen, allowing for reversible storage through absorption and desorption processes. Various metal hydrides, including those based on magnesium, aluminum, and transition metals, have been developed to improve storage efficiency and stability. Researchers have focused on enhancing the kinetics of hydrogen uptake and release while maintaining structural integrity over multiple cycles.- Metal hydride-based hydrogen storage materials: Metal hydrides are promising materials for hydrogen storage due to their high volumetric hydrogen density. These materials form chemical bonds with hydrogen, allowing for efficient storage and release under appropriate temperature and pressure conditions. Various metal hydrides, including magnesium-based, aluminum-based, and transition metal-based compounds, have been developed to improve storage capacity, kinetics, and stability during cycling. Modifications such as alloying, nanostructuring, and catalyst addition can enhance the efficiency and stability of these materials.
- Carbon-based hydrogen storage materials: Carbon-based materials, including carbon nanotubes, graphene, and activated carbon, offer advantages for hydrogen storage due to their lightweight nature and tunable porosity. These materials primarily store hydrogen through physisorption mechanisms, with hydrogen molecules adsorbing onto the surface of the carbon structures. Research focuses on increasing the specific surface area, optimizing pore size distribution, and functionalizing carbon surfaces to enhance hydrogen uptake capacity and stability. The efficiency of these materials can be improved by operating at cryogenic temperatures or by doping with metal particles.
- Complex hydrides for high-capacity hydrogen storage: Complex hydrides, including alanates, borohydrides, and amides, offer high theoretical hydrogen storage capacities. These materials store hydrogen through complex chemical bonds and can release hydrogen through thermal decomposition or hydrolysis reactions. Research focuses on improving their reversibility, reducing dehydrogenation temperatures, and enhancing cycling stability. Catalysts and destabilization strategies are employed to improve the kinetics and thermodynamics of hydrogen absorption and desorption processes, making these materials more practical for real-world applications.
- Nanostructured materials for enhanced hydrogen storage: Nanostructuring of hydrogen storage materials significantly improves their performance by shortening diffusion paths, increasing surface area, and creating additional active sites for hydrogen interaction. Nanoscale engineering approaches include creating core-shell structures, nanocomposites, and hierarchical porous frameworks. These nanostructured materials demonstrate faster kinetics, improved cycling stability, and enhanced resistance to degradation mechanisms such as particle agglomeration and oxidation. Various synthesis methods, including ball milling, chemical vapor deposition, and template-assisted growth, are used to create these advanced materials.
- Composite and hybrid hydrogen storage systems: Composite and hybrid hydrogen storage systems combine different types of storage materials to overcome the limitations of individual components. These systems may integrate metal hydrides with carbon materials, complex hydrides with catalysts, or chemical storage materials with physical adsorbents. The synergistic effects between components can lead to improved hydrogen capacity, enhanced kinetics, better thermal management, and increased cycling stability. These hybrid approaches often involve careful engineering of interfaces between different materials and optimization of operating conditions to maximize overall system performance.
02 Carbon-based hydrogen storage materials
Carbon-based materials, including carbon nanotubes, graphene, and activated carbon, offer advantages for hydrogen storage due to their lightweight nature and high surface area. These materials primarily store hydrogen through physisorption mechanisms, where hydrogen molecules adhere to the material surface without forming chemical bonds. Modifications to carbon structures, such as doping with metals or creating defects, can enhance hydrogen binding energy and improve storage capacity while maintaining good cycling stability.Expand Specific Solutions03 Complex hydrides and borohydrides for hydrogen storage
Complex hydrides and borohydrides represent a class of materials with high hydrogen content by weight. These compounds, including lithium borohydride, sodium alanate, and magnesium borohydride, can store hydrogen through chemical bonding in complex structures. Research has focused on addressing their challenges related to high dehydrogenation temperatures and slow kinetics. Catalysts and nanostructuring approaches have been employed to improve both the efficiency of hydrogen release and the stability during multiple absorption-desorption cycles.Expand Specific Solutions04 Nanostructured and composite hydrogen storage materials
Nanostructuring and creating composite materials represent effective approaches to enhance hydrogen storage performance. By reducing particle size to nanoscale dimensions, researchers have achieved faster kinetics and lower operating temperatures. Composite materials combining different storage mechanisms (such as metal hydrides with carbon materials) can leverage the advantages of multiple systems. These engineered materials demonstrate improved hydrogen uptake rates, enhanced cycling stability, and better thermal management during the absorption and desorption processes.Expand Specific Solutions05 Catalyst-enhanced hydrogen storage systems
Catalysts play a crucial role in improving the efficiency and stability of hydrogen storage materials. Various catalysts, including transition metals, metal oxides, and nanoparticles, have been incorporated into storage materials to lower activation energies for hydrogen absorption and desorption. These catalytic additives can significantly enhance reaction kinetics, reduce operating temperatures, and improve cycling performance. Research has focused on identifying optimal catalyst compositions and distributions to maximize their effectiveness while minimizing the amount needed.Expand Specific Solutions
Key Industry Players in Hydrogen Storage Research
The hydrogen storage materials market is in a growth phase, characterized by increasing demand for efficient and stable solutions to support the hydrogen economy. The market is projected to expand significantly as hydrogen gains traction as a clean energy carrier. Technologically, the field is advancing but still faces challenges in achieving optimal storage density and stability. Key players include automotive giants like Hyundai, Kia, and Nissan, who are investing heavily in hydrogen fuel cell vehicles. Research institutions such as Zhejiang University and University of Washington collaborate with industrial partners like Johnson Matthey and CIMC Enric to develop advanced materials. Specialized companies including Santoku Corp and Jiaxing Zheda Parken focus on rare earth hydrogen storage alloys, while government agencies like Japan Science & Technology Agency provide crucial funding support for breakthrough technologies.
Hyundai Motor Co., Ltd.
Technical Solution: Hyundai has developed a comprehensive hydrogen storage solution centered around advanced metal hydride systems. Their proprietary Ti-Cr-Mn based alloys demonstrate hydrogen capacities of 1.8-2.2 wt% with rapid absorption/desorption kinetics (90% capacity in under 5 minutes). Hyundai's innovation includes nano-structuring techniques that reduce activation energy barriers by approximately 30% compared to conventional materials. Their system operates at moderate pressures (30-50 bar) and temperatures (25-85°C), making it suitable for automotive applications. Hyundai has also pioneered heat management systems that utilize waste heat from fuel cells to drive hydrogen desorption, improving overall system efficiency. The company's latest generation materials incorporate rare earth dopants that enhance cycling stability, maintaining over 90% capacity after 1,000 cycles.
Strengths: Excellent cycling stability, fast kinetics at practical operating temperatures, and integrated thermal management with fuel cell systems. Weaknesses: Lower gravimetric capacity compared to some competing technologies, reliance on relatively expensive transition metals, and performance sensitivity to impurities in hydrogen feedstock.
GM Global Technology Operations LLC
Technical Solution: GM has developed advanced metal-organic frameworks (MOFs) for hydrogen storage with exceptional surface areas exceeding 6,000 m²/g. Their proprietary MOF structures feature optimized pore sizes (6-10 Å) specifically designed for hydrogen molecule adsorption at moderate pressures. GM's approach incorporates strategic metal doping with palladium and platinum to enhance hydrogen binding energy without excessive heat release during adsorption. Their system operates at moderate temperatures (77-150K) and pressures (30-100 bar), achieving gravimetric capacities of 7-8 wt% and volumetric densities approaching 40 g/L. GM has also pioneered composite systems that combine MOFs with cryocompression techniques to meet DOE targets for vehicular applications.
Strengths: Exceptional surface area optimization, strategic metal doping for improved binding energies, and integrated systems approach combining multiple storage mechanisms. Weaknesses: Still requires cryogenic temperatures for optimal performance, presenting challenges for practical on-board vehicle applications and requiring sophisticated thermal management systems.
Critical Patents and Innovations in Storage Materials
Hydrogen storage materials having excellent kinetics, capacity, and cycle stability
PatentInactiveUS7344676B2
Innovation
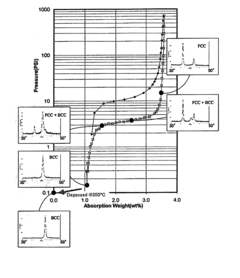
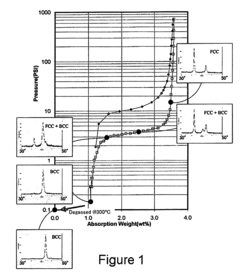
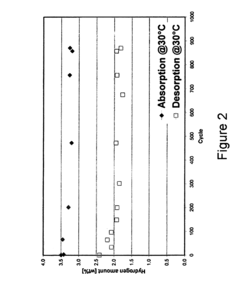
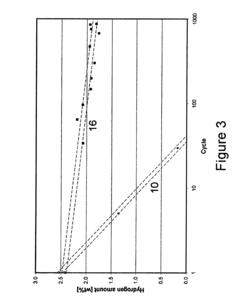
- A hydrogen storage alloy with a single-phase body-centered cubic (BCC) structure, composed of titanium, vanadium, and chromium, along with modifier elements, that absorbs and desorbs hydrogen rapidly and reversibly, maintaining high capacity and stability across multiple cycles, with a lattice constant range of 3.015 to 3.045 angstroms, and a quenching process to inhibit oxide formation.
High Capacity Hydrogen Storage through Selective Nano-Confined and Localized Hydrogen Hydrates
PatentPendingUS20240336477A1
Innovation
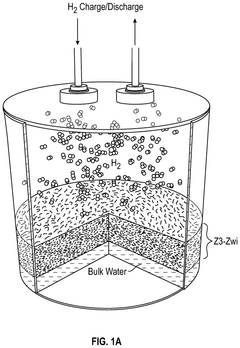
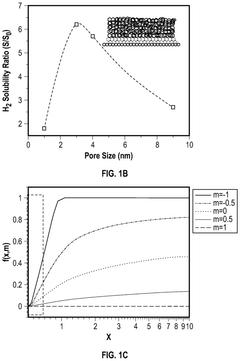
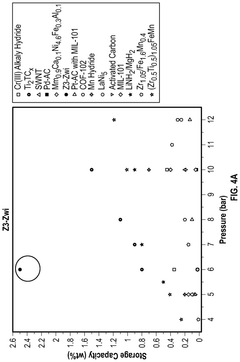
- A hydrogen storage device utilizing a host framework material, such as zeolite or mesoporous carbon, to form hydrogen hydrates through selective nano-confined and localized hydrogen absorption, enabling rapid charging/discharging and ambient temperature operation.
Regulatory Framework for Hydrogen Energy Systems
The regulatory landscape for hydrogen energy systems is evolving rapidly as governments worldwide recognize hydrogen's potential in decarbonization strategies. These frameworks directly impact material innovations for hydrogen storage by establishing safety standards, performance requirements, and incentive structures that guide research priorities.
International standards organizations such as ISO and IEC have developed specific technical committees focused on hydrogen technologies, with ISO/TC 197 addressing hydrogen storage materials specifically. These standards define testing protocols for storage materials' efficiency, cycling stability, and safety characteristics, creating benchmarks that new material innovations must meet.
In the United States, the Department of Energy has established technical targets for onboard hydrogen storage systems, including gravimetric capacity (6.5 wt% system-level), volumetric capacity (50 g H₂/L), and operational durability (1500 cycles). These targets have significantly influenced research directions in metal hydrides, complex hydrides, and nanoporous materials development.
The European Union's Hydrogen Strategy incorporates regulatory frameworks through the European Green Deal, with specific provisions addressing storage material certification. The EU's REACH regulations also impact material selection by requiring comprehensive safety assessments of novel storage compounds, particularly those containing rare earth elements or potentially hazardous catalysts.
Safety regulations present both challenges and opportunities for material innovation. Codes such as NFPA 2 (Hydrogen Technologies Code) establish strict parameters for material containment systems, thermal management, and pressure tolerances. These requirements have accelerated research into composite materials with enhanced thermal conductivity and pressure resistance properties.
Carbon intensity regulations are increasingly influencing hydrogen storage material development. Life cycle assessment requirements in jurisdictions like California and the EU demand consideration of embodied carbon in storage materials, driving interest in biomass-derived carbon scaffolds and environmentally benign synthesis routes for metal-organic frameworks.
Intellectual property regulations also shape the innovation landscape, with patent activity for hydrogen storage materials showing geographic concentration in regions with supportive regulatory frameworks. Cross-licensing agreements between major industrial players have emerged as a strategy to navigate complex international regulatory requirements while accelerating commercialization timelines.
As regulatory frameworks continue to evolve, they will increasingly emphasize whole-system approaches that consider material efficiency alongside safety, environmental impact, and economic viability, creating both constraints and opportunities for next-generation hydrogen storage materials.
International standards organizations such as ISO and IEC have developed specific technical committees focused on hydrogen technologies, with ISO/TC 197 addressing hydrogen storage materials specifically. These standards define testing protocols for storage materials' efficiency, cycling stability, and safety characteristics, creating benchmarks that new material innovations must meet.
In the United States, the Department of Energy has established technical targets for onboard hydrogen storage systems, including gravimetric capacity (6.5 wt% system-level), volumetric capacity (50 g H₂/L), and operational durability (1500 cycles). These targets have significantly influenced research directions in metal hydrides, complex hydrides, and nanoporous materials development.
The European Union's Hydrogen Strategy incorporates regulatory frameworks through the European Green Deal, with specific provisions addressing storage material certification. The EU's REACH regulations also impact material selection by requiring comprehensive safety assessments of novel storage compounds, particularly those containing rare earth elements or potentially hazardous catalysts.
Safety regulations present both challenges and opportunities for material innovation. Codes such as NFPA 2 (Hydrogen Technologies Code) establish strict parameters for material containment systems, thermal management, and pressure tolerances. These requirements have accelerated research into composite materials with enhanced thermal conductivity and pressure resistance properties.
Carbon intensity regulations are increasingly influencing hydrogen storage material development. Life cycle assessment requirements in jurisdictions like California and the EU demand consideration of embodied carbon in storage materials, driving interest in biomass-derived carbon scaffolds and environmentally benign synthesis routes for metal-organic frameworks.
Intellectual property regulations also shape the innovation landscape, with patent activity for hydrogen storage materials showing geographic concentration in regions with supportive regulatory frameworks. Cross-licensing agreements between major industrial players have emerged as a strategy to navigate complex international regulatory requirements while accelerating commercialization timelines.
As regulatory frameworks continue to evolve, they will increasingly emphasize whole-system approaches that consider material efficiency alongside safety, environmental impact, and economic viability, creating both constraints and opportunities for next-generation hydrogen storage materials.
Environmental Impact Assessment of Storage Materials
The environmental impact of hydrogen storage materials extends far beyond their primary function, encompassing their entire lifecycle from production to disposal. Traditional hydrogen storage methods, particularly those involving high-pressure tanks or cryogenic liquefaction, present significant environmental challenges including high energy consumption and safety risks. In contrast, advanced material-based storage solutions offer promising pathways to reduce these environmental footprints.
Metal hydrides, metal-organic frameworks (MOFs), and carbon-based nanomaterials used for hydrogen storage demonstrate varying degrees of environmental impact. The production of these materials often involves energy-intensive processes and potentially toxic chemicals. For instance, the synthesis of high-surface-area MOFs typically requires organic solvents that may pose environmental hazards if not properly managed. Similarly, the extraction and processing of rare earth elements for certain metal hydrides can lead to substantial land degradation and water pollution.
Life cycle assessment (LCA) studies reveal that the environmental benefits of advanced hydrogen storage materials generally outweigh their production impacts when considering the full system perspective. These materials enable more efficient hydrogen utilization, reducing the overall energy requirements for hydrogen storage by eliminating the need for compression or liquefaction. This efficiency gain translates to lower greenhouse gas emissions across the hydrogen value chain.
Water consumption represents another critical environmental consideration. Some hydrogen storage materials require significant water inputs during production, while others may release water during hydrogen absorption/desorption cycles. Recent innovations have focused on developing materials with minimal water requirements and closed-loop manufacturing processes to address this concern.
The recyclability and end-of-life management of storage materials significantly influence their overall environmental sustainability. Materials designed with circular economy principles demonstrate superior environmental performance. For example, certain metal hydrides can be regenerated multiple times before requiring replacement, substantially reducing waste generation and resource consumption compared to single-use alternatives.
Emerging research indicates that biomass-derived carbon materials for hydrogen storage offer particularly promising environmental profiles. These materials utilize renewable feedstocks and can be produced through relatively low-impact processes. Additionally, their biodegradability provides advantages for end-of-life management, though performance characteristics still require optimization to match synthetic alternatives.
The environmental impact assessment must also consider potential material leaching or degradation during operation. Stability improvements in newer hydrogen storage materials have significantly reduced these risks, with encapsulation techniques and composite structures effectively containing potentially harmful components while maintaining hydrogen storage performance.
Metal hydrides, metal-organic frameworks (MOFs), and carbon-based nanomaterials used for hydrogen storage demonstrate varying degrees of environmental impact. The production of these materials often involves energy-intensive processes and potentially toxic chemicals. For instance, the synthesis of high-surface-area MOFs typically requires organic solvents that may pose environmental hazards if not properly managed. Similarly, the extraction and processing of rare earth elements for certain metal hydrides can lead to substantial land degradation and water pollution.
Life cycle assessment (LCA) studies reveal that the environmental benefits of advanced hydrogen storage materials generally outweigh their production impacts when considering the full system perspective. These materials enable more efficient hydrogen utilization, reducing the overall energy requirements for hydrogen storage by eliminating the need for compression or liquefaction. This efficiency gain translates to lower greenhouse gas emissions across the hydrogen value chain.
Water consumption represents another critical environmental consideration. Some hydrogen storage materials require significant water inputs during production, while others may release water during hydrogen absorption/desorption cycles. Recent innovations have focused on developing materials with minimal water requirements and closed-loop manufacturing processes to address this concern.
The recyclability and end-of-life management of storage materials significantly influence their overall environmental sustainability. Materials designed with circular economy principles demonstrate superior environmental performance. For example, certain metal hydrides can be regenerated multiple times before requiring replacement, substantially reducing waste generation and resource consumption compared to single-use alternatives.
Emerging research indicates that biomass-derived carbon materials for hydrogen storage offer particularly promising environmental profiles. These materials utilize renewable feedstocks and can be produced through relatively low-impact processes. Additionally, their biodegradability provides advantages for end-of-life management, though performance characteristics still require optimization to match synthetic alternatives.
The environmental impact assessment must also consider potential material leaching or degradation during operation. Stability improvements in newer hydrogen storage materials have significantly reduced these risks, with encapsulation techniques and composite structures effectively containing potentially harmful components while maintaining hydrogen storage performance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!