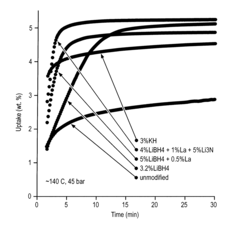
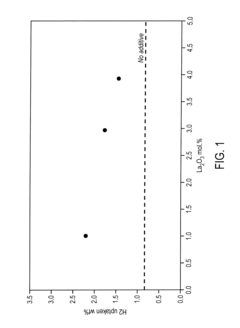
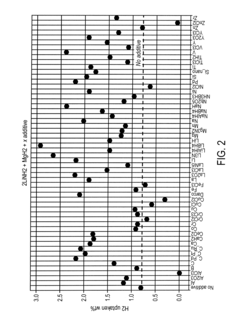
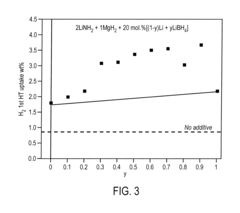
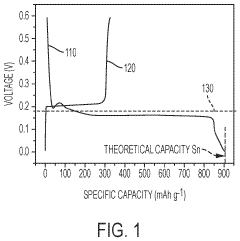
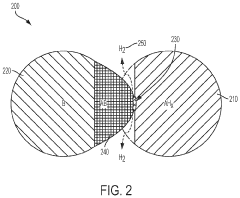
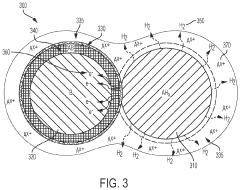
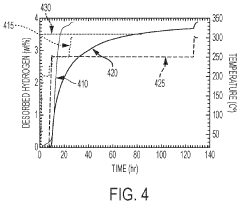
What are the key material parameters affecting Hydrogen storage materials
SEP 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogen Storage Materials Background and Objectives
Hydrogen storage has emerged as a critical component in the global transition towards sustainable energy systems. The journey of hydrogen storage technology dates back to the early 20th century, but significant advancements have primarily occurred in the past few decades as environmental concerns and energy security issues have intensified. The evolution of hydrogen storage materials has progressed from conventional physical storage methods to more sophisticated material-based approaches, reflecting the growing recognition of hydrogen's potential as a clean energy carrier.
The fundamental challenge in hydrogen storage lies in achieving high volumetric and gravimetric storage capacities while maintaining practical operating conditions. Material parameters such as binding energy, surface area, pore structure, and catalytic properties have proven to be decisive factors affecting storage performance. Recent technological breakthroughs in nanomaterials and composite structures have opened new pathways for enhancing these parameters, pushing the boundaries of what is theoretically achievable.
Current research objectives in hydrogen storage materials focus on meeting the U.S. Department of Energy's targets, which include achieving a system gravimetric capacity of 6.5 wt% and a volumetric capacity of 50 g/L by 2025. These ambitious targets necessitate innovative approaches to material design and synthesis. The scientific community is increasingly exploring multi-functional materials that can simultaneously address multiple challenges in hydrogen storage, including thermodynamics, kinetics, and system integration.
The global research landscape shows a concentrated effort on developing materials that operate under moderate temperature and pressure conditions. Metal-organic frameworks (MOFs), complex metal hydrides, and functionalized carbon nanostructures represent the frontier of current research. Each material class presents unique advantages and limitations, with their performance heavily dependent on specific material parameters such as crystal structure, chemical composition, and surface properties.
Looking forward, the technological trajectory points toward hybrid systems that combine different storage mechanisms to overcome the limitations of individual approaches. The ultimate goal remains developing materials that enable safe, efficient, and economical hydrogen storage for various applications, from portable devices to stationary power systems and transportation. This requires a comprehensive understanding of how key material parameters interact and influence overall system performance under real-world conditions.
The interdisciplinary nature of hydrogen storage research necessitates collaboration across materials science, chemistry, physics, and engineering disciplines. As computational capabilities advance, predictive modeling is increasingly guiding experimental efforts, accelerating the discovery and optimization of novel hydrogen storage materials with enhanced performance characteristics.
The fundamental challenge in hydrogen storage lies in achieving high volumetric and gravimetric storage capacities while maintaining practical operating conditions. Material parameters such as binding energy, surface area, pore structure, and catalytic properties have proven to be decisive factors affecting storage performance. Recent technological breakthroughs in nanomaterials and composite structures have opened new pathways for enhancing these parameters, pushing the boundaries of what is theoretically achievable.
Current research objectives in hydrogen storage materials focus on meeting the U.S. Department of Energy's targets, which include achieving a system gravimetric capacity of 6.5 wt% and a volumetric capacity of 50 g/L by 2025. These ambitious targets necessitate innovative approaches to material design and synthesis. The scientific community is increasingly exploring multi-functional materials that can simultaneously address multiple challenges in hydrogen storage, including thermodynamics, kinetics, and system integration.
The global research landscape shows a concentrated effort on developing materials that operate under moderate temperature and pressure conditions. Metal-organic frameworks (MOFs), complex metal hydrides, and functionalized carbon nanostructures represent the frontier of current research. Each material class presents unique advantages and limitations, with their performance heavily dependent on specific material parameters such as crystal structure, chemical composition, and surface properties.
Looking forward, the technological trajectory points toward hybrid systems that combine different storage mechanisms to overcome the limitations of individual approaches. The ultimate goal remains developing materials that enable safe, efficient, and economical hydrogen storage for various applications, from portable devices to stationary power systems and transportation. This requires a comprehensive understanding of how key material parameters interact and influence overall system performance under real-world conditions.
The interdisciplinary nature of hydrogen storage research necessitates collaboration across materials science, chemistry, physics, and engineering disciplines. As computational capabilities advance, predictive modeling is increasingly guiding experimental efforts, accelerating the discovery and optimization of novel hydrogen storage materials with enhanced performance characteristics.
Market Analysis for Hydrogen Storage Technologies
The global hydrogen storage market is experiencing significant growth, driven by the increasing focus on clean energy solutions and the transition away from fossil fuels. Currently valued at approximately $14.8 billion in 2023, the market is projected to reach $31.4 billion by 2030, representing a compound annual growth rate (CAGR) of 11.3%. This growth trajectory is primarily fueled by governmental policies promoting hydrogen as a key component of future energy systems, particularly in regions like Europe, Japan, South Korea, and parts of North America.
The market segmentation for hydrogen storage technologies reveals distinct categories based on storage methods: physical-based storage (including compression, liquefaction, and adsorption) currently dominates with about 65% market share, while material-based storage (metal hydrides, chemical hydrides, and liquid organic hydrogen carriers) accounts for approximately 35%. Among these, compressed hydrogen storage represents the largest segment due to its technological maturity and established infrastructure.
From an application perspective, transportation emerges as the fastest-growing sector, with a projected CAGR of 13.7% through 2030. This is largely attributed to the increasing adoption of hydrogen fuel cell vehicles in commercial fleets and the gradual expansion of hydrogen refueling infrastructure. Stationary power storage represents the second-largest application segment, growing steadily at 10.2% annually.
Regional analysis indicates that Asia-Pacific currently leads the market with approximately 42% share, followed by Europe (31%) and North America (21%). China, Japan, and South Korea are particularly aggressive in developing hydrogen infrastructure, while Germany leads European investments in this technology.
Key market drivers include stringent emission regulations, declining renewable energy costs enabling economical green hydrogen production, and increasing industrial demand for clean hydrogen. However, significant barriers remain, including high costs associated with material development for efficient hydrogen storage, limited infrastructure, and safety concerns related to hydrogen handling.
The competitive landscape features established industrial gas companies like Air Liquide and Linde, specialized hydrogen technology firms such as Hexagon Purus and McPhy Energy, and automotive manufacturers investing heavily in hydrogen fuel cell technology, including Toyota, Hyundai, and BMW. Recent market trends show increasing strategic partnerships between material science companies and energy providers to develop advanced storage materials with improved capacity and safety profiles.
The market segmentation for hydrogen storage technologies reveals distinct categories based on storage methods: physical-based storage (including compression, liquefaction, and adsorption) currently dominates with about 65% market share, while material-based storage (metal hydrides, chemical hydrides, and liquid organic hydrogen carriers) accounts for approximately 35%. Among these, compressed hydrogen storage represents the largest segment due to its technological maturity and established infrastructure.
From an application perspective, transportation emerges as the fastest-growing sector, with a projected CAGR of 13.7% through 2030. This is largely attributed to the increasing adoption of hydrogen fuel cell vehicles in commercial fleets and the gradual expansion of hydrogen refueling infrastructure. Stationary power storage represents the second-largest application segment, growing steadily at 10.2% annually.
Regional analysis indicates that Asia-Pacific currently leads the market with approximately 42% share, followed by Europe (31%) and North America (21%). China, Japan, and South Korea are particularly aggressive in developing hydrogen infrastructure, while Germany leads European investments in this technology.
Key market drivers include stringent emission regulations, declining renewable energy costs enabling economical green hydrogen production, and increasing industrial demand for clean hydrogen. However, significant barriers remain, including high costs associated with material development for efficient hydrogen storage, limited infrastructure, and safety concerns related to hydrogen handling.
The competitive landscape features established industrial gas companies like Air Liquide and Linde, specialized hydrogen technology firms such as Hexagon Purus and McPhy Energy, and automotive manufacturers investing heavily in hydrogen fuel cell technology, including Toyota, Hyundai, and BMW. Recent market trends show increasing strategic partnerships between material science companies and energy providers to develop advanced storage materials with improved capacity and safety profiles.
Current Challenges in Hydrogen Storage Material Development
Despite significant advancements in hydrogen storage technologies, several critical challenges continue to impede the widespread adoption of hydrogen as a mainstream energy carrier. The development of efficient hydrogen storage materials faces multifaceted obstacles that span scientific, engineering, and economic domains.
Material stability represents a primary challenge, with many promising storage materials exhibiting degradation after multiple hydrogen absorption-desorption cycles. This cycling stability issue is particularly pronounced in complex hydrides and chemical hydrogen storage materials, where structural changes during hydrogen uptake and release can lead to capacity loss over time.
Kinetics limitations present another significant barrier. Many materials with theoretically high storage capacities suffer from slow hydrogen absorption and desorption rates, particularly at practical operating temperatures. This kinetic constraint necessitates elevated temperatures or sophisticated catalysts to achieve acceptable charging and discharging rates, adding complexity and cost to storage systems.
Thermodynamic constraints further complicate material development. The ideal hydrogen storage material must bind hydrogen strongly enough for stable storage yet release it easily when needed. Finding materials with this optimal binding energy window (approximately 20-40 kJ/mol H₂) remains challenging, as most materials either bind hydrogen too strongly, requiring high desorption temperatures, or too weakly, resulting in low storage capacities under practical conditions.
Heat management during hydrogen charging and discharging processes presents additional engineering challenges. The exothermic nature of hydrogen absorption and endothermic character of desorption necessitates effective thermal management systems, adding weight and complexity to storage solutions.
Material synthesis and scalability issues also persist. Many promising laboratory-scale materials involve complex synthesis procedures using expensive precursors or requiring precise control of reaction conditions, making large-scale production economically unfeasible.
Contamination sensitivity represents another significant hurdle. Many advanced storage materials, particularly those based on complex hydrides or nanoporous structures, exhibit performance degradation when exposed to common impurities in hydrogen gas streams, such as oxygen, water vapor, or carbon monoxide.
Finally, comprehensive characterization of hydrogen storage materials remains challenging. The dynamic nature of hydrogen storage processes and the light weight of hydrogen atoms make in-situ monitoring of structural changes and hydrogen diffusion pathways difficult, requiring sophisticated analytical techniques and often limiting our fundamental understanding of material behavior.
Material stability represents a primary challenge, with many promising storage materials exhibiting degradation after multiple hydrogen absorption-desorption cycles. This cycling stability issue is particularly pronounced in complex hydrides and chemical hydrogen storage materials, where structural changes during hydrogen uptake and release can lead to capacity loss over time.
Kinetics limitations present another significant barrier. Many materials with theoretically high storage capacities suffer from slow hydrogen absorption and desorption rates, particularly at practical operating temperatures. This kinetic constraint necessitates elevated temperatures or sophisticated catalysts to achieve acceptable charging and discharging rates, adding complexity and cost to storage systems.
Thermodynamic constraints further complicate material development. The ideal hydrogen storage material must bind hydrogen strongly enough for stable storage yet release it easily when needed. Finding materials with this optimal binding energy window (approximately 20-40 kJ/mol H₂) remains challenging, as most materials either bind hydrogen too strongly, requiring high desorption temperatures, or too weakly, resulting in low storage capacities under practical conditions.
Heat management during hydrogen charging and discharging processes presents additional engineering challenges. The exothermic nature of hydrogen absorption and endothermic character of desorption necessitates effective thermal management systems, adding weight and complexity to storage solutions.
Material synthesis and scalability issues also persist. Many promising laboratory-scale materials involve complex synthesis procedures using expensive precursors or requiring precise control of reaction conditions, making large-scale production economically unfeasible.
Contamination sensitivity represents another significant hurdle. Many advanced storage materials, particularly those based on complex hydrides or nanoporous structures, exhibit performance degradation when exposed to common impurities in hydrogen gas streams, such as oxygen, water vapor, or carbon monoxide.
Finally, comprehensive characterization of hydrogen storage materials remains challenging. The dynamic nature of hydrogen storage processes and the light weight of hydrogen atoms make in-situ monitoring of structural changes and hydrogen diffusion pathways difficult, requiring sophisticated analytical techniques and often limiting our fundamental understanding of material behavior.
Current Material Solutions for Hydrogen Storage
01 Metal hydrides for hydrogen storage
Metal hydrides are compounds formed by hydrogen and metals that can store hydrogen at high densities. These materials can absorb and release hydrogen through chemical reactions, making them suitable for hydrogen storage applications. Key material parameters include hydrogen storage capacity, absorption/desorption kinetics, thermodynamic stability, and cycling durability. Various metal hydride compositions have been developed to optimize these parameters for practical hydrogen storage applications.- Metal hydride-based hydrogen storage materials: Metal hydrides are compounds formed by metals or metal alloys that can absorb and release hydrogen under specific conditions. These materials are characterized by their high volumetric hydrogen density and moderate operating temperatures. Key material parameters include hydrogen storage capacity, absorption/desorption kinetics, cycling stability, and thermal conductivity. Various metal hydride systems such as AB5, AB2, and AB compounds have been developed with different thermodynamic and kinetic properties for hydrogen storage applications.
- Carbon-based hydrogen storage materials: Carbon-based materials including activated carbon, carbon nanotubes, graphene, and carbon aerogels are being investigated for hydrogen storage. These materials store hydrogen through physisorption mechanisms, with key parameters including specific surface area, pore size distribution, and binding energy. The advantages of carbon-based materials include lightweight properties, good cycling stability, and fast kinetics, though they typically require low temperatures to achieve significant storage capacities. Surface modifications and doping with other elements can enhance their hydrogen storage performance.
- Complex hydrides for hydrogen storage: Complex hydrides, including alanates, borohydrides, and amides, offer high gravimetric hydrogen storage capacities. These materials store hydrogen through chemical bonds and release it through decomposition reactions. Critical material parameters include decomposition temperature, reaction enthalpy, hydrogen content, and reversibility. While these materials can achieve high theoretical storage capacities, challenges include high desorption temperatures, slow kinetics, and limited cycling stability. Catalysts and destabilization strategies are employed to improve their performance characteristics.
- Nanostructured hydrogen storage materials: Nanostructuring of hydrogen storage materials can significantly improve their performance by reducing diffusion distances and altering thermodynamic properties. Key parameters for nanostructured materials include particle size, morphology, surface area, and interface characteristics. Nanoconfinement and core-shell structures can modify the hydrogen sorption properties, leading to faster kinetics and altered thermodynamics. Various synthesis methods including ball milling, chemical vapor deposition, and template-assisted growth are used to create these nanostructured materials with enhanced hydrogen storage properties.
- Composite and hybrid hydrogen storage systems: Composite and hybrid hydrogen storage systems combine different types of storage materials to overcome limitations of individual materials. These systems integrate multiple storage mechanisms to achieve improved overall performance. Important parameters include system integration, heat management, and synergistic effects between components. Examples include metal hydride-carbon composites, reactive hydride composites, and liquid organic hydrogen carrier systems. These hybrid approaches aim to optimize hydrogen storage density, operating conditions, and system efficiency by leveraging the complementary properties of different materials.
02 Carbon-based hydrogen storage materials
Carbon-based materials such as carbon nanotubes, graphene, and activated carbon can store hydrogen through physisorption mechanisms. These materials offer advantages including lightweight structure, high surface area, and tunable porosity. Material parameters of interest include specific surface area, pore size distribution, adsorption enthalpy, and hydrogen uptake capacity. Modifications to carbon structures through doping or functionalization can enhance hydrogen storage performance by increasing binding energy with hydrogen molecules.Expand Specific Solutions03 Complex hydrides for hydrogen storage
Complex hydrides, including alanates, borohydrides, and amides, represent advanced hydrogen storage materials with high theoretical storage capacities. These materials store hydrogen through complex chemical bonds and can release hydrogen through thermal decomposition. Critical material parameters include decomposition temperature, hydrogen content, reversibility, and reaction kinetics. Catalysts are often incorporated to improve desorption properties and lower operating temperatures for practical applications.Expand Specific Solutions04 Composite and nanostructured hydrogen storage materials
Composite and nanostructured materials combine different hydrogen storage mechanisms to achieve enhanced performance. These materials often integrate multiple components such as metal hydrides with carbon materials or catalysts to improve kinetics and thermodynamics. Key material parameters include particle size, distribution of components, interface properties, and thermal conductivity. Nanostructuring can significantly improve hydrogen sorption kinetics by reducing diffusion distances and increasing active surface area for hydrogen interaction.Expand Specific Solutions05 Characterization and testing methods for hydrogen storage materials
Various techniques are employed to characterize and evaluate hydrogen storage materials, including volumetric and gravimetric measurements, temperature-programmed desorption, X-ray diffraction, and electron microscopy. These methods help determine critical material parameters such as hydrogen capacity, cycling stability, kinetics, and structural properties. Standardized testing protocols are essential for comparing different materials and assessing their suitability for practical hydrogen storage applications under various operating conditions.Expand Specific Solutions
Critical Material Parameters and Their Mechanisms
Hydrogen storage materials and related methods and systems
PatentInactiveUS8454855B1
Innovation
- Development of hydrogen storage materials with a mixed imide formula LiiMgiNkHl, combined with specific additives such as oxides, hydrides, nitrides, and supported metals, synthesized through ball milling, to achieve high gravimetric capacity, fast kinetics, and stability, allowing for efficient hydrogen absorption and retention under moderate temperatures and pressures.
Hydrogen storage materials containing liquid electrolytes
PatentActiveUS11050075B1
Innovation
- A hydrogen-storage material formulation comprising a solid hydrogen-storage material bonded ionically, covalently, or interstitially with a metal or metalloid, combined with a liquid electrolyte that is ionically conductive, enhancing hydrogen evolution rates and allowing for reversible dehydrogenation-hydrogenation cycles at practical temperatures and pressures.
Environmental Impact and Sustainability Considerations
The environmental impact of hydrogen storage materials extends far beyond their immediate application in energy systems. As these materials become increasingly central to the global energy transition, their full lifecycle environmental footprint demands rigorous assessment. The production processes for advanced hydrogen storage materials—particularly metal hydrides, complex hydrides, and nanoporous materials—often require energy-intensive manufacturing and rare earth elements, creating significant upstream environmental concerns. Mining operations for these elements frequently result in habitat disruption, water pollution, and substantial carbon emissions that must be factored into sustainability evaluations.
Material parameters directly influence environmental performance throughout the hydrogen storage lifecycle. Higher gravimetric and volumetric capacities reduce the material quantity needed for equivalent storage, thereby minimizing resource extraction impacts. Similarly, materials with lower operating temperatures and pressures decrease the energy required for hydrogen charging and discharging cycles, improving overall system efficiency and reducing operational carbon footprints.
Recyclability emerges as a critical parameter affecting long-term sustainability. Materials designed with end-of-life considerations demonstrate superior environmental profiles, particularly those allowing for component separation and recovery without energy-intensive processes. The durability parameter—measured through cycle stability—directly correlates with sustainability, as longer-lasting materials reduce replacement frequency and associated manufacturing impacts.
Water consumption presents another significant environmental consideration, particularly for hydrogen storage systems requiring cooling or those utilizing water-reactive materials. In water-scarce regions, this parameter becomes especially critical for deployment feasibility and ecological impact assessment.
The potential for toxic byproduct formation during degradation or disposal phases represents a substantial environmental risk factor. Materials engineered to minimize hazardous waste generation or leaching demonstrate superior environmental performance metrics, though comprehensive lifecycle analysis remains essential for accurate impact assessment.
Carbon intensity of manufacturing processes varies dramatically across different hydrogen storage material classes. Recent innovations in green synthesis pathways have demonstrated potential for reducing embedded carbon by up to 40% for certain metal-organic frameworks and composite materials, highlighting the importance of manufacturing parameter optimization for environmental sustainability.
As hydrogen infrastructure expands globally, these environmental considerations will increasingly influence material selection decisions, regulatory frameworks, and market adoption rates, underscoring the need for continued research into environmentally optimized material parameters for next-generation hydrogen storage solutions.
Material parameters directly influence environmental performance throughout the hydrogen storage lifecycle. Higher gravimetric and volumetric capacities reduce the material quantity needed for equivalent storage, thereby minimizing resource extraction impacts. Similarly, materials with lower operating temperatures and pressures decrease the energy required for hydrogen charging and discharging cycles, improving overall system efficiency and reducing operational carbon footprints.
Recyclability emerges as a critical parameter affecting long-term sustainability. Materials designed with end-of-life considerations demonstrate superior environmental profiles, particularly those allowing for component separation and recovery without energy-intensive processes. The durability parameter—measured through cycle stability—directly correlates with sustainability, as longer-lasting materials reduce replacement frequency and associated manufacturing impacts.
Water consumption presents another significant environmental consideration, particularly for hydrogen storage systems requiring cooling or those utilizing water-reactive materials. In water-scarce regions, this parameter becomes especially critical for deployment feasibility and ecological impact assessment.
The potential for toxic byproduct formation during degradation or disposal phases represents a substantial environmental risk factor. Materials engineered to minimize hazardous waste generation or leaching demonstrate superior environmental performance metrics, though comprehensive lifecycle analysis remains essential for accurate impact assessment.
Carbon intensity of manufacturing processes varies dramatically across different hydrogen storage material classes. Recent innovations in green synthesis pathways have demonstrated potential for reducing embedded carbon by up to 40% for certain metal-organic frameworks and composite materials, highlighting the importance of manufacturing parameter optimization for environmental sustainability.
As hydrogen infrastructure expands globally, these environmental considerations will increasingly influence material selection decisions, regulatory frameworks, and market adoption rates, underscoring the need for continued research into environmentally optimized material parameters for next-generation hydrogen storage solutions.
Safety Standards and Regulatory Framework
The safety standards and regulatory framework surrounding hydrogen storage materials are critical for the widespread adoption of hydrogen as an energy carrier. As hydrogen storage technologies advance, international organizations and national governments have established comprehensive guidelines that specifically address the unique material parameters affecting storage safety. The International Organization for Standardization (ISO) has developed ISO 16111 for hydrogen absorbed in reversible metal hydrides, which outlines specific requirements for material integrity, thermal stability, and pressure resistance based on the key material parameters.
National regulations, such as those from the U.S. Department of Energy and the European Commission's Regulations on Hydrogen and Fuel Cell Vehicles, have established threshold values for material degradation rates, thermal conductivity, and reaction kinetics in hydrogen storage systems. These regulations typically require storage materials to maintain structural integrity under repeated hydrogen absorption-desorption cycles, with specific focus on parameters like crystal structure stability and surface area consistency.
Risk assessment protocols mandated by these frameworks require extensive testing of material parameters under various environmental conditions. For instance, the SAE J2579 standard specifies that materials must undergo thermal cycling tests between -40°C and 85°C while maintaining hydrogen containment integrity. This directly addresses how temperature fluctuations affect critical material parameters such as lattice expansion and phase transitions in metal hydrides.
Certification processes for hydrogen storage materials involve rigorous evaluation of parameters including volumetric and gravimetric capacity, cycling stability, and impurity tolerance. The UN Global Technical Regulation No. 13 specifically addresses how material composition affects safety performance, requiring manufacturers to document the effects of trace elements on long-term storage stability and release characteristics.
Recent regulatory developments have begun incorporating emerging research on nanomaterial-based storage solutions, with specific provisions addressing potential risks associated with nanoparticle agglomeration and surface area changes during cycling. The Japanese High Pressure Gas Safety Act now includes specific clauses for composite materials and metal-organic frameworks used in hydrogen storage, focusing on their unique structural parameters and degradation mechanisms.
Harmonization efforts between different regulatory bodies are currently underway through the International Partnership for Hydrogen and Fuel Cells in the Economy (IPHE), which aims to standardize testing protocols for key material parameters across jurisdictions. This includes unified methodologies for measuring parameters such as hydrogen embrittlement susceptibility, thermal conductivity under loading conditions, and reaction enthalpy in various storage materials.
National regulations, such as those from the U.S. Department of Energy and the European Commission's Regulations on Hydrogen and Fuel Cell Vehicles, have established threshold values for material degradation rates, thermal conductivity, and reaction kinetics in hydrogen storage systems. These regulations typically require storage materials to maintain structural integrity under repeated hydrogen absorption-desorption cycles, with specific focus on parameters like crystal structure stability and surface area consistency.
Risk assessment protocols mandated by these frameworks require extensive testing of material parameters under various environmental conditions. For instance, the SAE J2579 standard specifies that materials must undergo thermal cycling tests between -40°C and 85°C while maintaining hydrogen containment integrity. This directly addresses how temperature fluctuations affect critical material parameters such as lattice expansion and phase transitions in metal hydrides.
Certification processes for hydrogen storage materials involve rigorous evaluation of parameters including volumetric and gravimetric capacity, cycling stability, and impurity tolerance. The UN Global Technical Regulation No. 13 specifically addresses how material composition affects safety performance, requiring manufacturers to document the effects of trace elements on long-term storage stability and release characteristics.
Recent regulatory developments have begun incorporating emerging research on nanomaterial-based storage solutions, with specific provisions addressing potential risks associated with nanoparticle agglomeration and surface area changes during cycling. The Japanese High Pressure Gas Safety Act now includes specific clauses for composite materials and metal-organic frameworks used in hydrogen storage, focusing on their unique structural parameters and degradation mechanisms.
Harmonization efforts between different regulatory bodies are currently underway through the International Partnership for Hydrogen and Fuel Cells in the Economy (IPHE), which aims to standardize testing protocols for key material parameters across jurisdictions. This includes unified methodologies for measuring parameters such as hydrogen embrittlement susceptibility, thermal conductivity under loading conditions, and reaction enthalpy in various storage materials.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!