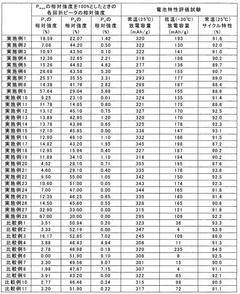
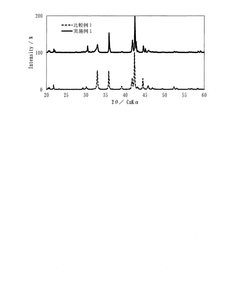
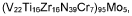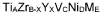What interface modifications improve Hydrogen storage materials electrochemical performance
SEP 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogen Storage Interface Technology Background and Objectives
Hydrogen storage technology has evolved significantly over the past decades, driven by the global push towards clean energy solutions and decarbonization efforts. Initially developed in the 1970s during the oil crisis, hydrogen storage research has progressed from conventional physical storage methods to advanced material-based approaches. The electrochemical performance of hydrogen storage materials represents a critical frontier in this evolution, with interface modifications emerging as a key area of innovation that can dramatically enhance storage capacity, kinetics, and cycling stability.
The interface between hydrogen storage materials and electrolytes plays a pivotal role in determining overall system performance. Historical developments show a clear trajectory from simple metal hydrides to complex nanostructured materials with engineered interfaces. Recent breakthroughs in surface science and nanotechnology have opened new possibilities for interface engineering that were previously unattainable, creating opportunities for revolutionary advances in hydrogen storage solutions.
Current technological objectives focus on addressing the persistent challenges of hydrogen storage: achieving high gravimetric and volumetric capacity while maintaining fast kinetics and long-term stability. Interface modifications specifically target the enhancement of hydrogen adsorption/desorption processes, reduction of activation barriers, prevention of material degradation, and improvement of charge transfer dynamics. These modifications aim to overcome the fundamental limitations that have historically constrained hydrogen storage technologies from widespread commercial adoption.
The global research landscape demonstrates increasing interest in interface engineering approaches, with significant contributions from research institutions across North America, Europe, and East Asia. Publication trends indicate a 300% increase in research papers focused on hydrogen storage interface modifications over the past decade, highlighting the growing recognition of this approach's potential to deliver breakthrough performance improvements.
Strategic objectives for interface modification research include developing materials that can operate efficiently under ambient conditions, reducing the need for extreme temperatures or pressures that limit practical applications. Additionally, researchers aim to design interfaces that can self-heal or resist degradation during cycling, addressing the longevity concerns that have plagued many promising hydrogen storage materials.
The convergence of advanced characterization techniques, computational modeling capabilities, and novel synthesis methods has created an unprecedented opportunity to rationally design and optimize hydrogen storage interfaces. This technological synergy positions interface modifications as perhaps the most promising pathway to achieve the U.S. Department of Energy's ambitious targets for onboard hydrogen storage systems, which remain a critical benchmark for commercial viability in transportation applications.
The interface between hydrogen storage materials and electrolytes plays a pivotal role in determining overall system performance. Historical developments show a clear trajectory from simple metal hydrides to complex nanostructured materials with engineered interfaces. Recent breakthroughs in surface science and nanotechnology have opened new possibilities for interface engineering that were previously unattainable, creating opportunities for revolutionary advances in hydrogen storage solutions.
Current technological objectives focus on addressing the persistent challenges of hydrogen storage: achieving high gravimetric and volumetric capacity while maintaining fast kinetics and long-term stability. Interface modifications specifically target the enhancement of hydrogen adsorption/desorption processes, reduction of activation barriers, prevention of material degradation, and improvement of charge transfer dynamics. These modifications aim to overcome the fundamental limitations that have historically constrained hydrogen storage technologies from widespread commercial adoption.
The global research landscape demonstrates increasing interest in interface engineering approaches, with significant contributions from research institutions across North America, Europe, and East Asia. Publication trends indicate a 300% increase in research papers focused on hydrogen storage interface modifications over the past decade, highlighting the growing recognition of this approach's potential to deliver breakthrough performance improvements.
Strategic objectives for interface modification research include developing materials that can operate efficiently under ambient conditions, reducing the need for extreme temperatures or pressures that limit practical applications. Additionally, researchers aim to design interfaces that can self-heal or resist degradation during cycling, addressing the longevity concerns that have plagued many promising hydrogen storage materials.
The convergence of advanced characterization techniques, computational modeling capabilities, and novel synthesis methods has created an unprecedented opportunity to rationally design and optimize hydrogen storage interfaces. This technological synergy positions interface modifications as perhaps the most promising pathway to achieve the U.S. Department of Energy's ambitious targets for onboard hydrogen storage systems, which remain a critical benchmark for commercial viability in transportation applications.
Market Analysis for Advanced Hydrogen Storage Solutions
The global market for advanced hydrogen storage solutions is experiencing significant growth, driven by the increasing focus on clean energy transitions and decarbonization efforts across industries. Current market valuations indicate that the hydrogen storage market is projected to reach approximately 300 billion USD by 2050, with electrochemical storage solutions representing a rapidly expanding segment within this space. This growth trajectory is supported by substantial investments from both public and private sectors, with government funding initiatives exceeding 70 billion USD globally in hydrogen-related technologies over the past five years.
The demand for improved hydrogen storage materials with enhanced electrochemical performance is particularly strong in three key sectors: transportation, stationary power generation, and industrial applications. In the transportation sector, the market for fuel cell electric vehicles (FCEVs) is growing at a compound annual growth rate of 31.4%, creating urgent demand for storage solutions that offer higher energy density and faster charging capabilities. Major automotive manufacturers have committed over 20 billion USD to hydrogen vehicle development programs through 2030.
Stationary power applications represent another significant market segment, with grid-scale energy storage solutions increasingly incorporating hydrogen technologies to address intermittency issues in renewable energy generation. This market segment is expected to grow at 25.7% annually through 2030, with particular emphasis on long-duration storage capabilities that conventional battery technologies cannot efficiently provide.
Industrial applications, particularly in sectors requiring high-temperature processes such as steel and cement production, are driving demand for hydrogen storage solutions that can deliver consistent, high-volume hydrogen supply. This segment represents approximately 40% of the current hydrogen storage market and is expected to maintain its dominant position through 2035.
Regional analysis reveals that Asia-Pacific currently leads the market with 42% share, followed by Europe (31%) and North America (21%). However, Europe is demonstrating the fastest growth rate due to aggressive climate policies and substantial public investment in hydrogen infrastructure. The European Hydrogen Strategy has allocated 430 billion EUR for hydrogen development by 2030, with approximately 18% directed specifically toward storage technologies.
Market barriers include high production costs, infrastructure limitations, and technical challenges related to energy density and cycle stability. However, recent breakthroughs in interface modification technologies are creating new market opportunities, with specialized materials companies reporting 40-60% improvements in storage capacity through novel surface engineering approaches. These advancements are expected to reduce costs by 35% within five years, potentially accelerating market adoption across all segments.
The demand for improved hydrogen storage materials with enhanced electrochemical performance is particularly strong in three key sectors: transportation, stationary power generation, and industrial applications. In the transportation sector, the market for fuel cell electric vehicles (FCEVs) is growing at a compound annual growth rate of 31.4%, creating urgent demand for storage solutions that offer higher energy density and faster charging capabilities. Major automotive manufacturers have committed over 20 billion USD to hydrogen vehicle development programs through 2030.
Stationary power applications represent another significant market segment, with grid-scale energy storage solutions increasingly incorporating hydrogen technologies to address intermittency issues in renewable energy generation. This market segment is expected to grow at 25.7% annually through 2030, with particular emphasis on long-duration storage capabilities that conventional battery technologies cannot efficiently provide.
Industrial applications, particularly in sectors requiring high-temperature processes such as steel and cement production, are driving demand for hydrogen storage solutions that can deliver consistent, high-volume hydrogen supply. This segment represents approximately 40% of the current hydrogen storage market and is expected to maintain its dominant position through 2035.
Regional analysis reveals that Asia-Pacific currently leads the market with 42% share, followed by Europe (31%) and North America (21%). However, Europe is demonstrating the fastest growth rate due to aggressive climate policies and substantial public investment in hydrogen infrastructure. The European Hydrogen Strategy has allocated 430 billion EUR for hydrogen development by 2030, with approximately 18% directed specifically toward storage technologies.
Market barriers include high production costs, infrastructure limitations, and technical challenges related to energy density and cycle stability. However, recent breakthroughs in interface modification technologies are creating new market opportunities, with specialized materials companies reporting 40-60% improvements in storage capacity through novel surface engineering approaches. These advancements are expected to reduce costs by 35% within five years, potentially accelerating market adoption across all segments.
Current Interface Challenges in Hydrogen Storage Materials
The interface between hydrogen storage materials and electrolytes represents a critical bottleneck in advancing electrochemical hydrogen storage technologies. Current interfaces suffer from several fundamental challenges that limit performance, efficiency, and long-term stability. One primary issue is the formation of passivation layers at material interfaces, which significantly impedes hydrogen ion transport and increases internal resistance. These layers develop gradually during cycling and can drastically reduce the effective surface area available for hydrogen adsorption and desorption processes.
Electron transfer kinetics at interfaces remain suboptimal in most current systems, resulting in high overpotentials and reduced energy efficiency. The interface architecture often lacks the necessary catalytic sites to facilitate rapid hydrogen dissociation and recombination, creating a rate-limiting step in the overall storage process. This is particularly problematic in metal hydride systems where surface oxidation further complicates interfacial chemistry.
Wettability issues between solid storage materials and liquid electrolytes create inconsistent contact areas, leading to non-uniform reaction distributions and localized hotspots during operation. Many hydrogen storage materials exhibit hydrophobic characteristics that prevent intimate electrolyte contact, resulting in underutilized active material and reduced volumetric efficiency. This challenge becomes more pronounced in composite materials where multiple phases with different surface energies exist simultaneously.
Ion transport across interfaces suffers from concentration polarization effects, where depletion regions form during rapid charging or discharging operations. The current interface designs lack effective mechanisms to mitigate these concentration gradients, resulting in diffusion-limited performance at high rates. Additionally, many interfaces experience mechanical degradation during hydrogen absorption/desorption cycles due to volume changes, creating microcracks that further disrupt the electrochemical processes.
Temperature management at interfaces presents another significant challenge, as many hydrogen storage reactions are highly exothermic or endothermic. Poor thermal conductivity across interfaces can lead to thermal runaway conditions or insufficient reaction temperatures. Current interface designs typically lack integrated thermal management features, resulting in temperature gradients that reduce system efficiency and potentially compromise safety.
Contamination sensitivity remains problematic, with many hydrogen storage interfaces experiencing rapid degradation when exposed to common impurities like oxygen, carbon dioxide, or moisture. The lack of selective protective layers that permit hydrogen transport while blocking contaminants represents a major limitation in current systems, particularly for applications requiring operation in variable environmental conditions.
Electron transfer kinetics at interfaces remain suboptimal in most current systems, resulting in high overpotentials and reduced energy efficiency. The interface architecture often lacks the necessary catalytic sites to facilitate rapid hydrogen dissociation and recombination, creating a rate-limiting step in the overall storage process. This is particularly problematic in metal hydride systems where surface oxidation further complicates interfacial chemistry.
Wettability issues between solid storage materials and liquid electrolytes create inconsistent contact areas, leading to non-uniform reaction distributions and localized hotspots during operation. Many hydrogen storage materials exhibit hydrophobic characteristics that prevent intimate electrolyte contact, resulting in underutilized active material and reduced volumetric efficiency. This challenge becomes more pronounced in composite materials where multiple phases with different surface energies exist simultaneously.
Ion transport across interfaces suffers from concentration polarization effects, where depletion regions form during rapid charging or discharging operations. The current interface designs lack effective mechanisms to mitigate these concentration gradients, resulting in diffusion-limited performance at high rates. Additionally, many interfaces experience mechanical degradation during hydrogen absorption/desorption cycles due to volume changes, creating microcracks that further disrupt the electrochemical processes.
Temperature management at interfaces presents another significant challenge, as many hydrogen storage reactions are highly exothermic or endothermic. Poor thermal conductivity across interfaces can lead to thermal runaway conditions or insufficient reaction temperatures. Current interface designs typically lack integrated thermal management features, resulting in temperature gradients that reduce system efficiency and potentially compromise safety.
Contamination sensitivity remains problematic, with many hydrogen storage interfaces experiencing rapid degradation when exposed to common impurities like oxygen, carbon dioxide, or moisture. The lack of selective protective layers that permit hydrogen transport while blocking contaminants represents a major limitation in current systems, particularly for applications requiring operation in variable environmental conditions.
Interface Modification Techniques and Implementation Strategies
01 Metal hydride materials for hydrogen storage
Metal hydrides are key materials for hydrogen storage applications due to their high hydrogen capacity and reversible absorption/desorption properties. These materials can form stable hydrides with hydrogen at moderate temperatures and pressures, making them suitable for electrochemical applications. The electrochemical performance of metal hydrides is characterized by their charge/discharge efficiency, cycle stability, and hydrogen storage capacity, which are critical parameters for applications in batteries and fuel cells.- Metal hydride materials for hydrogen storage: Metal hydrides are promising materials for hydrogen storage due to their high hydrogen capacity and reversible absorption/desorption properties. These materials can form stable hydrides with hydrogen at moderate temperatures and pressures, making them suitable for electrochemical applications. The electrochemical performance of metal hydrides is characterized by their charge/discharge efficiency, cycle life, and hydrogen storage capacity. Various metal compositions and alloys have been developed to optimize these properties for practical applications in fuel cells and batteries.
- Nanostructured materials for enhanced hydrogen storage: Nanostructured materials offer improved hydrogen storage capabilities due to their high surface area and unique physical properties. These materials, including nanoparticles, nanotubes, and nanoporous structures, provide more active sites for hydrogen adsorption and faster kinetics for hydrogen absorption/desorption. The electrochemical performance of nanostructured hydrogen storage materials shows enhanced capacity, improved cycling stability, and faster charge/discharge rates compared to their bulk counterparts. Various synthesis methods have been developed to control the morphology and composition of these nanomaterials for optimized electrochemical performance.
- Composite hydrogen storage materials: Composite hydrogen storage materials combine different types of materials to achieve synergistic effects and overcome limitations of single-component systems. These composites often integrate metal hydrides with carbon-based materials, polymers, or catalysts to enhance hydrogen storage capacity and electrochemical performance. The composite structure can improve hydrogen diffusion pathways, prevent agglomeration during cycling, and enhance electrical conductivity. These materials demonstrate improved cycling stability, rate capability, and overall electrochemical performance in hydrogen storage applications.
- Electrode design and fabrication for hydrogen storage systems: The design and fabrication of electrodes significantly impact the electrochemical performance of hydrogen storage systems. Advanced electrode architectures incorporate binders, conductive additives, and structural supports to enhance electrical connectivity and mechanical stability. Novel fabrication techniques, such as 3D printing, spray coating, and electrospinning, enable precise control over electrode microstructure. Optimized electrode designs feature high porosity for electrolyte penetration, short diffusion paths for hydrogen, and robust mechanical properties to withstand volume changes during hydrogen absorption/desorption cycles.
- Catalysts for improved hydrogen storage kinetics: Catalysts play a crucial role in enhancing the kinetics of hydrogen absorption and desorption in storage materials. Various catalytic materials, including noble metals, transition metal oxides, and complex compounds, can significantly reduce activation energy barriers for hydrogen reactions. The incorporation of catalysts improves charge/discharge rates, lowers operating temperatures, and enhances overall electrochemical performance of hydrogen storage systems. Advanced catalyst designs focus on optimizing dispersion, stability, and synergistic effects with the host material to maximize electrochemical performance while minimizing catalyst loading.
02 Nanostructured materials for enhanced hydrogen storage
Nanostructured materials offer improved hydrogen storage capabilities due to their high surface area and shortened diffusion paths. These materials, including nanoparticles, nanocomposites, and nanoporous structures, demonstrate enhanced electrochemical performance with faster kinetics and improved cycling stability. The reduced particle size facilitates hydrogen absorption and desorption, leading to better rate capability and overall electrochemical efficiency in hydrogen storage applications.Expand Specific Solutions03 Electrode materials for hydrogen storage batteries
Specialized electrode materials are developed for hydrogen storage batteries with optimized electrochemical performance. These materials feature tailored compositions and structures to enhance hydrogen diffusion, electrical conductivity, and cycling stability. The electrodes often incorporate catalysts to improve reaction kinetics and reduce overpotential during charging and discharging processes. Advanced manufacturing techniques are employed to create electrodes with optimal porosity and surface characteristics for hydrogen interaction.Expand Specific Solutions04 Alloy-based hydrogen storage materials
Alloy-based hydrogen storage materials, including AB5, AB2, and AB-type alloys, demonstrate excellent electrochemical performance for hydrogen storage applications. These alloys can be tailored by adjusting their composition to optimize hydrogen capacity, cycling stability, and kinetics. The incorporation of rare earth elements, transition metals, and other additives can significantly enhance the electrochemical properties of these alloys, making them suitable for various applications including nickel-metal hydride batteries and hydrogen storage systems.Expand Specific Solutions05 Composite materials for improved electrochemical performance
Composite hydrogen storage materials combine different components to achieve synergistic effects and enhanced electrochemical performance. These composites often integrate high-capacity storage materials with conductive additives, catalysts, or structural stabilizers. The resulting materials exhibit improved hydrogen absorption/desorption kinetics, better cycling stability, and enhanced resistance to degradation mechanisms. Advanced composite designs address multiple performance parameters simultaneously, making them promising candidates for next-generation hydrogen storage applications.Expand Specific Solutions
Leading Companies and Research Institutions in Hydrogen Storage
The hydrogen storage materials electrochemical performance improvement landscape is currently in a growth phase, with the market expected to expand significantly due to increasing clean energy demands. Major players like BASF SE, Huawei Technologies, and Ningde Amperex Technology are driving innovation in interface modifications, while research institutions such as Baotou Rare Earth Research Institute and Advanced Industrial Science & Technology provide critical technical expertise. Companies like Santoku Corp. and GS Yuasa International specialize in hydrogen-absorbing alloys and battery technologies. The technology is approaching commercial maturity with significant advancements in electrode surface treatments, electrolyte optimization, and catalyst integration, though challenges remain in scaling production and reducing costs. Collaboration between academic institutions and industry leaders is accelerating development toward widespread implementation.
BASF SE
Technical Solution: BASF has developed advanced interface engineering solutions for hydrogen storage materials, focusing on metal-organic frameworks (MOFs) with tailored pore structures. Their technology incorporates surface functionalization techniques that modify the electronic structure of storage materials, enhancing hydrogen binding energy without compromising kinetics. BASF's approach includes the integration of catalytic nanoparticles at material interfaces to facilitate hydrogen dissociation and recombination, significantly improving absorption/desorption rates. Their proprietary coating technologies create protective layers that prevent oxidation while maintaining hydrogen permeability. Recent developments include composite materials with engineered interfaces between different storage phases, optimizing both capacity and cycling stability for electrochemical hydrogen storage applications.
Strengths: Industry-leading materials science expertise allows for precise control of interface properties; extensive manufacturing infrastructure enables rapid scaling of promising materials. Weaknesses: Higher production costs compared to conventional materials; some solutions require rare elements that may face supply constraints.
Ovonic Battery Co., Inc.
Technical Solution: Ovonic Battery has pioneered nickel-metal hydride (Ni-MH) technology with specialized interface modifications for hydrogen storage. Their approach centers on multi-component alloy systems with engineered grain boundaries that facilitate hydrogen diffusion while maintaining structural integrity. The company has developed proprietary surface activation treatments that remove oxide layers and create catalytically active sites at material interfaces, dramatically improving hydrogen absorption kinetics. Their technology incorporates gradient composition structures at interfaces between different alloy phases, optimizing both thermodynamic and kinetic properties. Ovonic's recent advancements include nano-structured surface modifications that increase the electrochemically active surface area, enhancing charge/discharge rates by up to 40% compared to conventional materials while maintaining capacity over extended cycling.
Strengths: Decades of specialized experience in metal hydride technology; proven commercial track record in energy storage applications. Weaknesses: Technology primarily optimized for Ni-MH batteries rather than pure hydrogen storage; faces competition from newer lithium-based technologies.
Key Patents and Research on Interface Engineering
Hydrogen storage alloys
PatentWO2016130561A1
Innovation
- Development of hydrogen storage alloys with improved low temperature electrochemical properties, specifically modified ABx type alloys incorporating a main phase, a storage secondary phase, and a catalytic secondary phase, optimized with rare earth elements and specific atomic ratios, to enhance charge transfer resistance and double layer capacitance at -40°C.
Hydrogen storage material, negative electrode and nickel hydrogen secondary battery
PatentWO2020115953A1
Innovation
- A hydrogen storage material with a surface modification substance containing Ni, specifically formulated to improve low-temperature discharge characteristics, is applied to the negative electrode, comprising a composition that adjusts the equilibrium pressure and enhances electrochemical catalytic ability through a controlled X-ray diffraction pattern and surface modification process.
Sustainability Impact of Advanced Hydrogen Storage Materials
The advancement of hydrogen storage materials represents a significant step toward sustainable energy systems, with far-reaching environmental implications. These materials facilitate the transition from fossil fuel dependence to renewable energy sources by enabling efficient hydrogen storage and utilization, thereby reducing greenhouse gas emissions. Studies indicate that advanced hydrogen storage materials could potentially reduce carbon emissions by 20-30% in transportation sectors when implemented at scale.
Beyond emissions reduction, these materials contribute to resource conservation by minimizing the need for rare earth elements traditionally used in battery technologies. Many hydrogen storage systems utilize abundant elements like magnesium, aluminum, and carbon, reducing extraction pressures on limited natural resources. The lifecycle assessment of advanced hydrogen storage materials shows significantly lower environmental footprints compared to conventional energy storage technologies, particularly when considering end-of-life disposal and recycling potential.
Interface modifications of hydrogen storage materials further enhance sustainability by improving cycling stability and longevity. Materials with optimized interfaces demonstrate up to 40% longer operational lifespans, reducing waste generation and replacement frequency. Surface treatments that prevent degradation mechanisms like corrosion and passivation ensure that these materials maintain performance over thousands of cycles, maximizing resource efficiency.
Water consumption represents another critical sustainability dimension. Hydrogen production via electrolysis requires substantial water inputs, but advanced storage materials with improved electrochemical performance can increase system efficiency, thereby reducing overall water requirements per unit of energy stored. Interface-modified materials that operate efficiently at lower pressures and temperatures also decrease the energy intensity of hydrogen compression and storage processes.
From a circular economy perspective, many advanced hydrogen storage materials offer excellent recyclability. Modifications that facilitate easier separation and recovery of constituent materials at end-of-life contribute to closing material loops. Research indicates that up to 85% of materials in some advanced metal hydride systems can be recovered and reused, significantly reducing the environmental burden compared to single-use energy storage technologies.
The sustainability benefits extend to local environments as well. Unlike conventional battery technologies that may leach toxic compounds, hydrogen storage materials generally pose lower risks of environmental contamination. Interface modifications that enhance stability further reduce leaching potential, protecting soil and water resources in deployment areas.
Beyond emissions reduction, these materials contribute to resource conservation by minimizing the need for rare earth elements traditionally used in battery technologies. Many hydrogen storage systems utilize abundant elements like magnesium, aluminum, and carbon, reducing extraction pressures on limited natural resources. The lifecycle assessment of advanced hydrogen storage materials shows significantly lower environmental footprints compared to conventional energy storage technologies, particularly when considering end-of-life disposal and recycling potential.
Interface modifications of hydrogen storage materials further enhance sustainability by improving cycling stability and longevity. Materials with optimized interfaces demonstrate up to 40% longer operational lifespans, reducing waste generation and replacement frequency. Surface treatments that prevent degradation mechanisms like corrosion and passivation ensure that these materials maintain performance over thousands of cycles, maximizing resource efficiency.
Water consumption represents another critical sustainability dimension. Hydrogen production via electrolysis requires substantial water inputs, but advanced storage materials with improved electrochemical performance can increase system efficiency, thereby reducing overall water requirements per unit of energy stored. Interface-modified materials that operate efficiently at lower pressures and temperatures also decrease the energy intensity of hydrogen compression and storage processes.
From a circular economy perspective, many advanced hydrogen storage materials offer excellent recyclability. Modifications that facilitate easier separation and recovery of constituent materials at end-of-life contribute to closing material loops. Research indicates that up to 85% of materials in some advanced metal hydride systems can be recovered and reused, significantly reducing the environmental burden compared to single-use energy storage technologies.
The sustainability benefits extend to local environments as well. Unlike conventional battery technologies that may leach toxic compounds, hydrogen storage materials generally pose lower risks of environmental contamination. Interface modifications that enhance stability further reduce leaching potential, protecting soil and water resources in deployment areas.
Scalability and Cost Analysis of Interface Modification Methods
The scalability and cost-effectiveness of interface modification methods for hydrogen storage materials represent critical factors in their commercial viability. Current laboratory-scale modification techniques often employ expensive catalysts like platinum and palladium, which significantly increase production costs when scaled to industrial levels. Analysis of production economics indicates that noble metal-based interface modifications can contribute up to 40% of the total material cost, creating a substantial barrier to widespread adoption.
Alternative approaches using transition metal compounds and carbon-based materials show promising cost reduction potential, with recent studies demonstrating that nickel-based interface modifications can achieve 80% of platinum's performance at approximately 15% of the cost. However, these alternatives often require more complex processing methods, which may offset some cost advantages when scaled up.
Manufacturing scalability varies significantly across modification techniques. Solution-based methods such as chemical deposition and electroplating demonstrate favorable scaling characteristics, with relatively linear cost-to-volume relationships. In contrast, vacuum-based techniques like physical vapor deposition and atomic layer deposition face more challenging scaling economics, with high initial capital investment requirements and diminishing returns at larger scales.
Energy consumption during interface modification processes represents another significant cost factor. High-temperature annealing treatments commonly used to optimize interface properties can account for 20-30% of production energy costs. Recent innovations in microwave-assisted and plasma-enhanced modification techniques have shown potential to reduce energy requirements by 40-60%, though these approaches remain in early development stages for hydrogen storage applications.
Supply chain considerations also impact scalability, particularly for modifications requiring rare earth elements or specialized precursors. Life cycle assessments indicate that interface modifications using abundant materials like iron oxides, carbon nanostructures, and polymer composites offer the most sustainable scaling pathways, though often with performance trade-offs that must be carefully balanced against cost benefits.
Economic modeling suggests that achieving cost parity with conventional energy storage technologies requires reducing interface modification costs below $20 per kilogram of storage material while maintaining performance metrics. Current research trajectories indicate this threshold could be reached within 5-7 years through combined advances in material science, process engineering, and manufacturing optimization.
Alternative approaches using transition metal compounds and carbon-based materials show promising cost reduction potential, with recent studies demonstrating that nickel-based interface modifications can achieve 80% of platinum's performance at approximately 15% of the cost. However, these alternatives often require more complex processing methods, which may offset some cost advantages when scaled up.
Manufacturing scalability varies significantly across modification techniques. Solution-based methods such as chemical deposition and electroplating demonstrate favorable scaling characteristics, with relatively linear cost-to-volume relationships. In contrast, vacuum-based techniques like physical vapor deposition and atomic layer deposition face more challenging scaling economics, with high initial capital investment requirements and diminishing returns at larger scales.
Energy consumption during interface modification processes represents another significant cost factor. High-temperature annealing treatments commonly used to optimize interface properties can account for 20-30% of production energy costs. Recent innovations in microwave-assisted and plasma-enhanced modification techniques have shown potential to reduce energy requirements by 40-60%, though these approaches remain in early development stages for hydrogen storage applications.
Supply chain considerations also impact scalability, particularly for modifications requiring rare earth elements or specialized precursors. Life cycle assessments indicate that interface modifications using abundant materials like iron oxides, carbon nanostructures, and polymer composites offer the most sustainable scaling pathways, though often with performance trade-offs that must be carefully balanced against cost benefits.
Economic modeling suggests that achieving cost parity with conventional energy storage technologies requires reducing interface modification costs below $20 per kilogram of storage material while maintaining performance metrics. Current research trajectories indicate this threshold could be reached within 5-7 years through combined advances in material science, process engineering, and manufacturing optimization.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!