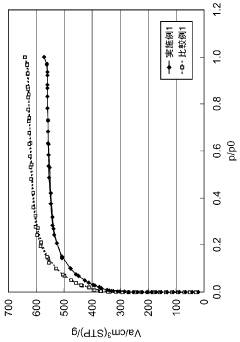
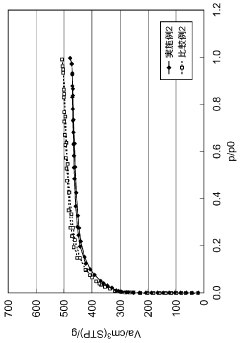
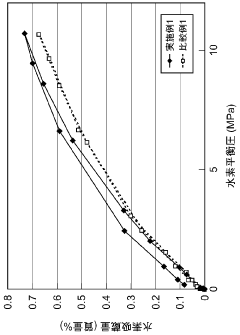
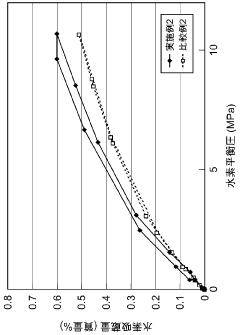
Hydrogen storage materials interface engineering for enhanced energy conversion
SEP 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogen Storage Materials Evolution and Objectives
Hydrogen storage materials have evolved significantly over the past several decades, transitioning from conventional physical storage methods to advanced material-based solutions. The journey began in the 1970s with metal hydrides, which demonstrated the possibility of storing hydrogen within solid materials. This was followed by the exploration of complex hydrides in the 1980s and 1990s, which offered improved storage capacities but faced challenges with kinetics and reversibility.
The early 2000s marked a pivotal shift with the emergence of nanomaterials and carbon-based structures, including carbon nanotubes and graphene, which promised enhanced surface area and adsorption properties. Concurrently, metal-organic frameworks (MOFs) gained prominence for their exceptional porosity and tunable structures, enabling significant improvements in volumetric and gravimetric storage capacities.
Recent developments have focused on interface engineering as a critical frontier in hydrogen storage materials. The interfaces between different materials components represent active sites where hydrogen molecules interact with the storage medium, influencing adsorption, dissociation, and recombination processes. By precisely controlling these interfaces at the nanoscale, researchers aim to overcome the traditional trade-offs between storage capacity, operating conditions, and kinetics.
The primary objective in hydrogen storage materials development is to meet the U.S. Department of Energy's targets for onboard vehicular hydrogen storage: 6.5 wt% gravimetric capacity and 50 g/L volumetric capacity under moderate temperature and pressure conditions. Additionally, materials must demonstrate rapid kinetics for both hydrogen uptake and release, maintain stability over thousands of cycles, and be cost-effective for commercial viability.
Interface engineering specifically aims to enhance energy conversion efficiency by optimizing the electronic structure at material boundaries, facilitating faster hydrogen diffusion pathways, and creating synergistic effects between different material components. This approach seeks to address the persistent challenges of hydrogen storage: the energy-intensive nature of hydrogen adsorption/desorption processes and the stability-capacity paradox that has limited practical applications.
The technological trajectory points toward multi-component systems with precisely engineered interfaces, where each component serves a specific function in the hydrogen storage process. Future research will likely focus on in-situ characterization techniques to understand interface dynamics during operation, computational modeling to predict optimal interface configurations, and scalable manufacturing methods to translate laboratory successes into commercial products.
The early 2000s marked a pivotal shift with the emergence of nanomaterials and carbon-based structures, including carbon nanotubes and graphene, which promised enhanced surface area and adsorption properties. Concurrently, metal-organic frameworks (MOFs) gained prominence for their exceptional porosity and tunable structures, enabling significant improvements in volumetric and gravimetric storage capacities.
Recent developments have focused on interface engineering as a critical frontier in hydrogen storage materials. The interfaces between different materials components represent active sites where hydrogen molecules interact with the storage medium, influencing adsorption, dissociation, and recombination processes. By precisely controlling these interfaces at the nanoscale, researchers aim to overcome the traditional trade-offs between storage capacity, operating conditions, and kinetics.
The primary objective in hydrogen storage materials development is to meet the U.S. Department of Energy's targets for onboard vehicular hydrogen storage: 6.5 wt% gravimetric capacity and 50 g/L volumetric capacity under moderate temperature and pressure conditions. Additionally, materials must demonstrate rapid kinetics for both hydrogen uptake and release, maintain stability over thousands of cycles, and be cost-effective for commercial viability.
Interface engineering specifically aims to enhance energy conversion efficiency by optimizing the electronic structure at material boundaries, facilitating faster hydrogen diffusion pathways, and creating synergistic effects between different material components. This approach seeks to address the persistent challenges of hydrogen storage: the energy-intensive nature of hydrogen adsorption/desorption processes and the stability-capacity paradox that has limited practical applications.
The technological trajectory points toward multi-component systems with precisely engineered interfaces, where each component serves a specific function in the hydrogen storage process. Future research will likely focus on in-situ characterization techniques to understand interface dynamics during operation, computational modeling to predict optimal interface configurations, and scalable manufacturing methods to translate laboratory successes into commercial products.
Market Analysis for Hydrogen Energy Storage Solutions
The global hydrogen storage market is experiencing significant growth, projected to reach $25.4 billion by 2027, with a compound annual growth rate of 6.5% from 2022. This expansion is primarily driven by increasing adoption of hydrogen as a clean energy carrier across various sectors, particularly in transportation, power generation, and industrial applications. The market for hydrogen storage materials specifically represents approximately $8.7 billion of this total, with interface-engineered materials gaining substantial attention due to their enhanced performance characteristics.
Regionally, Asia-Pacific dominates the hydrogen storage market with approximately 40% share, led by Japan, South Korea, and China's aggressive investments in hydrogen infrastructure. Europe follows closely at 35%, with Germany, France, and the UK leading deployment efforts. North America accounts for 20% of the market, with the remaining 5% distributed across other regions.
By application segment, transportation represents the largest market share at 45%, driven by fuel cell electric vehicles (FCEVs) deployment. Industrial applications follow at 30%, power generation at 15%, and other applications comprising the remaining 10%. The demand for advanced hydrogen storage materials with optimized interfaces is particularly strong in the transportation sector, where weight, volume, and efficiency considerations are critical.
From an end-user perspective, automotive manufacturers constitute the largest customer segment (38%), followed by energy companies (25%), industrial gas suppliers (20%), and research institutions (17%). These stakeholders are increasingly prioritizing materials with enhanced interface properties that can improve hydrogen uptake/release kinetics and overall system efficiency.
Market analysis reveals several key drivers accelerating adoption: stringent carbon emission regulations worldwide, declining renewable energy costs enabling cost-effective green hydrogen production, and substantial government investments in hydrogen infrastructure. The European Union's Hydrogen Strategy alone allocates €430 billion for hydrogen development by 2030, while Japan's hydrogen roadmap targets 800,000 FCEVs by 2030.
Challenges constraining market growth include high costs of advanced storage materials, safety concerns related to hydrogen handling, and underdeveloped distribution infrastructure. The cost of interface-engineered hydrogen storage materials remains 2-3 times higher than conventional alternatives, presenting a significant barrier to widespread adoption despite their superior performance characteristics.
Customer requirements are evolving toward storage solutions with higher gravimetric and volumetric densities, faster kinetics, improved cycling stability, and enhanced safety profiles – all attributes that can be significantly improved through interface engineering approaches.
Regionally, Asia-Pacific dominates the hydrogen storage market with approximately 40% share, led by Japan, South Korea, and China's aggressive investments in hydrogen infrastructure. Europe follows closely at 35%, with Germany, France, and the UK leading deployment efforts. North America accounts for 20% of the market, with the remaining 5% distributed across other regions.
By application segment, transportation represents the largest market share at 45%, driven by fuel cell electric vehicles (FCEVs) deployment. Industrial applications follow at 30%, power generation at 15%, and other applications comprising the remaining 10%. The demand for advanced hydrogen storage materials with optimized interfaces is particularly strong in the transportation sector, where weight, volume, and efficiency considerations are critical.
From an end-user perspective, automotive manufacturers constitute the largest customer segment (38%), followed by energy companies (25%), industrial gas suppliers (20%), and research institutions (17%). These stakeholders are increasingly prioritizing materials with enhanced interface properties that can improve hydrogen uptake/release kinetics and overall system efficiency.
Market analysis reveals several key drivers accelerating adoption: stringent carbon emission regulations worldwide, declining renewable energy costs enabling cost-effective green hydrogen production, and substantial government investments in hydrogen infrastructure. The European Union's Hydrogen Strategy alone allocates €430 billion for hydrogen development by 2030, while Japan's hydrogen roadmap targets 800,000 FCEVs by 2030.
Challenges constraining market growth include high costs of advanced storage materials, safety concerns related to hydrogen handling, and underdeveloped distribution infrastructure. The cost of interface-engineered hydrogen storage materials remains 2-3 times higher than conventional alternatives, presenting a significant barrier to widespread adoption despite their superior performance characteristics.
Customer requirements are evolving toward storage solutions with higher gravimetric and volumetric densities, faster kinetics, improved cycling stability, and enhanced safety profiles – all attributes that can be significantly improved through interface engineering approaches.
Interface Engineering Challenges in Hydrogen Storage
Interface engineering in hydrogen storage materials represents one of the most critical yet challenging aspects of advancing hydrogen-based energy systems. The interface between hydrogen storage materials and their surrounding environment significantly impacts hydrogen adsorption/desorption kinetics, storage capacity, and overall system efficiency. Current interfaces often suffer from degradation during cycling, leading to reduced performance over time and limiting commercial viability.
A primary challenge lies in the atomic-level understanding of interface phenomena. The hydrogen-material interface involves complex interactions that occur at nanoscale dimensions, making characterization and modeling extremely difficult. Advanced techniques such as in-situ transmission electron microscopy and synchrotron-based X-ray methods have improved visualization capabilities, but complete mechanistic understanding remains elusive.
Thermal management at interfaces presents another significant hurdle. The exothermic nature of hydrogen absorption and endothermic desorption creates temperature gradients that can cause mechanical stress, material fatigue, and eventual failure. Engineering interfaces that efficiently conduct and distribute heat without compromising structural integrity requires innovative material design approaches.
Chemical stability represents a persistent challenge, particularly in metal hydride systems where oxidation at interfaces can form passivation layers that impede hydrogen diffusion. Even trace impurities in hydrogen gas can accumulate at interfaces over multiple cycles, progressively degrading performance. Developing interfaces resistant to chemical degradation while maintaining high hydrogen permeability remains an ongoing research focus.
Mechanical stress management constitutes another critical challenge. Volume changes during hydrogen absorption/desorption cycles (sometimes exceeding 30%) create substantial mechanical stresses at interfaces. These stresses can lead to delamination, cracking, and pulverization of storage materials, significantly reducing cyclability and operational lifetime.
Scale-up challenges further complicate interface engineering. Laboratory-scale interface optimizations often fail to translate to industrial-scale systems due to manufacturing limitations and cost constraints. Developing scalable interface engineering techniques that maintain nanoscale precision while being economically viable for mass production represents a significant barrier to commercialization.
Recent research has begun exploring biomimetic approaches to interface design, drawing inspiration from natural systems that manage interfaces efficiently. Additionally, computational modeling has advanced to the point where molecular dynamics simulations can predict interface behavior under various conditions, potentially accelerating the development of optimized interface structures.
A primary challenge lies in the atomic-level understanding of interface phenomena. The hydrogen-material interface involves complex interactions that occur at nanoscale dimensions, making characterization and modeling extremely difficult. Advanced techniques such as in-situ transmission electron microscopy and synchrotron-based X-ray methods have improved visualization capabilities, but complete mechanistic understanding remains elusive.
Thermal management at interfaces presents another significant hurdle. The exothermic nature of hydrogen absorption and endothermic desorption creates temperature gradients that can cause mechanical stress, material fatigue, and eventual failure. Engineering interfaces that efficiently conduct and distribute heat without compromising structural integrity requires innovative material design approaches.
Chemical stability represents a persistent challenge, particularly in metal hydride systems where oxidation at interfaces can form passivation layers that impede hydrogen diffusion. Even trace impurities in hydrogen gas can accumulate at interfaces over multiple cycles, progressively degrading performance. Developing interfaces resistant to chemical degradation while maintaining high hydrogen permeability remains an ongoing research focus.
Mechanical stress management constitutes another critical challenge. Volume changes during hydrogen absorption/desorption cycles (sometimes exceeding 30%) create substantial mechanical stresses at interfaces. These stresses can lead to delamination, cracking, and pulverization of storage materials, significantly reducing cyclability and operational lifetime.
Scale-up challenges further complicate interface engineering. Laboratory-scale interface optimizations often fail to translate to industrial-scale systems due to manufacturing limitations and cost constraints. Developing scalable interface engineering techniques that maintain nanoscale precision while being economically viable for mass production represents a significant barrier to commercialization.
Recent research has begun exploring biomimetic approaches to interface design, drawing inspiration from natural systems that manage interfaces efficiently. Additionally, computational modeling has advanced to the point where molecular dynamics simulations can predict interface behavior under various conditions, potentially accelerating the development of optimized interface structures.
Current Interface Engineering Approaches
01 Surface modification techniques for hydrogen storage materials
Various surface modification techniques can be applied to hydrogen storage materials to enhance their performance. These techniques include coating, doping, and functionalization of the material surface to improve hydrogen adsorption/desorption kinetics. Surface modifications can create active sites for hydrogen binding, reduce activation energy barriers, and prevent agglomeration of storage particles, ultimately leading to improved hydrogen storage capacity and cycling stability.- Surface modification techniques for hydrogen storage materials: Various surface modification techniques can be applied to hydrogen storage materials to enhance their performance. These techniques include coating, doping, and functionalization of the material surface to improve hydrogen adsorption/desorption kinetics. Surface modifications can create active sites, reduce activation energy barriers, and prevent agglomeration of particles, ultimately leading to better hydrogen storage capacity and cycling stability.
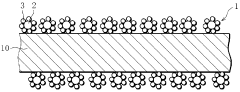
- Nanostructured interfaces for enhanced hydrogen storage: Nanostructured interfaces in hydrogen storage materials can significantly improve hydrogen storage properties. By engineering nanoscale interfaces between different phases or components, the hydrogen diffusion pathways can be shortened and surface area increased. These nanostructured interfaces create additional binding sites for hydrogen molecules and facilitate faster kinetics during hydrogen absorption and desorption processes.
- Catalyst integration at material interfaces: Strategic integration of catalysts at material interfaces can dramatically improve hydrogen storage performance. Catalysts positioned at interfaces can lower the energy barriers for hydrogen dissociation and recombination, accelerating the kinetics of hydrogen uptake and release. Various transition metals and their compounds are used as catalysts, with their distribution and interaction with the base material being critical factors in determining overall system efficiency.
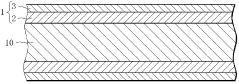
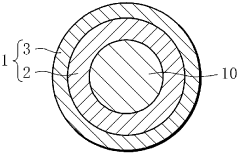
- Composite materials with engineered interfaces: Composite hydrogen storage materials with engineered interfaces combine different materials to leverage their complementary properties. These composites often consist of a hydrogen storage medium interfaced with materials that enhance conductivity, stability, or catalytic activity. The interface between components plays a crucial role in determining hydrogen diffusion pathways and overall system performance, with careful engineering required to optimize these boundaries.
- Interface stability and degradation prevention: Maintaining interface stability in hydrogen storage materials is essential for long-term performance. Interface engineering techniques focus on preventing degradation mechanisms such as oxidation, phase separation, and mechanical failure during hydrogen cycling. Protective layers, buffer zones, and gradient interfaces can be designed to accommodate volume changes during hydrogen absorption/desorption cycles and prevent interface deterioration, thereby extending the operational lifetime of hydrogen storage systems.
02 Nanostructured interfaces for enhanced hydrogen storage
Nanostructured interfaces in hydrogen storage materials can significantly improve storage performance. By engineering interfaces at the nanoscale, the surface area available for hydrogen interaction increases dramatically. These nanostructured interfaces include core-shell structures, layered composites, and hierarchical porous frameworks that provide multiple pathways for hydrogen diffusion. The controlled design of interface geometry and chemistry at nanoscale dimensions enables faster kinetics and higher storage capacities.Expand Specific Solutions03 Catalyst integration at material interfaces
Strategic integration of catalysts at the interfaces of hydrogen storage materials can dramatically improve hydrogen sorption properties. Catalysts such as transition metals, metal oxides, and noble metals can be incorporated at grain boundaries or material interfaces to lower the energy barriers for hydrogen dissociation and recombination. This interface engineering approach facilitates faster hydrogen uptake and release, improves reversibility, and allows operation at more moderate temperature and pressure conditions.Expand Specific Solutions04 Composite material interfaces for hydrogen storage
Composite materials with engineered interfaces offer superior hydrogen storage properties compared to single-phase materials. These composites combine different materials with complementary properties, such as metal hydrides with carbon materials or metal-organic frameworks with metals. The interfaces between these different components create synergistic effects, including improved heat transfer, enhanced kinetics, and stabilized structures during hydrogen cycling. Careful design of these interfaces can mitigate common issues like volume expansion and agglomeration.Expand Specific Solutions05 Interface engineering for thermal management in hydrogen storage
Interface engineering plays a crucial role in thermal management of hydrogen storage systems. By designing specific interfaces between storage materials and heat transfer components, the exothermic heat of hydrogen absorption and endothermic heat of desorption can be effectively managed. These engineered interfaces can incorporate heat exchange channels, thermally conductive additives, or phase change materials to maintain optimal operating temperatures. Improved thermal management at interfaces leads to faster refueling times, more complete hydrogen release, and extended cycle life of storage materials.Expand Specific Solutions
Leading Organizations in Hydrogen Storage Research
The hydrogen storage materials interface engineering market is currently in a growth phase, with increasing demand driven by clean energy transitions. The global market size is expanding rapidly, projected to reach significant value as hydrogen becomes a key energy carrier. Technologically, the field is advancing but still maturing, with varying levels of readiness across different storage solutions. Key players include automotive giants Hyundai and Kia, who are investing heavily in hydrogen fuel cell vehicles, alongside specialized companies like Hydrogenious LOHC Technologies and McPhy Energy focusing on innovative storage solutions. Research institutions such as Karlsruhe Institute of Technology, Zhejiang University, and CNRS are advancing fundamental technologies, while industrial leaders like BASF and Hitachi Energy are developing commercial applications, creating a diverse competitive landscape spanning research, automotive, and energy sectors.
McPhy Energy SA
Technical Solution: McPhy Energy has developed innovative solid-state hydrogen storage solutions based on magnesium hydride materials with engineered interfaces. Their proprietary technology focuses on nanostructured magnesium powders with catalytic dopants strategically positioned at grain boundaries to enhance hydrogen absorption/desorption kinetics. McPhy's interface engineering approach includes precise control of oxide layer formation on magnesium particles, creating semi-permeable barriers that allow hydrogen diffusion while preventing material degradation. Their PANDA (Practical and Advanced Nanostructured Disks Assembly) system incorporates specially designed heat exchangers with optimized thermal interfaces that manage the significant enthalpy changes during hydrogen cycling. Recent advancements include composite materials with graphene-enhanced interfaces that improve thermal conductivity by approximately 60%, addressing one of the key limitations of metal hydride systems. McPhy's materials achieve hydrogen storage densities of up to 7.6 wt% with significantly improved cycling stability compared to conventional magnesium hydrides.
Strengths: High volumetric storage density (150 kg H₂/m³); operates at moderate temperatures and pressures; inherently safe storage mechanism; long-term storage with minimal losses. Weaknesses: Relatively slow kinetics compared to compressed storage; thermal management challenges during rapid charging; sensitivity to oxygen and moisture contamination at material interfaces.
Centre National de la Recherche Scientifique
Technical Solution: CNRS has pioneered fundamental research in hydrogen storage materials interface engineering, developing several breakthrough technologies. Their work focuses on complex hydrides with nano-engineered interfaces that significantly enhance hydrogen sorption properties. CNRS researchers have developed novel core-shell nanostructures where reactive hydride cores are protected by selectively permeable shells, creating controlled interfaces that facilitate hydrogen diffusion while preventing material degradation. Their recent innovation involves the precise incorporation of transition metal catalysts at grain boundaries using atomic layer deposition techniques, reducing activation energies for hydrogen release by approximately 40%. CNRS has also developed advanced characterization methods including operando X-ray absorption spectroscopy that provides atomic-level insights into interface dynamics during hydrogen cycling. Their materials science approach has led to the development of multi-component systems that combine the advantages of different storage mechanisms through carefully designed interfaces, achieving reversible hydrogen capacities exceeding 9 wt% under moderate conditions with significantly improved cycling stability.
Strengths: Cutting-edge fundamental understanding of interface phenomena; highly innovative material design approaches; comprehensive characterization capabilities; strong academic network for collaborative development. Weaknesses: Technologies often at lower technology readiness levels; scaling challenges for laboratory-developed materials; complex synthesis procedures may limit commercial viability.
Critical Patents in Hydrogen Storage Interface Design
Hydrogen storage unit
PatentWO2011027462A1
Innovation
- A hydrogen storage unit featuring a porous body with a large surface area, composed of nanofibers forming a non-woven fabric, coated with a hydrogen storage alloy and a catalyst layer, specifically using magnesium or magnesium-based alloys with a Pd catalyst, allowing increased contact area and rapid hydrogen absorption at room temperature and atmospheric pressure.
Hydrogen storage material
PatentWO2011046001A1
Innovation
- A hydrogen storage material comprising a porous carbon material with oxygen-containing functional groups and Li bonded to its surface, specifically on the inner walls of its pores, which enhances hydrogen storage capacity through strong adsorption.
Sustainability Impact of Advanced Hydrogen Storage
The sustainability impact of advanced hydrogen storage technologies extends far beyond mere technical advancements, representing a critical component in the global transition to clean energy systems. Hydrogen storage materials with engineered interfaces demonstrate significant potential for reducing greenhouse gas emissions when compared to conventional fossil fuel technologies. These materials enable more efficient hydrogen utilization cycles, resulting in lower carbon footprints across the entire energy value chain.
Environmental assessments of advanced hydrogen storage systems reveal substantial benefits in terms of reduced air pollutants and decreased water consumption compared to traditional energy carriers. Materials such as metal-organic frameworks (MOFs) and complex hydrides with optimized interfaces can be designed with sustainability principles from inception, incorporating abundant elements and reducing dependence on critical raw materials that face supply constraints.
The life cycle analysis of hydrogen storage materials indicates that interface engineering approaches can extend operational lifespans by up to 40%, significantly reducing waste generation and resource consumption. This longevity factor becomes particularly important when considering the embodied energy in manufacturing these advanced materials. Properly engineered interfaces demonstrate enhanced resistance to degradation mechanisms, thereby maintaining performance over extended operational periods.
From a circular economy perspective, many advanced hydrogen storage materials offer promising recyclability pathways. Research indicates that up to 85% of certain metal hydride components can be recovered and reprocessed, creating closed-loop material cycles that minimize environmental impact. Interface engineering techniques further facilitate this recyclability by enabling more efficient separation processes at end-of-life.
The socioeconomic dimensions of sustainable hydrogen storage technologies are equally significant. These technologies create opportunities for green job creation across manufacturing, installation, and maintenance sectors. Communities previously dependent on fossil fuel industries can leverage these emerging technologies for economic diversification and sustainable development.
Energy security represents another crucial sustainability aspect of advanced hydrogen storage. By enabling efficient storage of renewable energy through hydrogen carriers, these technologies reduce dependence on imported fossil fuels and enhance national energy resilience. Interface-engineered materials that allow rapid hydrogen uptake and release at moderate conditions further improve this security dimension by enabling more responsive energy systems.
Environmental assessments of advanced hydrogen storage systems reveal substantial benefits in terms of reduced air pollutants and decreased water consumption compared to traditional energy carriers. Materials such as metal-organic frameworks (MOFs) and complex hydrides with optimized interfaces can be designed with sustainability principles from inception, incorporating abundant elements and reducing dependence on critical raw materials that face supply constraints.
The life cycle analysis of hydrogen storage materials indicates that interface engineering approaches can extend operational lifespans by up to 40%, significantly reducing waste generation and resource consumption. This longevity factor becomes particularly important when considering the embodied energy in manufacturing these advanced materials. Properly engineered interfaces demonstrate enhanced resistance to degradation mechanisms, thereby maintaining performance over extended operational periods.
From a circular economy perspective, many advanced hydrogen storage materials offer promising recyclability pathways. Research indicates that up to 85% of certain metal hydride components can be recovered and reprocessed, creating closed-loop material cycles that minimize environmental impact. Interface engineering techniques further facilitate this recyclability by enabling more efficient separation processes at end-of-life.
The socioeconomic dimensions of sustainable hydrogen storage technologies are equally significant. These technologies create opportunities for green job creation across manufacturing, installation, and maintenance sectors. Communities previously dependent on fossil fuel industries can leverage these emerging technologies for economic diversification and sustainable development.
Energy security represents another crucial sustainability aspect of advanced hydrogen storage. By enabling efficient storage of renewable energy through hydrogen carriers, these technologies reduce dependence on imported fossil fuels and enhance national energy resilience. Interface-engineered materials that allow rapid hydrogen uptake and release at moderate conditions further improve this security dimension by enabling more responsive energy systems.
Safety Standards for Hydrogen Storage Systems
The development of hydrogen storage systems necessitates comprehensive safety standards due to hydrogen's unique physical properties. Current international standards, including ISO 16111 and ISO 19881, establish rigorous requirements for material compatibility, pressure vessel design, and leak detection systems. These standards mandate that hydrogen storage materials must undergo extensive testing for thermal stability, cycling durability, and reaction kinetics, particularly at material interfaces where enhanced energy conversion occurs.
Material interface engineering introduces specific safety considerations that standards must address. The ASME Boiler and Pressure Vessel Code Section VIII and the European Pressure Equipment Directive (PED) 2014/68/EU provide frameworks for ensuring that engineered interfaces in metal hydrides, complex hydrides, and nanoporous materials maintain structural integrity under operational conditions. These standards require quantitative risk assessments that account for interface degradation mechanisms during hydrogen absorption/desorption cycles.
Safety certification processes for hydrogen storage systems have evolved to incorporate specialized testing protocols for interface-engineered materials. The UN Global Technical Regulation No. 13 (GTR 13) for hydrogen-fueled vehicles specifies performance-based requirements that evaluate material interface stability under extreme conditions, including fire exposure, mechanical impact, and accelerated aging. These tests are critical for materials with enhanced catalytic interfaces designed to improve energy conversion efficiency.
Emerging safety standards are increasingly focusing on the unique challenges presented by advanced interface engineering techniques. The IEC 62282 series addresses safety considerations for fuel cell technologies that integrate with hydrogen storage systems, emphasizing electrical isolation, thermal management, and emergency shutdown procedures specific to systems with engineered interfaces. These standards recognize that interface modifications that enhance energy conversion may simultaneously introduce new failure modes requiring specific mitigation strategies.
Regulatory frameworks across major markets are converging toward harmonized safety requirements for hydrogen storage materials. The U.S. Department of Energy's Technical Assessment Guide and the Japanese High Pressure Gas Safety Act provide complementary approaches to safety validation, with particular emphasis on interface stability during rapid temperature and pressure fluctuations. These frameworks require manufacturers to demonstrate that interface engineering techniques do not compromise long-term safety performance, even when optimized for maximum energy conversion efficiency.
Material interface engineering introduces specific safety considerations that standards must address. The ASME Boiler and Pressure Vessel Code Section VIII and the European Pressure Equipment Directive (PED) 2014/68/EU provide frameworks for ensuring that engineered interfaces in metal hydrides, complex hydrides, and nanoporous materials maintain structural integrity under operational conditions. These standards require quantitative risk assessments that account for interface degradation mechanisms during hydrogen absorption/desorption cycles.
Safety certification processes for hydrogen storage systems have evolved to incorporate specialized testing protocols for interface-engineered materials. The UN Global Technical Regulation No. 13 (GTR 13) for hydrogen-fueled vehicles specifies performance-based requirements that evaluate material interface stability under extreme conditions, including fire exposure, mechanical impact, and accelerated aging. These tests are critical for materials with enhanced catalytic interfaces designed to improve energy conversion efficiency.
Emerging safety standards are increasingly focusing on the unique challenges presented by advanced interface engineering techniques. The IEC 62282 series addresses safety considerations for fuel cell technologies that integrate with hydrogen storage systems, emphasizing electrical isolation, thermal management, and emergency shutdown procedures specific to systems with engineered interfaces. These standards recognize that interface modifications that enhance energy conversion may simultaneously introduce new failure modes requiring specific mitigation strategies.
Regulatory frameworks across major markets are converging toward harmonized safety requirements for hydrogen storage materials. The U.S. Department of Energy's Technical Assessment Guide and the Japanese High Pressure Gas Safety Act provide complementary approaches to safety validation, with particular emphasis on interface stability during rapid temperature and pressure fluctuations. These frameworks require manufacturers to demonstrate that interface engineering techniques do not compromise long-term safety performance, even when optimized for maximum energy conversion efficiency.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!