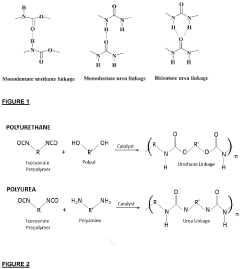
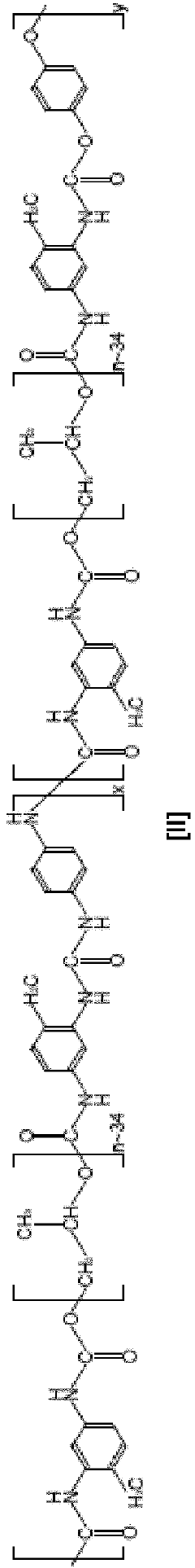
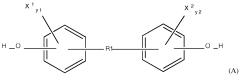
Analysis of Mechanical Properties in Biomedical Polymers
OCT 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biomedical Polymer Evolution and Research Objectives
Biomedical polymers have undergone significant evolution since their initial introduction in the 1960s. The field began with simple applications such as sutures and basic implants, utilizing primarily non-degradable polymers like polyethylene and polyurethane. The 1970s and 1980s witnessed the emergence of biodegradable polymers, notably polylactic acid (PLA) and polyglycolic acid (PGA), which revolutionized temporary medical applications by eliminating the need for removal surgeries.
The 1990s marked a pivotal shift toward functionalized polymers with enhanced biocompatibility and specific biological interactions. This era introduced smart polymers responsive to environmental stimuli such as temperature, pH, and electrical signals, expanding the potential applications in drug delivery systems and tissue engineering scaffolds.
The early 2000s saw the integration of nanotechnology with polymer science, creating nanostructured polymeric materials with unprecedented control over mechanical properties. This convergence enabled the development of materials with precisely engineered strength, elasticity, and degradation profiles tailored to specific anatomical requirements.
Current trends in biomedical polymer development focus on biomimetic approaches, where materials emulate the mechanical and biological properties of natural tissues. These advanced polymers incorporate gradient structures, anisotropic mechanical properties, and dynamic responsiveness that more accurately replicate the complex mechanical behavior of native tissues.
The mechanical properties of biomedical polymers represent a critical determinant of their clinical success. These properties include tensile strength, elastic modulus, fatigue resistance, creep behavior, and viscoelasticity. The analysis of these properties has become increasingly sophisticated, moving beyond simple stress-strain measurements to include dynamic mechanical analysis, nanoindentation, and in situ testing under physiological conditions.
This technical research aims to comprehensively evaluate the relationship between polymer structure and mechanical performance in biomedical applications. The primary objectives include: identifying structure-property relationships that govern mechanical behavior in various biomedical environments; developing predictive models for long-term mechanical performance under physiological conditions; establishing standardized testing protocols that accurately reflect in vivo mechanical demands; and exploring novel polymer architectures that can achieve previously unattainable combinations of mechanical properties.
Additionally, this research seeks to address the persistent challenges of mechanical property mismatch between synthetic polymers and natural tissues, mechanical property degradation over time, and the integration of multiple mechanical functionalities within a single polymer system. The ultimate goal is to develop design principles for next-generation biomedical polymers with optimized mechanical properties for specific clinical applications.
The 1990s marked a pivotal shift toward functionalized polymers with enhanced biocompatibility and specific biological interactions. This era introduced smart polymers responsive to environmental stimuli such as temperature, pH, and electrical signals, expanding the potential applications in drug delivery systems and tissue engineering scaffolds.
The early 2000s saw the integration of nanotechnology with polymer science, creating nanostructured polymeric materials with unprecedented control over mechanical properties. This convergence enabled the development of materials with precisely engineered strength, elasticity, and degradation profiles tailored to specific anatomical requirements.
Current trends in biomedical polymer development focus on biomimetic approaches, where materials emulate the mechanical and biological properties of natural tissues. These advanced polymers incorporate gradient structures, anisotropic mechanical properties, and dynamic responsiveness that more accurately replicate the complex mechanical behavior of native tissues.
The mechanical properties of biomedical polymers represent a critical determinant of their clinical success. These properties include tensile strength, elastic modulus, fatigue resistance, creep behavior, and viscoelasticity. The analysis of these properties has become increasingly sophisticated, moving beyond simple stress-strain measurements to include dynamic mechanical analysis, nanoindentation, and in situ testing under physiological conditions.
This technical research aims to comprehensively evaluate the relationship between polymer structure and mechanical performance in biomedical applications. The primary objectives include: identifying structure-property relationships that govern mechanical behavior in various biomedical environments; developing predictive models for long-term mechanical performance under physiological conditions; establishing standardized testing protocols that accurately reflect in vivo mechanical demands; and exploring novel polymer architectures that can achieve previously unattainable combinations of mechanical properties.
Additionally, this research seeks to address the persistent challenges of mechanical property mismatch between synthetic polymers and natural tissues, mechanical property degradation over time, and the integration of multiple mechanical functionalities within a single polymer system. The ultimate goal is to develop design principles for next-generation biomedical polymers with optimized mechanical properties for specific clinical applications.
Market Demand Analysis for High-Performance Biomedical Polymers
The global market for high-performance biomedical polymers has experienced significant growth over the past decade, driven by increasing demand for advanced medical devices, implants, and drug delivery systems. Current market estimates value this sector at approximately 12 billion USD, with projections indicating a compound annual growth rate of 7.8% through 2028. This robust growth trajectory reflects the expanding applications of specialized polymers in healthcare settings worldwide.
Healthcare providers and medical device manufacturers are increasingly seeking biomedical polymers with enhanced mechanical properties to address specific clinical challenges. The aging global population, rising prevalence of chronic diseases, and growing demand for minimally invasive surgical procedures have collectively fueled the need for polymers that can withstand complex biomechanical environments while maintaining biocompatibility.
Orthopedic applications represent the largest market segment, accounting for nearly 30% of the total biomedical polymers market. The demand for materials with precise tensile strength, fatigue resistance, and wear characteristics has intensified as joint replacement surgeries become more common globally. Particularly, polymers that can mimic the mechanical behavior of natural bone tissue while resisting degradation in physiological environments are commanding premium pricing in the marketplace.
Cardiovascular applications constitute another rapidly expanding segment, with particular emphasis on polymers that combine flexibility with durability for applications such as heart valves, vascular grafts, and stents. Market research indicates that polymers demonstrating superior mechanical performance under cyclical loading conditions are experiencing demand growth rates exceeding 9% annually in this segment.
Regional analysis reveals that North America currently dominates the market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is demonstrating the fastest growth rate, driven by expanding healthcare infrastructure, increasing medical tourism, and growing domestic manufacturing capabilities in countries like China, India, and South Korea.
Consumer preferences are shifting toward customized solutions that address patient-specific anatomical and physiological requirements. This trend has created a growing niche for polymers whose mechanical properties can be precisely tailored through advanced manufacturing techniques such as 3D printing and microinjection molding. Market surveys indicate that medical device manufacturers are willing to pay premium prices for polymers offering such customization potential.
Regulatory considerations significantly influence market dynamics, with materials demonstrating consistent mechanical performance under standardized testing protocols gaining faster market acceptance. The implementation of more stringent regulatory frameworks in major markets has increased demand for polymers with well-characterized mechanical property profiles and predictable long-term performance in biological environments.
Healthcare providers and medical device manufacturers are increasingly seeking biomedical polymers with enhanced mechanical properties to address specific clinical challenges. The aging global population, rising prevalence of chronic diseases, and growing demand for minimally invasive surgical procedures have collectively fueled the need for polymers that can withstand complex biomechanical environments while maintaining biocompatibility.
Orthopedic applications represent the largest market segment, accounting for nearly 30% of the total biomedical polymers market. The demand for materials with precise tensile strength, fatigue resistance, and wear characteristics has intensified as joint replacement surgeries become more common globally. Particularly, polymers that can mimic the mechanical behavior of natural bone tissue while resisting degradation in physiological environments are commanding premium pricing in the marketplace.
Cardiovascular applications constitute another rapidly expanding segment, with particular emphasis on polymers that combine flexibility with durability for applications such as heart valves, vascular grafts, and stents. Market research indicates that polymers demonstrating superior mechanical performance under cyclical loading conditions are experiencing demand growth rates exceeding 9% annually in this segment.
Regional analysis reveals that North America currently dominates the market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is demonstrating the fastest growth rate, driven by expanding healthcare infrastructure, increasing medical tourism, and growing domestic manufacturing capabilities in countries like China, India, and South Korea.
Consumer preferences are shifting toward customized solutions that address patient-specific anatomical and physiological requirements. This trend has created a growing niche for polymers whose mechanical properties can be precisely tailored through advanced manufacturing techniques such as 3D printing and microinjection molding. Market surveys indicate that medical device manufacturers are willing to pay premium prices for polymers offering such customization potential.
Regulatory considerations significantly influence market dynamics, with materials demonstrating consistent mechanical performance under standardized testing protocols gaining faster market acceptance. The implementation of more stringent regulatory frameworks in major markets has increased demand for polymers with well-characterized mechanical property profiles and predictable long-term performance in biological environments.
Current Mechanical Testing Challenges in Biomedical Polymers
The mechanical testing of biomedical polymers presents numerous challenges that significantly impact research accuracy and product development. Traditional testing methods often fail to adequately simulate the complex physiological environments in which these materials must function. Temperature variations, humidity levels, and the presence of biological fluids can dramatically alter polymer mechanical properties, yet standardized testing protocols rarely account for these variables comprehensively.
One critical challenge is the viscoelastic behavior exhibited by most biomedical polymers. These materials demonstrate time-dependent mechanical responses that are difficult to characterize using conventional testing approaches. Stress relaxation, creep, and hysteresis phenomena require specialized testing methodologies that many laboratories lack the equipment or expertise to implement correctly.
Scale-dependent mechanical properties present another significant obstacle. Nano and micro-scale mechanical behaviors often differ substantially from bulk properties, yet these smaller-scale characteristics frequently determine clinical performance. Current testing technologies struggle to bridge this multi-scale gap effectively, leading to potential disconnects between laboratory results and in vivo performance.
The heterogeneous nature of many biomedical polymers, particularly composites and those with engineered microstructures, creates additional testing complexities. Standard testing methods typically assume material homogeneity, potentially missing critical localized mechanical behaviors that could lead to premature failure in clinical applications.
Cyclic loading conditions that simulate physiological stresses remain poorly standardized across the industry. While implantable devices may undergo millions of loading cycles during their lifetime, accelerated testing protocols often fail to accurately predict long-term mechanical degradation and fatigue behavior. This disconnect between testing timeframes and actual service conditions creates significant uncertainty in product development.
Interface testing between polymers and other materials (metals, ceramics, biological tissues) represents another frontier challenge. Adhesion strength, interface stability, and mechanical property transitions across boundaries are difficult to quantify with current methodologies, yet these characteristics often determine device success or failure in clinical settings.
Regulatory requirements add another layer of complexity, with different global markets requiring different testing standards. This fragmentation creates significant challenges for companies developing products for international markets, as testing protocols may need to be duplicated or modified to satisfy various regulatory bodies, increasing development costs and timelines.
One critical challenge is the viscoelastic behavior exhibited by most biomedical polymers. These materials demonstrate time-dependent mechanical responses that are difficult to characterize using conventional testing approaches. Stress relaxation, creep, and hysteresis phenomena require specialized testing methodologies that many laboratories lack the equipment or expertise to implement correctly.
Scale-dependent mechanical properties present another significant obstacle. Nano and micro-scale mechanical behaviors often differ substantially from bulk properties, yet these smaller-scale characteristics frequently determine clinical performance. Current testing technologies struggle to bridge this multi-scale gap effectively, leading to potential disconnects between laboratory results and in vivo performance.
The heterogeneous nature of many biomedical polymers, particularly composites and those with engineered microstructures, creates additional testing complexities. Standard testing methods typically assume material homogeneity, potentially missing critical localized mechanical behaviors that could lead to premature failure in clinical applications.
Cyclic loading conditions that simulate physiological stresses remain poorly standardized across the industry. While implantable devices may undergo millions of loading cycles during their lifetime, accelerated testing protocols often fail to accurately predict long-term mechanical degradation and fatigue behavior. This disconnect between testing timeframes and actual service conditions creates significant uncertainty in product development.
Interface testing between polymers and other materials (metals, ceramics, biological tissues) represents another frontier challenge. Adhesion strength, interface stability, and mechanical property transitions across boundaries are difficult to quantify with current methodologies, yet these characteristics often determine device success or failure in clinical settings.
Regulatory requirements add another layer of complexity, with different global markets requiring different testing standards. This fragmentation creates significant challenges for companies developing products for international markets, as testing protocols may need to be duplicated or modified to satisfy various regulatory bodies, increasing development costs and timelines.
Current Methodologies for Mechanical Property Analysis
01 Biodegradable polymers for medical implants
Biodegradable polymers are widely used in medical implants due to their ability to gradually dissolve in the body, eliminating the need for removal surgery. These polymers can be engineered to have specific mechanical properties such as tensile strength, elasticity, and degradation rates to match the requirements of different implant applications. The mechanical properties can be tailored by adjusting the polymer composition, molecular weight, and processing conditions to ensure optimal performance during the healing process.- Biodegradable polymers for tissue engineering: Biodegradable polymers are widely used in biomedical applications, particularly for tissue engineering scaffolds. These polymers provide temporary mechanical support while gradually degrading as new tissue forms. The mechanical properties of these polymers can be tailored by adjusting their composition, molecular weight, and processing conditions to match the requirements of specific tissues. Common biodegradable polymers include polylactic acid (PLA), polyglycolic acid (PGA), and their copolymers, which offer controllable degradation rates and mechanical strength.
- Hydrogels with tunable mechanical properties: Hydrogels represent an important class of biomedical polymers with adjustable mechanical properties. These water-swollen polymer networks can be engineered to exhibit a wide range of stiffness, elasticity, and strength by modifying their crosslinking density, polymer concentration, and chemical composition. The mechanical properties of hydrogels can be designed to mimic various soft tissues in the body, making them suitable for applications such as drug delivery systems, wound dressings, and artificial cartilage. Smart hydrogels that respond to environmental stimuli like temperature, pH, or electrical signals offer additional functionality for biomedical applications.
- Nanocomposite polymers for enhanced mechanical strength: Incorporating nanoparticles into biomedical polymers creates nanocomposites with significantly enhanced mechanical properties. Materials such as carbon nanotubes, graphene, hydroxyapatite, and silica nanoparticles can be dispersed within polymer matrices to improve tensile strength, modulus, and fracture toughness while maintaining biocompatibility. These nanocomposite systems are particularly valuable for load-bearing applications such as orthopedic implants and dental materials where both strength and biocompatibility are essential. The interface between the nanoparticles and polymer matrix plays a crucial role in determining the overall mechanical performance of these materials.
- Characterization methods for polymer mechanical properties: Various techniques are employed to characterize the mechanical properties of biomedical polymers, including tensile testing, compression testing, dynamic mechanical analysis (DMA), nanoindentation, and atomic force microscopy (AFM). These methods provide critical information about elastic modulus, yield strength, elongation at break, viscoelastic behavior, and fatigue resistance. Understanding these properties is essential for predicting polymer performance in biological environments and ensuring their suitability for specific biomedical applications. Advanced characterization approaches also include in situ testing under physiologically relevant conditions to better simulate the actual performance of materials in the body.
- Stimuli-responsive polymers with adaptive mechanical properties: Stimuli-responsive biomedical polymers can change their mechanical properties in response to external triggers such as temperature, pH, light, or electrical signals. These smart materials offer unique advantages for applications like drug delivery systems, tissue engineering scaffolds, and soft robotics. For example, shape memory polymers can transition between rigid and flexible states based on temperature changes, while electroactive polymers can contract or expand in response to electrical stimulation. The ability to dynamically control mechanical properties enables the development of adaptive biomedical devices that can respond to changing physiological conditions or therapeutic needs.
02 Hydrogels with tunable mechanical properties
Hydrogels are water-swollen polymeric networks that can be designed with specific mechanical properties for biomedical applications. These materials can mimic the mechanical properties of natural tissues, making them suitable for tissue engineering, drug delivery, and wound healing. The mechanical strength, elasticity, and swelling behavior of hydrogels can be controlled by adjusting crosslinking density, polymer concentration, and incorporating reinforcing components. Advanced hydrogels can respond to environmental stimuli such as temperature, pH, or mechanical stress, enabling smart biomedical applications.Expand Specific Solutions03 Composite polymers for enhanced mechanical performance
Composite polymers combine different materials to achieve enhanced mechanical properties that cannot be obtained from a single polymer. These composites often incorporate nanoparticles, fibers, or other reinforcing agents to improve strength, stiffness, and durability while maintaining biocompatibility. The synergistic effect of the components allows for the development of materials with optimized mechanical properties for specific biomedical applications such as orthopedic implants, dental materials, and cardiovascular devices. The interface between the polymer matrix and reinforcing components plays a crucial role in determining the overall mechanical performance.Expand Specific Solutions04 Mechanical characterization methods for biomedical polymers
Various testing methods are employed to characterize the mechanical properties of biomedical polymers, including tensile testing, compression testing, dynamic mechanical analysis, and nanoindentation. These techniques provide essential data on parameters such as Young's modulus, yield strength, elongation at break, and viscoelastic behavior. Advanced characterization methods also consider the performance of polymers under physiological conditions, including temperature, hydration, and cyclic loading. Standardized testing protocols ensure reproducibility and comparability of mechanical property data across different research and development settings.Expand Specific Solutions05 Structure-property relationships in biomedical polymers
Understanding the relationship between polymer structure and mechanical properties is crucial for designing biomedical materials with optimal performance. Factors such as molecular weight, crystallinity, chain orientation, and crosslinking density significantly influence mechanical behavior. Research focuses on establishing predictive models that correlate polymer chemistry and processing with resulting mechanical properties. This knowledge enables the rational design of polymers with tailored mechanical characteristics for specific biomedical applications, reducing the need for extensive trial-and-error experimentation and accelerating the development of new materials.Expand Specific Solutions
Leading Institutions and Companies in Biomedical Polymer Research
The biomedical polymer mechanical properties market is currently in a growth phase, characterized by increasing demand driven by medical device innovations and aging populations. The global market size is estimated to exceed $5 billion, with projected annual growth of 8-10%. Technical maturity varies across applications, with established players like Medtronic and Abbott Cardiovascular Systems leading commercial implementation while academic institutions drive fundamental research. Universities (MIT, Rutgers, Sichuan University) focus on novel polymer development, while companies like Tepha, REVA Medical, and SupraPolix specialize in proprietary biomaterial technologies. The ecosystem demonstrates a productive collaboration between academic research and industrial application, with increasing focus on biodegradable and biocompatible polymers with tailored mechanical properties.
Medtronic, Inc.
Technical Solution: Medtronic has developed advanced characterization methodologies for biomedical polymers used in implantable medical devices. Their technical approach combines dynamic mechanical analysis (DMA) with accelerated aging protocols to predict long-term mechanical performance of polymeric components in physiological environments. The company employs a multi-scale testing framework that correlates molecular structure with macroscopic mechanical properties, particularly focusing on viscoelastic behavior critical for cardiovascular and neurological applications. Their proprietary testing protocols include cyclic loading under simulated physiological conditions to evaluate fatigue resistance and stress relaxation behaviors. Medtronic has pioneered the use of nanoindentation techniques to map mechanical property gradients across polymer interfaces, which is crucial for multi-material medical devices. Their analytical framework incorporates both experimental data and computational modeling to establish structure-property relationships for next-generation biomedical polymers.
Strengths: Comprehensive testing infrastructure that simulates in vivo conditions; extensive clinical data to validate mechanical property requirements; ability to correlate polymer processing parameters with final mechanical performance. Weaknesses: Proprietary testing methodologies limit academic collaboration; focus primarily on established polymer systems rather than novel biomaterials; testing protocols optimized for specific device applications may not be broadly applicable.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered multiscale mechanical characterization techniques for biomedical polymers that bridge molecular architecture to macroscopic performance. Their approach integrates atomic force microscopy (AFM) with traditional mechanical testing to provide comprehensive property mapping across different length scales. MIT researchers have developed novel rheological methods to characterize the viscoelastic properties of hydrogels and other soft biomedical polymers under physiologically relevant conditions. Their technical framework includes custom-built biaxial testing apparatus that can simultaneously measure mechanical properties while imaging microstructural changes in real-time. MIT's polymer characterization platform incorporates machine learning algorithms to predict mechanical property evolution during degradation, which is particularly valuable for biodegradable implants. They have also established correlations between processing parameters, resulting microstructure, and mechanical performance for 3D printed biomedical polymers, enabling precise control of spatial mechanical property gradients for tissue engineering applications.
Strengths: Cutting-edge instrumentation and analytical techniques; strong interdisciplinary approach combining materials science, mechanical engineering, and biological perspectives; ability to develop custom testing methodologies for novel biomaterials. Weaknesses: Research often focuses on fundamental understanding rather than specific commercial applications; some advanced characterization techniques require specialized equipment not readily available in industrial settings; translation pathway from research findings to standardized testing protocols can be lengthy.
Key Innovations in Polymer Mechanical Testing Technologies
An implantable biomaterial, and method of manufacturing thereof
PatentActiveEP4169538A1
Innovation
- Development of a two-step process for creating a linear polyurethane or polyurethane-urea polymer with a hydrogen-bonded, phase-separated structure, using a specific chain extender like benzene 1,4-diol, which enhances fatigue resistance and biocompatibility by maintaining molecular weight distribution and preventing oligomer leaching.
Monomers and phase-separated biocompatible polymer compositions prepared therefrom for medical uses
PatentWO2011014860A1
Innovation
- Development of novel monomers and phase-separated polymer compositions with specific structures, including polycarbonates, polyarylates, polyiminocarbonates, and polyphosphazenes, that exhibit improved mechanical properties and are adapted for radio-opacity, suitable for medical device applications and controlled release formulations.
Biocompatibility and Mechanical Property Correlation
The correlation between biocompatibility and mechanical properties represents a critical intersection in biomedical polymer research. Biocompatibility—the ability of a material to perform with an appropriate host response in a specific application—directly influences the mechanical performance requirements for implantable devices and tissue engineering scaffolds. Recent studies have demonstrated that surface chemistry and topography, which affect biocompatibility, simultaneously impact mechanical behavior through interface interactions and stress distribution patterns.
Mechanical properties such as tensile strength, elastic modulus, and fatigue resistance must be optimized while maintaining cellular compatibility. Research indicates that polymers exhibiting elastic moduli similar to native tissues (0.1-100 MPa range) generally demonstrate superior integration outcomes. However, this mechanical matching often requires compositional modifications that may introduce biocompatibility challenges through altered protein adsorption profiles or inflammatory responses.
Degradation kinetics further complicate this relationship. As biomedical polymers degrade in vivo, their mechanical properties undergo temporal changes that must be precisely controlled to maintain functional integrity. Studies of poly(lactic-co-glycolic acid) (PLGA) and polycaprolactone (PCL) demonstrate that degradation rates directly influence both mechanical stability and biocompatibility through the release of degradation products and changing surface properties.
Cross-linking density emerges as a pivotal parameter affecting both domains. Higher cross-linking typically enhances mechanical strength but may reduce biocompatibility by limiting cellular infiltration and nutrient transport. Conversely, hydrogels with lower cross-linking density often exhibit superior cell compatibility but insufficient mechanical integrity for load-bearing applications.
Advanced characterization techniques now enable simultaneous assessment of mechanical properties and biocompatibility markers. Atomic force microscopy combined with immunofluorescence staining allows researchers to correlate local stiffness with cellular adhesion patterns at the micro-scale. Similarly, nanoindentation techniques coupled with cell viability assays provide insights into how mechanical heterogeneity influences biological responses.
Computational models increasingly integrate both mechanical and biological parameters to predict in vivo performance. Finite element analysis incorporating both stress distribution and cellular migration patterns has proven valuable for optimizing scaffold designs that balance mechanical requirements with biological functionality. These models suggest that gradient structures, rather than homogeneous materials, may better accommodate the dual demands of mechanical support and biocompatibility.
Mechanical properties such as tensile strength, elastic modulus, and fatigue resistance must be optimized while maintaining cellular compatibility. Research indicates that polymers exhibiting elastic moduli similar to native tissues (0.1-100 MPa range) generally demonstrate superior integration outcomes. However, this mechanical matching often requires compositional modifications that may introduce biocompatibility challenges through altered protein adsorption profiles or inflammatory responses.
Degradation kinetics further complicate this relationship. As biomedical polymers degrade in vivo, their mechanical properties undergo temporal changes that must be precisely controlled to maintain functional integrity. Studies of poly(lactic-co-glycolic acid) (PLGA) and polycaprolactone (PCL) demonstrate that degradation rates directly influence both mechanical stability and biocompatibility through the release of degradation products and changing surface properties.
Cross-linking density emerges as a pivotal parameter affecting both domains. Higher cross-linking typically enhances mechanical strength but may reduce biocompatibility by limiting cellular infiltration and nutrient transport. Conversely, hydrogels with lower cross-linking density often exhibit superior cell compatibility but insufficient mechanical integrity for load-bearing applications.
Advanced characterization techniques now enable simultaneous assessment of mechanical properties and biocompatibility markers. Atomic force microscopy combined with immunofluorescence staining allows researchers to correlate local stiffness with cellular adhesion patterns at the micro-scale. Similarly, nanoindentation techniques coupled with cell viability assays provide insights into how mechanical heterogeneity influences biological responses.
Computational models increasingly integrate both mechanical and biological parameters to predict in vivo performance. Finite element analysis incorporating both stress distribution and cellular migration patterns has proven valuable for optimizing scaffold designs that balance mechanical requirements with biological functionality. These models suggest that gradient structures, rather than homogeneous materials, may better accommodate the dual demands of mechanical support and biocompatibility.
Regulatory Framework for Biomedical Polymer Approval
The regulatory landscape for biomedical polymers represents a complex and evolving framework that manufacturers must navigate to bring products to market. In the United States, the Food and Drug Administration (FDA) oversees the approval process through various pathways depending on the risk classification of the polymer-based medical device. Class I devices undergo general controls, Class II devices require special controls and often 510(k) clearance, while Class III devices demand rigorous premarket approval (PMA) with comprehensive mechanical property testing.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), replacing the previous Medical Device Directive (MDD). These regulations place greater emphasis on mechanical property validation throughout the product lifecycle, requiring manufacturers to demonstrate ongoing compliance rather than point-in-time testing.
International Organization for Standardization (ISO) standards play a crucial role in harmonizing regulatory requirements across different jurisdictions. ISO 10993 series specifically addresses biocompatibility testing, while ISO 13485 establishes quality management systems for medical devices. For mechanical properties, ISO 527 and ISO 178 provide standardized testing protocols for tensile and flexural properties respectively.
Regulatory bodies increasingly require real-world evidence and post-market surveillance data to complement laboratory testing of mechanical properties. This shift acknowledges that traditional bench testing may not fully predict in vivo performance of biomedical polymers under physiological conditions.
The approval process typically involves multiple stages of mechanical testing, including initial material characterization, prototype evaluation, design verification, validation testing, and accelerated aging studies. Manufacturers must demonstrate that polymers maintain their mechanical integrity throughout the intended product lifecycle under expected use conditions.
Emerging regulatory trends include increased scrutiny of additives and processing aids in biomedical polymers, particularly concerning leachables and extractables that might affect mechanical performance over time. Additionally, there is growing regulatory interest in the environmental impact of biomedical polymers, with some jurisdictions implementing sustainability requirements alongside traditional safety and efficacy standards.
Regulatory divergence remains a significant challenge for global manufacturers, as requirements for mechanical property testing can vary substantially between markets. This necessitates strategic planning of testing programs to satisfy multiple regulatory frameworks while minimizing redundant testing and associated costs.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), replacing the previous Medical Device Directive (MDD). These regulations place greater emphasis on mechanical property validation throughout the product lifecycle, requiring manufacturers to demonstrate ongoing compliance rather than point-in-time testing.
International Organization for Standardization (ISO) standards play a crucial role in harmonizing regulatory requirements across different jurisdictions. ISO 10993 series specifically addresses biocompatibility testing, while ISO 13485 establishes quality management systems for medical devices. For mechanical properties, ISO 527 and ISO 178 provide standardized testing protocols for tensile and flexural properties respectively.
Regulatory bodies increasingly require real-world evidence and post-market surveillance data to complement laboratory testing of mechanical properties. This shift acknowledges that traditional bench testing may not fully predict in vivo performance of biomedical polymers under physiological conditions.
The approval process typically involves multiple stages of mechanical testing, including initial material characterization, prototype evaluation, design verification, validation testing, and accelerated aging studies. Manufacturers must demonstrate that polymers maintain their mechanical integrity throughout the intended product lifecycle under expected use conditions.
Emerging regulatory trends include increased scrutiny of additives and processing aids in biomedical polymers, particularly concerning leachables and extractables that might affect mechanical performance over time. Additionally, there is growing regulatory interest in the environmental impact of biomedical polymers, with some jurisdictions implementing sustainability requirements alongside traditional safety and efficacy standards.
Regulatory divergence remains a significant challenge for global manufacturers, as requirements for mechanical property testing can vary substantially between markets. This necessitates strategic planning of testing programs to satisfy multiple regulatory frameworks while minimizing redundant testing and associated costs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!