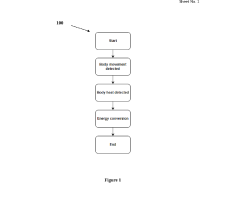
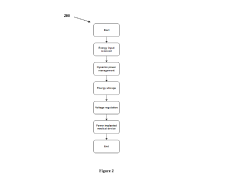
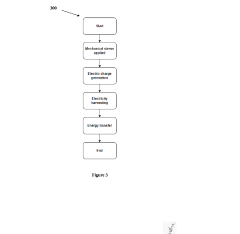
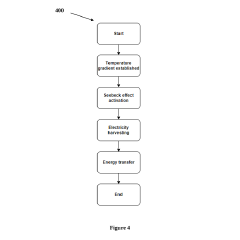
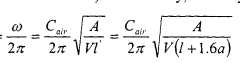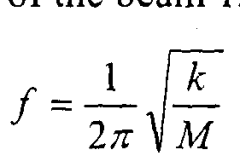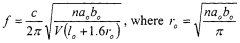Analysis of Micro Energy Harvesters in Medical Device Applications
OCT 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Micro Energy Harvesting Technology Background and Objectives
Micro energy harvesting technology has evolved significantly over the past two decades, transforming from theoretical concepts to practical applications across various industries. The fundamental principle involves capturing small amounts of energy from ambient sources such as vibrations, temperature differentials, electromagnetic radiation, and biological processes. In medical device applications specifically, this technology has progressed from rudimentary piezoelectric generators to sophisticated multi-modal harvesting systems capable of powering implantable and wearable medical devices.
The evolution trajectory shows a clear trend toward miniaturization, efficiency improvement, and integration with other technologies. Early developments in the 1990s focused primarily on basic piezoelectric materials, while the 2000s saw the emergence of electromagnetic and thermoelectric harvesters. The 2010s brought significant advancements in triboelectric nanogenerators and biofuel cells, particularly relevant for medical applications due to their biocompatibility and ability to operate in physiological environments.
Current technological trends indicate a convergence of multiple harvesting modalities into single systems, enhanced by advanced materials such as graphene, carbon nanotubes, and specialized polymers. The integration of artificial intelligence for optimizing energy capture and management represents the cutting edge of this field, allowing for adaptive harvesting based on changing physiological conditions and device requirements.
The primary objective of micro energy harvesting in medical devices is to achieve perpetual or at least long-term autonomous operation without battery replacement. This goal addresses critical challenges in medical device deployment, particularly for implantable devices where battery replacement requires invasive procedures. Secondary objectives include reducing device size, enhancing reliability, ensuring biocompatibility, and maintaining consistent power output despite variable physiological conditions.
For implantable medical devices such as pacemakers, neural stimulators, and drug delivery systems, energy harvesting aims to extend operational lifetimes from years to decades. In wearable health monitoring devices, the technology seeks to eliminate frequent charging requirements while maintaining continuous functionality. These objectives align with broader healthcare trends toward personalized, continuous, and preventative medicine.
The technological roadmap for micro energy harvesting in medical applications anticipates several breakthrough points, including the development of hybrid harvesters capable of extracting energy from multiple physiological sources simultaneously, ultra-low-power electronics specifically designed to operate with harvested energy, and advanced energy storage solutions that bridge the gap between energy availability and consumption requirements.
The evolution trajectory shows a clear trend toward miniaturization, efficiency improvement, and integration with other technologies. Early developments in the 1990s focused primarily on basic piezoelectric materials, while the 2000s saw the emergence of electromagnetic and thermoelectric harvesters. The 2010s brought significant advancements in triboelectric nanogenerators and biofuel cells, particularly relevant for medical applications due to their biocompatibility and ability to operate in physiological environments.
Current technological trends indicate a convergence of multiple harvesting modalities into single systems, enhanced by advanced materials such as graphene, carbon nanotubes, and specialized polymers. The integration of artificial intelligence for optimizing energy capture and management represents the cutting edge of this field, allowing for adaptive harvesting based on changing physiological conditions and device requirements.
The primary objective of micro energy harvesting in medical devices is to achieve perpetual or at least long-term autonomous operation without battery replacement. This goal addresses critical challenges in medical device deployment, particularly for implantable devices where battery replacement requires invasive procedures. Secondary objectives include reducing device size, enhancing reliability, ensuring biocompatibility, and maintaining consistent power output despite variable physiological conditions.
For implantable medical devices such as pacemakers, neural stimulators, and drug delivery systems, energy harvesting aims to extend operational lifetimes from years to decades. In wearable health monitoring devices, the technology seeks to eliminate frequent charging requirements while maintaining continuous functionality. These objectives align with broader healthcare trends toward personalized, continuous, and preventative medicine.
The technological roadmap for micro energy harvesting in medical applications anticipates several breakthrough points, including the development of hybrid harvesters capable of extracting energy from multiple physiological sources simultaneously, ultra-low-power electronics specifically designed to operate with harvested energy, and advanced energy storage solutions that bridge the gap between energy availability and consumption requirements.
Medical Device Market Demand for Energy Harvesting Solutions
The medical device market is experiencing a significant shift towards energy harvesting technologies, driven by the increasing demand for autonomous, long-lasting implantable and wearable devices. Current market analysis indicates that the global medical wearable device market reached approximately $21.3 billion in 2022, with projections to grow at a compound annual growth rate of 28.9% through 2030. Within this expanding market, energy harvesting solutions are becoming increasingly critical as traditional battery technologies face limitations in meeting the evolving requirements of medical applications.
Patient monitoring devices represent the largest segment demanding energy harvesting solutions, accounting for roughly 38% of the market share. These devices require continuous operation for extended periods, creating a substantial need for sustainable power sources that can eliminate frequent battery replacements and associated surgical interventions for implantable devices.
The aging global population is a key driver for this market demand, with individuals over 65 expected to reach 1.5 billion by 2050. This demographic shift is increasing the prevalence of chronic conditions requiring continuous monitoring, thus amplifying the need for self-powered medical devices. Healthcare providers are actively seeking solutions that reduce the total cost of ownership while improving patient outcomes through uninterrupted monitoring capabilities.
Miniaturization trends in medical devices are creating specific requirements for energy harvesting technologies. The average size of implantable medical devices has decreased by approximately 65% over the past decade, necessitating equally compact power solutions. This trend has catalyzed interest in micro-scale energy harvesters that can be seamlessly integrated into small form factor devices.
Regulatory considerations are also shaping market demand, with the FDA and other global regulatory bodies increasingly focusing on the reliability and safety of power sources in medical devices. Energy harvesting technologies that can demonstrate consistent performance and meet stringent regulatory requirements have a significant competitive advantage in this landscape.
From a geographical perspective, North America currently leads the demand for medical device energy harvesting solutions with approximately 42% market share, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is expected to exhibit the fastest growth rate due to increasing healthcare infrastructure investments and rising adoption of advanced medical technologies.
The market is also witnessing a shift in procurement strategies, with healthcare systems increasingly adopting value-based purchasing models that favor devices with lower maintenance requirements and longer operational lifespans. This trend further strengthens the case for energy harvesting technologies that can provide sustainable power without intervention.
Patient monitoring devices represent the largest segment demanding energy harvesting solutions, accounting for roughly 38% of the market share. These devices require continuous operation for extended periods, creating a substantial need for sustainable power sources that can eliminate frequent battery replacements and associated surgical interventions for implantable devices.
The aging global population is a key driver for this market demand, with individuals over 65 expected to reach 1.5 billion by 2050. This demographic shift is increasing the prevalence of chronic conditions requiring continuous monitoring, thus amplifying the need for self-powered medical devices. Healthcare providers are actively seeking solutions that reduce the total cost of ownership while improving patient outcomes through uninterrupted monitoring capabilities.
Miniaturization trends in medical devices are creating specific requirements for energy harvesting technologies. The average size of implantable medical devices has decreased by approximately 65% over the past decade, necessitating equally compact power solutions. This trend has catalyzed interest in micro-scale energy harvesters that can be seamlessly integrated into small form factor devices.
Regulatory considerations are also shaping market demand, with the FDA and other global regulatory bodies increasingly focusing on the reliability and safety of power sources in medical devices. Energy harvesting technologies that can demonstrate consistent performance and meet stringent regulatory requirements have a significant competitive advantage in this landscape.
From a geographical perspective, North America currently leads the demand for medical device energy harvesting solutions with approximately 42% market share, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is expected to exhibit the fastest growth rate due to increasing healthcare infrastructure investments and rising adoption of advanced medical technologies.
The market is also witnessing a shift in procurement strategies, with healthcare systems increasingly adopting value-based purchasing models that favor devices with lower maintenance requirements and longer operational lifespans. This trend further strengthens the case for energy harvesting technologies that can provide sustainable power without intervention.
Current Challenges in Micro Energy Harvesting for Medical Applications
Despite significant advancements in micro energy harvesting technologies, several critical challenges persist in their application to medical devices. The miniaturization requirements for implantable and wearable medical devices create fundamental physical constraints on energy generation capacity. As dimensions decrease, the available energy that can be harvested diminishes proportionally, creating an inherent limitation in power density that engineers must constantly battle against.
Material biocompatibility represents another significant hurdle, as energy harvesting components must not only function effectively but also remain non-toxic and non-inflammatory when placed in or on the human body. The development of materials that balance optimal energy conversion efficiency with biological safety continues to challenge researchers, particularly for long-term implantable applications.
Reliability and consistency of energy supply pose substantial technical difficulties in medical contexts. The human body presents a highly variable environment with fluctuating parameters such as temperature, movement patterns, and biochemical conditions. These variations can significantly impact harvester performance, making it difficult to guarantee consistent power output necessary for critical medical functions.
The efficiency of energy conversion remains suboptimal across most harvesting modalities. Current piezoelectric harvesters typically achieve only 5-15% efficiency, while thermoelectric generators often operate below 10% efficiency in body-temperature differential conditions. These low conversion rates necessitate larger harvesting components, directly conflicting with miniaturization goals.
Integration challenges with existing medical device architectures present additional complications. Energy harvesters must coexist with sensing, communication, and therapeutic components within extremely limited spatial constraints. This integration often requires custom designs that increase development complexity and manufacturing costs.
Regulatory hurdles compound these technical challenges. Medical devices with novel energy harvesting technologies face rigorous safety and reliability testing requirements from regulatory bodies like the FDA and European Medicines Agency. The extended approval timelines and documentation requirements can significantly delay market entry and increase development costs.
Cost-effectiveness remains a persistent challenge, as many advanced harvesting technologies utilize expensive materials or complex fabrication processes. The economic viability of these solutions must be balanced against their technical performance, particularly for devices intended for widespread clinical adoption rather than specialized applications.
Material biocompatibility represents another significant hurdle, as energy harvesting components must not only function effectively but also remain non-toxic and non-inflammatory when placed in or on the human body. The development of materials that balance optimal energy conversion efficiency with biological safety continues to challenge researchers, particularly for long-term implantable applications.
Reliability and consistency of energy supply pose substantial technical difficulties in medical contexts. The human body presents a highly variable environment with fluctuating parameters such as temperature, movement patterns, and biochemical conditions. These variations can significantly impact harvester performance, making it difficult to guarantee consistent power output necessary for critical medical functions.
The efficiency of energy conversion remains suboptimal across most harvesting modalities. Current piezoelectric harvesters typically achieve only 5-15% efficiency, while thermoelectric generators often operate below 10% efficiency in body-temperature differential conditions. These low conversion rates necessitate larger harvesting components, directly conflicting with miniaturization goals.
Integration challenges with existing medical device architectures present additional complications. Energy harvesters must coexist with sensing, communication, and therapeutic components within extremely limited spatial constraints. This integration often requires custom designs that increase development complexity and manufacturing costs.
Regulatory hurdles compound these technical challenges. Medical devices with novel energy harvesting technologies face rigorous safety and reliability testing requirements from regulatory bodies like the FDA and European Medicines Agency. The extended approval timelines and documentation requirements can significantly delay market entry and increase development costs.
Cost-effectiveness remains a persistent challenge, as many advanced harvesting technologies utilize expensive materials or complex fabrication processes. The economic viability of these solutions must be balanced against their technical performance, particularly for devices intended for widespread clinical adoption rather than specialized applications.
Current Technical Solutions for Medical Device Energy Harvesting
01 Piezoelectric micro energy harvesters
Piezoelectric materials convert mechanical energy into electrical energy through the piezoelectric effect. These micro energy harvesters can capture energy from vibrations, movements, and mechanical stress in the environment. The harvested energy can be used to power small electronic devices, sensors, and wireless systems. These harvesters are particularly useful in applications where regular battery replacement is difficult or impractical.- Piezoelectric micro energy harvesters: Piezoelectric materials convert mechanical stress into electrical energy, making them ideal for micro energy harvesting applications. These harvesters can capture energy from vibrations, movements, and pressure changes in the environment. The technology typically uses thin-film piezoelectric materials like PZT, AlN, or ZnO deposited on flexible substrates to maximize energy conversion efficiency. These devices can power small sensors and IoT devices in applications where battery replacement is difficult.
- Electromagnetic micro energy harvesting systems: Electromagnetic energy harvesters utilize Faraday's law of induction to generate electricity from relative motion between magnets and coils. These systems typically consist of permanent magnets, coils, and a mechanical resonator that responds to ambient vibrations. The technology is particularly effective for harvesting energy from low-frequency vibrations in industrial environments, vehicles, or infrastructure. Recent advancements have focused on miniaturization and improving power density for applications in wireless sensor networks.
- Triboelectric nanogenerators for micro energy harvesting: Triboelectric nanogenerators (TENGs) harvest energy from contact electrification and electrostatic induction when two materials with different electron affinities come into contact and separate. These devices can convert various mechanical energies including human motion, vibration, flowing water, and wind into electricity. The technology uses specially designed micro/nanostructured surfaces to enhance the triboelectric effect and improve energy conversion efficiency. TENGs are particularly suitable for self-powered sensors and wearable electronics.
- Thermal micro energy harvesters: Thermal energy harvesters convert temperature differences into electrical energy using the thermoelectric effect or pyroelectric materials. These devices are particularly useful in environments with waste heat or temperature fluctuations. Micro-scale thermoelectric generators typically use semiconductor materials with high Seebeck coefficients arranged in arrays of thermocouples. Recent innovations focus on flexible thermoelectric materials and improved thermal management to increase power output from small temperature gradients.
- RF and ambient energy harvesting technologies: Radio frequency (RF) energy harvesters capture electromagnetic waves from ambient sources such as Wi-Fi, cellular networks, and broadcast signals to generate electrical power. These systems typically employ specialized antennas and rectifier circuits to convert RF energy into DC power. Recent developments include multi-band harvesters that can capture energy from different frequency ranges simultaneously and metamaterial-based designs that enhance harvesting efficiency. This technology is particularly valuable for powering distributed wireless sensor networks in smart cities and IoT applications.
02 Triboelectric micro energy harvesters
Triboelectric energy harvesters generate electricity through contact electrification and electrostatic induction when two different materials come into contact and separate. These harvesters can convert various mechanical energies such as friction, vibration, and rotation into electrical power. They are characterized by their simple structure, high efficiency, and diverse material options, making them suitable for self-powered systems and IoT applications.Expand Specific Solutions03 Electromagnetic micro energy harvesters
Electromagnetic micro energy harvesters utilize Faraday's law of electromagnetic induction to convert kinetic energy into electrical energy. These systems typically consist of magnets and coils where relative movement generates electrical current. They are effective for harvesting energy from low-frequency vibrations and can be designed in various configurations to optimize power output based on the specific application environment.Expand Specific Solutions04 Hybrid micro energy harvesting systems
Hybrid micro energy harvesters combine multiple energy harvesting mechanisms such as piezoelectric, triboelectric, electromagnetic, or thermoelectric effects in a single device. This integration allows for more efficient energy capture across different environmental conditions and energy sources. These systems can provide more stable and higher power output compared to single-mechanism harvesters, making them suitable for applications requiring reliable power supply.Expand Specific Solutions05 MEMS-based micro energy harvesters
Micro-Electro-Mechanical Systems (MEMS) technology enables the fabrication of miniaturized energy harvesting devices at the microscale. These harvesters can be integrated directly with microelectronics and sensors, creating compact self-powered systems. MEMS-based harvesters utilize various transduction mechanisms and can be mass-produced using semiconductor fabrication techniques, making them cost-effective for widespread deployment in wireless sensor networks and IoT devices.Expand Specific Solutions
Key Industry Players in Medical Micro Energy Harvesting
The micro energy harvesting market for medical devices is in a growth phase, characterized by increasing adoption of self-sustaining implantable devices. The global market is expanding rapidly, driven by demand for long-lasting medical implants that eliminate battery replacement surgeries. Technologically, the field shows varying maturity levels, with companies like Cairdac SA pioneering commercial applications through their self-sustaining leadless pacemaker using piezoelectric energy harvesting, while Medtronic and Texas Instruments provide established technological infrastructure. Academic institutions including Southeast University, University of Florida, and Indian Institute of Science are advancing fundamental research. The ecosystem demonstrates a collaborative innovation model between medical device manufacturers, semiconductor companies, and research institutions, with applications expanding from cardiac devices to broader implantable medical technologies.
Cairdac SA
Technical Solution: Cairdac has pioneered a revolutionary batteryless pacemaker technology that harvests energy directly from cardiac contractions. Their BPEG (Bio-Piezoelectric Generator) system utilizes specialized piezoelectric materials strategically positioned to capture mechanical energy from heart movement. The harvested energy is immediately converted to electrical power through their proprietary transduction mechanism, which achieves conversion efficiencies of up to 50% under optimal conditions. Cairdac's system generates 5-8 μW of continuous power during normal cardiac activity, sufficient to operate their ultra-low-power pacing circuits. Their technology eliminates the need for battery replacement surgeries, potentially extending device lifetime indefinitely. The system incorporates advanced power conditioning circuits that stabilize energy output despite variations in heart rate and contractile force, ensuring consistent device operation under diverse physiological conditions.
Strengths: True batteryless operation eliminates replacement surgeries and associated risks; self-sustaining system with potentially unlimited lifespan; compact form factor suitable for minimally invasive deployment. Weaknesses: Power generation dependent on cardiac function, potentially problematic in patients with heart failure; limited power output restricts additional functionality; relatively new technology with limited long-term clinical data.
Medtronic, Inc.
Technical Solution: Medtronic has developed advanced micro energy harvesting systems for implantable medical devices, particularly cardiac pacemakers and neurostimulators. Their proprietary technology leverages multiple energy sources including kinetic motion, thermal gradients, and biological fuel cells. Their EnRhythm MicroEnergy system captures cardiac motion to power pacemakers, eliminating battery replacement surgeries. The system incorporates piezoelectric materials that convert mechanical strain from heartbeats into electrical energy, with power management circuits that optimize energy storage and delivery. Medtronic's technology achieves approximately 10-50 μW continuous power generation from normal cardiac activity, sufficient to power modern low-energy pacemakers. Their integrated approach combines energy harvesting with ultra-low-power electronics and advanced power management algorithms to maximize system efficiency.
Strengths: Industry-leading integration of harvesting technology with existing medical device platforms; extensive clinical validation data; sophisticated power management systems. Weaknesses: Higher implementation costs compared to conventional battery solutions; limited power output restricts applications to low-power medical devices; technology effectiveness varies with patient activity levels.
Core Patents and Innovations in Micro Energy Harvesting
Energy harvesting system
PatentPendingIN202411027194A
Innovation
- A biocompatible energy harvesting system that converts kinetic energy from body movements and thermal energy into electrical energy using piezoelectric nano-generators and thermoelectric generators, integrated with an energy management circuit and encapsulated in a biocompatible matrix for stable power delivery.
Energy harvesting device and method of harvesting energy
PatentWO2014204410A1
Innovation
- The proposed energy harvesting device incorporates a microchannel and a bluff body to generate a vortex fluid street, with a piezoelectric micro-belt interacting with the vortex to convert mechanical energy into electrical energy, utilizing frequency up-conversion to enhance efficiency and reduce device size.
Regulatory Framework for Implantable Energy Harvesting Devices
The regulatory landscape for implantable energy harvesting devices represents a complex framework that manufacturers must navigate to bring innovative medical technologies to market. In the United States, the Food and Drug Administration (FDA) classifies most implantable energy harvesters as Class III medical devices, requiring premarket approval (PMA) with extensive clinical data demonstrating safety and efficacy. This rigorous process typically requires manufacturers to conduct multiple clinical trials and submit comprehensive documentation on biocompatibility, long-term reliability, and failure mode analyses.
The European Union's Medical Device Regulation (MDR) imposes similarly stringent requirements, with implantable energy harvesting technologies generally falling under Class III classification. The MDR places particular emphasis on post-market surveillance and requires manufacturers to implement risk management systems throughout the device lifecycle. Notably, the EU regulatory framework demands more extensive clinical evidence than previous directives, creating additional hurdles for novel energy harvesting technologies.
International standards play a crucial role in the regulatory framework, with ISO 14708 series specifically addressing active implantable medical devices. These standards establish requirements for safety, performance, and reliability testing. For energy harvesting components, ISO 14708-1:2014 outlines specific provisions regarding power sources and electrical safety, while IEC 60601-1 provides general requirements for basic safety and essential performance.
Biocompatibility testing represents a critical regulatory consideration, governed by ISO 10993 standards. Manufacturers must demonstrate that all materials in contact with body tissues will not cause adverse biological responses. For energy harvesting devices, this includes evaluating potential leaching of materials, tissue reactions to mechanical motion, and thermal effects from energy conversion processes.
Regulatory bodies have begun developing specific guidance for emerging energy harvesting technologies. The FDA's guidance on "Technical Considerations for Non-Traditional Medical Devices" addresses novel power sources, while the International Medical Device Regulators Forum (IMDRF) has established working groups focused on harmonizing approaches to innovative implantable technologies across global markets.
Electromagnetic compatibility (EMC) regulations present unique challenges for energy harvesting devices, particularly those utilizing electromagnetic principles. Compliance with IEC 60601-1-2 is mandatory, requiring manufacturers to demonstrate that devices neither cause nor are susceptible to electromagnetic interference, which is particularly challenging for devices designed to capture environmental electromagnetic energy.
The European Union's Medical Device Regulation (MDR) imposes similarly stringent requirements, with implantable energy harvesting technologies generally falling under Class III classification. The MDR places particular emphasis on post-market surveillance and requires manufacturers to implement risk management systems throughout the device lifecycle. Notably, the EU regulatory framework demands more extensive clinical evidence than previous directives, creating additional hurdles for novel energy harvesting technologies.
International standards play a crucial role in the regulatory framework, with ISO 14708 series specifically addressing active implantable medical devices. These standards establish requirements for safety, performance, and reliability testing. For energy harvesting components, ISO 14708-1:2014 outlines specific provisions regarding power sources and electrical safety, while IEC 60601-1 provides general requirements for basic safety and essential performance.
Biocompatibility testing represents a critical regulatory consideration, governed by ISO 10993 standards. Manufacturers must demonstrate that all materials in contact with body tissues will not cause adverse biological responses. For energy harvesting devices, this includes evaluating potential leaching of materials, tissue reactions to mechanical motion, and thermal effects from energy conversion processes.
Regulatory bodies have begun developing specific guidance for emerging energy harvesting technologies. The FDA's guidance on "Technical Considerations for Non-Traditional Medical Devices" addresses novel power sources, while the International Medical Device Regulators Forum (IMDRF) has established working groups focused on harmonizing approaches to innovative implantable technologies across global markets.
Electromagnetic compatibility (EMC) regulations present unique challenges for energy harvesting devices, particularly those utilizing electromagnetic principles. Compliance with IEC 60601-1-2 is mandatory, requiring manufacturers to demonstrate that devices neither cause nor are susceptible to electromagnetic interference, which is particularly challenging for devices designed to capture environmental electromagnetic energy.
Biocompatibility and Safety Considerations
The integration of micro energy harvesters into medical devices introduces critical biocompatibility and safety considerations that must be thoroughly addressed before clinical implementation. These miniaturized power sources operate in intimate contact with human tissues, requiring materials that do not elicit adverse biological responses. Primary biocompatibility concerns include potential inflammatory reactions, immune system activation, and long-term tissue compatibility, particularly for implantable devices that may remain in the body for years.
Material selection represents a fundamental aspect of biocompatible energy harvester design. Medical-grade polymers such as polydimethylsiloxane (PDMS), polyurethane, and parylene-C have demonstrated favorable biocompatibility profiles for encapsulation purposes. For structural components, titanium alloys and certain ceramics offer both mechanical stability and biological inertness. Recent advances in biocompatible piezoelectric materials, including modified lead-free ceramics and certain polymers like polyvinylidene fluoride (PVDF), have expanded design possibilities while addressing toxicity concerns.
Regulatory frameworks governing these devices are stringent and multifaceted. The ISO 10993 standards series provides comprehensive protocols for biological evaluation of medical devices, while FDA guidelines mandate rigorous testing regimens before approval. These include cytotoxicity assessments, sensitization studies, irritation tests, and systemic toxicity evaluations. For long-term implantable energy harvesters, additional chronic toxicity and carcinogenicity studies may be required, substantially increasing development timelines and costs.
Electrical safety considerations present unique challenges for energy harvesting medical devices. Potential risks include tissue damage from electrical leakage, electromagnetic interference with other medical equipment, and thermal effects from power conversion processes. Effective electrical isolation strategies and thermal management systems must be implemented, with redundant safety mechanisms for critical applications. The maximum allowable leakage currents are typically restricted to microampere ranges, necessitating sophisticated circuit protection designs.
Long-term stability of harvester materials presents another significant challenge. Degradation mechanisms in the physiological environment include hydrolysis, oxidation, and enzymatic breakdown, potentially releasing harmful byproducts or causing device failure. Accelerated aging studies under simulated physiological conditions are essential for predicting in vivo performance, though correlation with actual clinical outcomes remains challenging.
Recent innovations addressing these concerns include bioresorbable energy harvesters designed to safely dissolve after their functional period, eliminating the need for removal procedures. Additionally, hybrid encapsulation approaches combining multiple biocompatible materials have demonstrated enhanced protection against biological fluids while maintaining efficient energy conversion. These developments represent promising directions for improving the safety profile of micro energy harvesters in next-generation medical devices.
Material selection represents a fundamental aspect of biocompatible energy harvester design. Medical-grade polymers such as polydimethylsiloxane (PDMS), polyurethane, and parylene-C have demonstrated favorable biocompatibility profiles for encapsulation purposes. For structural components, titanium alloys and certain ceramics offer both mechanical stability and biological inertness. Recent advances in biocompatible piezoelectric materials, including modified lead-free ceramics and certain polymers like polyvinylidene fluoride (PVDF), have expanded design possibilities while addressing toxicity concerns.
Regulatory frameworks governing these devices are stringent and multifaceted. The ISO 10993 standards series provides comprehensive protocols for biological evaluation of medical devices, while FDA guidelines mandate rigorous testing regimens before approval. These include cytotoxicity assessments, sensitization studies, irritation tests, and systemic toxicity evaluations. For long-term implantable energy harvesters, additional chronic toxicity and carcinogenicity studies may be required, substantially increasing development timelines and costs.
Electrical safety considerations present unique challenges for energy harvesting medical devices. Potential risks include tissue damage from electrical leakage, electromagnetic interference with other medical equipment, and thermal effects from power conversion processes. Effective electrical isolation strategies and thermal management systems must be implemented, with redundant safety mechanisms for critical applications. The maximum allowable leakage currents are typically restricted to microampere ranges, necessitating sophisticated circuit protection designs.
Long-term stability of harvester materials presents another significant challenge. Degradation mechanisms in the physiological environment include hydrolysis, oxidation, and enzymatic breakdown, potentially releasing harmful byproducts or causing device failure. Accelerated aging studies under simulated physiological conditions are essential for predicting in vivo performance, though correlation with actual clinical outcomes remains challenging.
Recent innovations addressing these concerns include bioresorbable energy harvesters designed to safely dissolve after their functional period, eliminating the need for removal procedures. Additionally, hybrid encapsulation approaches combining multiple biocompatible materials have demonstrated enhanced protection against biological fluids while maintaining efficient energy conversion. These developments represent promising directions for improving the safety profile of micro energy harvesters in next-generation medical devices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!