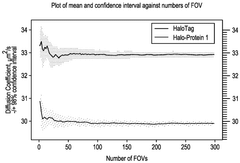
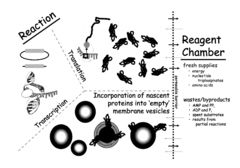
Analytical methods for intracellular processes in cell-free systems.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-Free Systems Background and Research Objectives
Cell-free systems represent a paradigm shift in synthetic biology, emerging from the pioneering work of Nirenberg and Matthaei in the 1960s who utilized cell extracts to decipher the genetic code. These systems have evolved from simple protein synthesis platforms to sophisticated biomolecular factories capable of complex reactions without cellular constraints. The fundamental principle involves extracting cellular machinery while eliminating cell walls and genomic material, creating a controlled environment for studying intracellular processes.
The evolution of cell-free systems has accelerated dramatically in the past decade, transitioning from academic curiosity to practical biotechnological tools. Modern cell-free platforms derive from various organisms including Escherichia coli, Saccharomyces cerevisiae, rabbit reticulocytes, wheat germ, and insect cells, each offering distinct advantages for specific applications. This diversity has expanded the utility of cell-free systems across pharmaceutical development, biosensing, and fundamental research.
Current analytical methods for intracellular processes in cell-free systems face significant limitations in real-time monitoring, quantitative precision, and multiplexed analysis capabilities. Traditional approaches often rely on endpoint measurements that fail to capture the dynamic nature of biochemical reactions. Furthermore, existing methods frequently require labeling strategies that may interfere with native biomolecular interactions or necessitate complex sample preparation procedures.
The primary research objectives in this field focus on developing novel analytical techniques that overcome these limitations. Specifically, we aim to establish methodologies that enable: (1) real-time monitoring of multiple reaction parameters simultaneously; (2) quantitative assessment of reaction kinetics with minimal interference; (3) spatial resolution of reaction components within the cell-free environment; and (4) integration of these analytical capabilities with automated high-throughput platforms.
Achieving these objectives would transform our understanding of fundamental cellular processes by providing unprecedented insights into reaction dynamics, metabolic pathways, and regulatory mechanisms. From an applied perspective, enhanced analytical methods would accelerate the optimization of cell-free systems for biomanufacturing applications, including the production of therapeutics, vaccines, and biomaterials under precisely controlled conditions.
The convergence of advanced analytical technologies—including microfluidics, label-free detection methods, and computational modeling—presents promising opportunities to address current challenges. By leveraging these interdisciplinary approaches, we anticipate developing comprehensive analytical frameworks that reveal the intricate mechanisms governing intracellular processes in cell-free environments, ultimately advancing both fundamental science and biotechnological applications.
The evolution of cell-free systems has accelerated dramatically in the past decade, transitioning from academic curiosity to practical biotechnological tools. Modern cell-free platforms derive from various organisms including Escherichia coli, Saccharomyces cerevisiae, rabbit reticulocytes, wheat germ, and insect cells, each offering distinct advantages for specific applications. This diversity has expanded the utility of cell-free systems across pharmaceutical development, biosensing, and fundamental research.
Current analytical methods for intracellular processes in cell-free systems face significant limitations in real-time monitoring, quantitative precision, and multiplexed analysis capabilities. Traditional approaches often rely on endpoint measurements that fail to capture the dynamic nature of biochemical reactions. Furthermore, existing methods frequently require labeling strategies that may interfere with native biomolecular interactions or necessitate complex sample preparation procedures.
The primary research objectives in this field focus on developing novel analytical techniques that overcome these limitations. Specifically, we aim to establish methodologies that enable: (1) real-time monitoring of multiple reaction parameters simultaneously; (2) quantitative assessment of reaction kinetics with minimal interference; (3) spatial resolution of reaction components within the cell-free environment; and (4) integration of these analytical capabilities with automated high-throughput platforms.
Achieving these objectives would transform our understanding of fundamental cellular processes by providing unprecedented insights into reaction dynamics, metabolic pathways, and regulatory mechanisms. From an applied perspective, enhanced analytical methods would accelerate the optimization of cell-free systems for biomanufacturing applications, including the production of therapeutics, vaccines, and biomaterials under precisely controlled conditions.
The convergence of advanced analytical technologies—including microfluidics, label-free detection methods, and computational modeling—presents promising opportunities to address current challenges. By leveraging these interdisciplinary approaches, we anticipate developing comprehensive analytical frameworks that reveal the intricate mechanisms governing intracellular processes in cell-free environments, ultimately advancing both fundamental science and biotechnological applications.
Market Analysis for Cell-Free Analytical Technologies
The cell-free analytical technologies market is experiencing robust growth, driven by increasing applications in synthetic biology, pharmaceutical research, and diagnostics. Currently valued at approximately $1.2 billion, this market is projected to grow at a CAGR of 8.5% over the next five years, potentially reaching $1.8 billion by 2028. This growth trajectory is supported by the expanding adoption of cell-free systems as alternatives to traditional cell-based methods across various research domains.
The pharmaceutical and biotechnology sectors represent the largest market segments, collectively accounting for over 60% of the total market share. These industries leverage cell-free analytical technologies for drug discovery, protein production, and toxicity screening, appreciating the reduced complexity and increased control offered by cell-free environments. Academic research institutions constitute another significant market segment, contributing approximately 25% of the market demand, primarily focused on fundamental research and method development.
Geographically, North America dominates the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and Japan, is expected to witness the fastest growth rate due to increasing investments in biotechnology research infrastructure and favorable government policies supporting innovation in life sciences.
Key market drivers include the growing demand for rapid and cost-effective drug development processes, increasing research in synthetic biology, and the rising prevalence of chronic diseases necessitating novel therapeutic approaches. The ability of cell-free systems to provide insights into intracellular processes without cellular complexity represents a significant value proposition for researchers and industry professionals alike.
Market challenges include the high cost of reagents and equipment, technical complexities in maintaining system stability, and standardization issues across different analytical platforms. Additionally, regulatory uncertainties regarding the validation of results obtained from cell-free systems for clinical applications present barriers to market expansion in certain segments.
Customer segments are diversifying beyond traditional research institutions to include biotechnology startups, contract research organizations, and diagnostic companies. This broadening customer base is driving demand for more specialized and application-specific cell-free analytical solutions, creating opportunities for market differentiation and niche product development.
The market exhibits moderate fragmentation with several established players and emerging companies competing through technological innovation and strategic partnerships. Price sensitivity varies across customer segments, with academic institutions being more price-conscious compared to pharmaceutical companies that prioritize performance and reliability.
The pharmaceutical and biotechnology sectors represent the largest market segments, collectively accounting for over 60% of the total market share. These industries leverage cell-free analytical technologies for drug discovery, protein production, and toxicity screening, appreciating the reduced complexity and increased control offered by cell-free environments. Academic research institutions constitute another significant market segment, contributing approximately 25% of the market demand, primarily focused on fundamental research and method development.
Geographically, North America dominates the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and Japan, is expected to witness the fastest growth rate due to increasing investments in biotechnology research infrastructure and favorable government policies supporting innovation in life sciences.
Key market drivers include the growing demand for rapid and cost-effective drug development processes, increasing research in synthetic biology, and the rising prevalence of chronic diseases necessitating novel therapeutic approaches. The ability of cell-free systems to provide insights into intracellular processes without cellular complexity represents a significant value proposition for researchers and industry professionals alike.
Market challenges include the high cost of reagents and equipment, technical complexities in maintaining system stability, and standardization issues across different analytical platforms. Additionally, regulatory uncertainties regarding the validation of results obtained from cell-free systems for clinical applications present barriers to market expansion in certain segments.
Customer segments are diversifying beyond traditional research institutions to include biotechnology startups, contract research organizations, and diagnostic companies. This broadening customer base is driving demand for more specialized and application-specific cell-free analytical solutions, creating opportunities for market differentiation and niche product development.
The market exhibits moderate fragmentation with several established players and emerging companies competing through technological innovation and strategic partnerships. Price sensitivity varies across customer segments, with academic institutions being more price-conscious compared to pharmaceutical companies that prioritize performance and reliability.
Current Challenges in Intracellular Process Analysis
Despite significant advancements in cell-free systems for studying intracellular processes, researchers face substantial analytical challenges that limit the full potential of these platforms. The primary obstacle remains the accurate quantification of dynamic biochemical reactions occurring within these artificial environments. Current analytical methods often lack the temporal resolution needed to capture rapid enzymatic cascades and transient molecular interactions that characterize many cellular pathways.
Sensitivity limitations present another critical challenge, particularly when analyzing low-abundance molecules such as signaling proteins or regulatory RNAs. While techniques like mass spectrometry offer impressive detection capabilities, they frequently require sample volumes or concentrations that exceed what is practically available in many cell-free experimental setups, creating a fundamental mismatch between analytical requirements and experimental constraints.
The complex matrix effects inherent to cell-free systems further complicate analysis. Components necessary for maintaining functionality—such as crowding agents, energy regeneration systems, and stabilizing proteins—can interfere with analytical measurements, producing artifacts or masking signals of interest. This matrix complexity necessitates extensive method development and validation for each specific cell-free system configuration.
Multiplexing capabilities remain insufficient for comprehensive pathway analysis. Most current methods excel at measuring one class of biomolecules but struggle to simultaneously track multiple molecular species (proteins, metabolites, nucleic acids) within the same sample. This limitation prevents researchers from obtaining integrated views of interconnected biochemical networks operating in cell-free systems.
Spatial resolution represents another significant gap in analytical capabilities. While cell-free systems are often treated as homogeneous solutions, evidence suggests that spatial organization and molecular crowding significantly influence reaction kinetics and pathway efficiency. Current analytical approaches largely fail to capture these spatial dimensions of biochemical processes.
Data integration across different analytical platforms presents additional challenges. Researchers typically employ multiple techniques to analyze different aspects of cell-free systems, but lack standardized frameworks for integrating these diverse datasets into coherent models of intracellular processes. This fragmentation hampers comprehensive understanding of system behavior.
Finally, real-time monitoring capabilities remain limited. Many analytical methods require sample processing steps that preclude continuous measurement, resulting in discrete time points rather than continuous reaction profiles. This limitation is particularly problematic for studying feedback mechanisms and oscillatory behaviors that characterize many cellular regulatory networks.
Sensitivity limitations present another critical challenge, particularly when analyzing low-abundance molecules such as signaling proteins or regulatory RNAs. While techniques like mass spectrometry offer impressive detection capabilities, they frequently require sample volumes or concentrations that exceed what is practically available in many cell-free experimental setups, creating a fundamental mismatch between analytical requirements and experimental constraints.
The complex matrix effects inherent to cell-free systems further complicate analysis. Components necessary for maintaining functionality—such as crowding agents, energy regeneration systems, and stabilizing proteins—can interfere with analytical measurements, producing artifacts or masking signals of interest. This matrix complexity necessitates extensive method development and validation for each specific cell-free system configuration.
Multiplexing capabilities remain insufficient for comprehensive pathway analysis. Most current methods excel at measuring one class of biomolecules but struggle to simultaneously track multiple molecular species (proteins, metabolites, nucleic acids) within the same sample. This limitation prevents researchers from obtaining integrated views of interconnected biochemical networks operating in cell-free systems.
Spatial resolution represents another significant gap in analytical capabilities. While cell-free systems are often treated as homogeneous solutions, evidence suggests that spatial organization and molecular crowding significantly influence reaction kinetics and pathway efficiency. Current analytical approaches largely fail to capture these spatial dimensions of biochemical processes.
Data integration across different analytical platforms presents additional challenges. Researchers typically employ multiple techniques to analyze different aspects of cell-free systems, but lack standardized frameworks for integrating these diverse datasets into coherent models of intracellular processes. This fragmentation hampers comprehensive understanding of system behavior.
Finally, real-time monitoring capabilities remain limited. Many analytical methods require sample processing steps that preclude continuous measurement, resulting in discrete time points rather than continuous reaction profiles. This limitation is particularly problematic for studying feedback mechanisms and oscillatory behaviors that characterize many cellular regulatory networks.
Established Analytical Techniques for Cell-Free Systems
01 Fluorescence-based techniques for intracellular analysis
Fluorescence-based methods are widely used for analyzing intracellular processes. These techniques involve the use of fluorescent probes or markers that can be detected within living cells. They allow for real-time visualization and quantification of cellular components, molecular interactions, and biochemical processes. These methods include fluorescence microscopy, flow cytometry, and fluorescence resonance energy transfer (FRET), which provide insights into protein localization, enzyme activity, and signaling pathways within cells.- Fluorescence-based analytical methods: Fluorescence-based techniques are widely used for analyzing intracellular processes. These methods involve the use of fluorescent probes or markers that can be detected within living cells. The fluorescent signals can be measured using various imaging techniques, allowing researchers to track molecular interactions, protein localization, and cellular dynamics in real-time. These methods provide high sensitivity and specificity for studying complex intracellular processes.
- Molecular biology techniques for intracellular analysis: Various molecular biology techniques are employed to analyze intracellular processes. These include PCR-based methods, gene expression analysis, RNA interference, and CRISPR-based approaches. These techniques allow researchers to manipulate gene expression, study protein-protein interactions, and investigate signaling pathways within cells. By combining these molecular approaches with advanced imaging and analytical tools, researchers can gain comprehensive insights into complex intracellular mechanisms.
- Cell-based assays for intracellular process monitoring: Cell-based assays provide valuable tools for monitoring intracellular processes in living cells. These assays can measure various cellular parameters such as enzyme activity, receptor signaling, metabolic changes, and cell viability. High-throughput screening platforms allow for rapid analysis of multiple samples simultaneously. These methods are particularly useful for drug discovery and for understanding how compounds affect specific intracellular pathways.
- Imaging technologies for visualizing intracellular dynamics: Advanced imaging technologies enable the visualization of intracellular dynamics with high spatial and temporal resolution. These include confocal microscopy, super-resolution microscopy, live-cell imaging, and electron microscopy. These techniques allow researchers to observe cellular structures, track protein movements, and analyze dynamic processes within cells. The integration of computational tools with imaging technologies further enhances the ability to quantify and interpret complex intracellular phenomena.
- Biochemical methods for analyzing intracellular components: Biochemical methods are essential for analyzing intracellular components and their interactions. These include protein purification, enzymatic assays, chromatography, mass spectrometry, and structural analysis techniques. These approaches allow researchers to isolate and characterize specific cellular components, determine their biochemical properties, and understand their roles in intracellular processes. The combination of biochemical methods with other analytical techniques provides comprehensive insights into cellular function and regulation.
02 Molecular biology techniques for studying gene expression
Various molecular biology techniques are employed to analyze gene expression and regulation within cells. These methods include polymerase chain reaction (PCR), RNA sequencing, microarrays, and in situ hybridization. They enable researchers to quantify gene expression levels, identify transcription factors, and understand gene regulatory networks. These techniques provide valuable insights into how cells respond to different stimuli and how genetic information is processed within the cellular environment.Expand Specific Solutions03 Imaging technologies for cellular structure and function
Advanced imaging technologies allow for detailed visualization of cellular structures and functions. These include confocal microscopy, super-resolution microscopy, electron microscopy, and live-cell imaging techniques. These methods enable researchers to observe cellular organelles, cytoskeletal arrangements, and dynamic processes such as vesicle trafficking and cell division. By providing high-resolution images of cellular components, these technologies help in understanding the spatial organization and temporal dynamics of intracellular processes.Expand Specific Solutions04 Biochemical assays for protein interactions and modifications
Biochemical assays are essential for studying protein interactions and post-translational modifications within cells. These include co-immunoprecipitation, yeast two-hybrid systems, protein arrays, and mass spectrometry-based proteomics. These methods help in identifying protein-protein interactions, characterizing enzyme activities, and detecting post-translational modifications such as phosphorylation, ubiquitination, and glycosylation. Understanding these protein-level processes is crucial for deciphering signaling pathways and regulatory mechanisms in cells.Expand Specific Solutions05 Single-cell analysis methods
Single-cell analysis methods allow for the study of cellular heterogeneity and individual cell responses. These techniques include single-cell RNA sequencing, mass cytometry, and microfluidic approaches. They enable researchers to analyze gene expression, protein levels, and metabolic states at the individual cell level, revealing cell-to-cell variations that might be masked in bulk analyses. These methods are particularly valuable for understanding complex tissues, cellular differentiation processes, and rare cell populations within heterogeneous samples.Expand Specific Solutions
Leading Research Groups and Commercial Entities
The analytical methods for intracellular processes in cell-free systems market is currently in a growth phase, with increasing adoption across pharmaceutical and biotechnology sectors. The global market size is estimated to be expanding at a CAGR of 8-10%, driven by demand for efficient drug development processes and personalized medicine applications. From a technological maturity perspective, the landscape shows varying degrees of advancement. Industry leaders like Agilent Technologies and Bio-Rad Laboratories have established comprehensive analytical platforms, while specialized players such as GreenLight Biosciences and CS Genetics are driving innovation in RNA-based technologies and single-cell analysis respectively. Academic institutions including MIT, Harvard, and Tsinghua University continue to contribute foundational research, creating a dynamic ecosystem where commercial applications are rapidly evolving from theoretical concepts.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has pioneered comprehensive analytical solutions for cell-free systems through their integrated workflow approach. Their technology combines high-sensitivity mass spectrometry, microfluidics, and automated sample preparation to enable detailed characterization of intracellular processes in cell-free environments. The Agilent Cell-Free Analysis Suite incorporates specialized reagent kits optimized for protein expression monitoring, metabolite profiling, and enzyme kinetics studies. Their analytical platform features real-time monitoring capabilities with temporal resolution down to seconds, allowing researchers to capture transient biochemical states that would be missed in traditional endpoint assays. The system employs machine learning algorithms to process complex multi-parameter data, identifying patterns and correlations across different molecular species. Agilent's technology enables multiplexed analysis of up to 100 different analytes simultaneously from a single cell-free reaction, providing unprecedented insight into pathway dynamics and regulatory mechanisms. The platform has been validated across diverse applications including drug metabolism studies, protein-protein interaction mapping, and synthetic biology circuit characterization.
Strengths: Exceptional analytical sensitivity detecting biomolecules at femtomolar concentrations; comprehensive integration across multiple analytical modalities; extensive validation across diverse research applications with proven reproducibility. Weaknesses: Significant capital investment required for full platform implementation; complex data analysis workflows require specialized expertise; reagent costs can be prohibitive for high-throughput applications.
GreenLight Biosciences, Inc.
Technical Solution: GreenLight Biosciences has developed a cell-free protein synthesis platform specifically optimized for analytical methods in intracellular processes. Their technology utilizes a cell lysate-based system that maintains the biochemical environment of cells while allowing direct access for analytical tools. The platform incorporates real-time monitoring capabilities through fluorescent reporters and mass spectrometry integration, enabling quantitative analysis of protein synthesis kinetics, metabolic flux, and enzymatic activities. GreenLight's system features standardized reaction conditions that ensure reproducibility across experiments, with proprietary lysate preparation methods that preserve critical cellular components. Their analytical framework includes computational models that predict reaction outcomes based on input parameters, allowing researchers to optimize conditions before physical experimentation. The company has demonstrated successful applications in vaccine development, enzyme engineering, and metabolic pathway optimization with significantly reduced development timelines compared to traditional cell-based methods.
Strengths: Superior scalability from microliter to liter scale while maintaining performance consistency; integrated analytical tools provide comprehensive data collection without disrupting reactions; reduced development time compared to cell-based systems. Weaknesses: Higher cost per reaction compared to some competing technologies; requires specialized equipment for optimal performance; limited shelf-life of reaction components necessitates careful logistics.
Key Innovations in Intracellular Process Detection
Methods and systems for single molecule tracking in cell-free systems
PatentWO2025006619A1
Innovation
- High-throughput methods involving the tracking of target molecules over time to generate spatiotemporal trajectories, with comparisons to reference movements to identify interactions, determine molecule forms, and assess dose responses, using techniques such as diffusion coefficient analysis and machine learning methods like variational Bayesian inference.
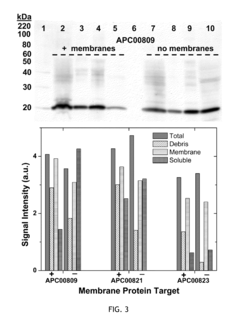
Cell-free system for synthesizing membrane proteins cell free method for synthesizing membrane proteins
PatentActiveUS20110244524A1
Innovation
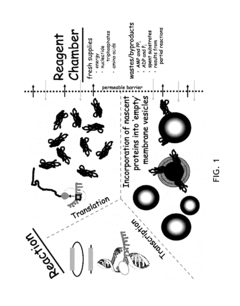
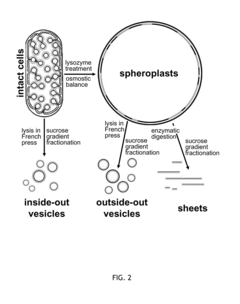
- A cell-free system utilizing Rhodobacter extracts with a coupled transcription/translation system, where intracytoplasmic membranes (ICM) from genetically modified organisms are used to produce and encapsulate heterologous membrane proteins, reducing native protein content and optimizing membrane space for maximal protein encapsulation, allowing for simultaneous production and sequestration.
Standardization and Reproducibility Considerations
One of the most significant challenges in cell-free systems research is the lack of standardized protocols and analytical methods, which hampers reproducibility across different laboratories and experimental setups. The field currently suffers from considerable variability in preparation methods, component concentrations, and analytical techniques, making direct comparison of results difficult. Establishing standardized protocols for cell extract preparation, reaction conditions, and analytical procedures is essential for advancing the field and enabling meaningful cross-laboratory validation.
Reproducibility issues in cell-free systems stem from multiple sources, including variations in cell growth conditions prior to extract preparation, differences in lysis methods, and inconsistencies in post-lysis processing. These variations can significantly impact the performance and characteristics of the resulting cell-free systems, affecting the reliability of analytical measurements of intracellular processes. The development of reference standards and control reactions that can be universally adopted would provide benchmarks against which different analytical methods could be calibrated.
Analytical method validation represents another critical aspect of standardization. Currently, many laboratories employ custom-developed assays without comprehensive validation of their sensitivity, specificity, and dynamic range. This practice introduces uncertainty when comparing data across studies. Implementing rigorous validation protocols for analytical methods used in cell-free systems would enhance confidence in reported results and facilitate meta-analyses across multiple studies.
Data reporting standards also require attention, as inconsistent reporting formats and incomplete experimental details hinder reproducibility efforts. The field would benefit from consensus guidelines specifying minimum information requirements for publications and data repositories. These standards should include detailed descriptions of extract preparation, reaction composition, analytical methods, and raw data processing algorithms to enable other researchers to replicate experiments accurately.
International collaborative initiatives are emerging to address these standardization challenges. These efforts aim to develop community-accepted protocols, reference materials, and quality control metrics specifically designed for cell-free systems. Such initiatives could significantly accelerate progress by reducing redundant method development and enabling researchers to build more effectively on previous work. The establishment of interlaboratory comparison studies would further strengthen confidence in analytical methods by identifying sources of variability and best practices for minimizing them.
Reproducibility issues in cell-free systems stem from multiple sources, including variations in cell growth conditions prior to extract preparation, differences in lysis methods, and inconsistencies in post-lysis processing. These variations can significantly impact the performance and characteristics of the resulting cell-free systems, affecting the reliability of analytical measurements of intracellular processes. The development of reference standards and control reactions that can be universally adopted would provide benchmarks against which different analytical methods could be calibrated.
Analytical method validation represents another critical aspect of standardization. Currently, many laboratories employ custom-developed assays without comprehensive validation of their sensitivity, specificity, and dynamic range. This practice introduces uncertainty when comparing data across studies. Implementing rigorous validation protocols for analytical methods used in cell-free systems would enhance confidence in reported results and facilitate meta-analyses across multiple studies.
Data reporting standards also require attention, as inconsistent reporting formats and incomplete experimental details hinder reproducibility efforts. The field would benefit from consensus guidelines specifying minimum information requirements for publications and data repositories. These standards should include detailed descriptions of extract preparation, reaction composition, analytical methods, and raw data processing algorithms to enable other researchers to replicate experiments accurately.
International collaborative initiatives are emerging to address these standardization challenges. These efforts aim to develop community-accepted protocols, reference materials, and quality control metrics specifically designed for cell-free systems. Such initiatives could significantly accelerate progress by reducing redundant method development and enabling researchers to build more effectively on previous work. The establishment of interlaboratory comparison studies would further strengthen confidence in analytical methods by identifying sources of variability and best practices for minimizing them.
Biosafety and Ethical Implications
The development of cell-free systems for analyzing intracellular processes brings significant biosafety and ethical considerations that must be addressed comprehensively. Unlike traditional cellular systems, cell-free approaches eliminate certain containment concerns by operating without intact, reproducing cells. However, this technology introduces novel biosafety challenges, particularly when working with components derived from pathogenic organisms or when engineering synthetic biological pathways that could potentially produce harmful substances.
Risk assessment frameworks specifically tailored to cell-free systems remain underdeveloped compared to those for intact cellular systems. The potential for reconstituting infectious agents or creating novel biological entities with unpredictable properties necessitates rigorous safety protocols. Current biosafety guidelines typically classify cell-free systems at lower containment levels than their cellular counterparts, yet this classification may underestimate certain risks, especially as analytical capabilities become more sophisticated.
Ethical implications extend beyond laboratory safety to broader societal concerns. The accessibility of cell-free technologies potentially democratizes biotechnology, enabling wider participation in biological research and development. While this democratization promotes innovation, it simultaneously raises concerns about potential misuse or accidental harm by inexperienced practitioners. The reduced technical barriers could enable both beneficial applications and potential biosecurity threats, creating a dual-use dilemma that requires careful governance.
Intellectual property considerations present another ethical dimension. As analytical methods for cell-free systems advance, questions arise regarding ownership of biological information, synthetic pathways, and engineered components. The tension between open science principles and commercial interests becomes particularly acute when fundamental biological processes are involved, potentially affecting access to these technologies in resource-limited settings.
Privacy concerns emerge when cell-free systems are used for diagnostic purposes or personalized medicine applications. The analysis of human-derived components raises questions about consent, data ownership, and potential discrimination based on biological information. Establishing ethical frameworks that balance innovation with protection of individual rights remains a critical challenge.
Regulatory frameworks must evolve to address these unique challenges. Current regulations often focus on organism-based risk assessments rather than process-based or component-based evaluations more appropriate for cell-free systems. International harmonization of these regulations becomes increasingly important as the technology crosses borders through collaborative research and commercial applications.
Risk assessment frameworks specifically tailored to cell-free systems remain underdeveloped compared to those for intact cellular systems. The potential for reconstituting infectious agents or creating novel biological entities with unpredictable properties necessitates rigorous safety protocols. Current biosafety guidelines typically classify cell-free systems at lower containment levels than their cellular counterparts, yet this classification may underestimate certain risks, especially as analytical capabilities become more sophisticated.
Ethical implications extend beyond laboratory safety to broader societal concerns. The accessibility of cell-free technologies potentially democratizes biotechnology, enabling wider participation in biological research and development. While this democratization promotes innovation, it simultaneously raises concerns about potential misuse or accidental harm by inexperienced practitioners. The reduced technical barriers could enable both beneficial applications and potential biosecurity threats, creating a dual-use dilemma that requires careful governance.
Intellectual property considerations present another ethical dimension. As analytical methods for cell-free systems advance, questions arise regarding ownership of biological information, synthetic pathways, and engineered components. The tension between open science principles and commercial interests becomes particularly acute when fundamental biological processes are involved, potentially affecting access to these technologies in resource-limited settings.
Privacy concerns emerge when cell-free systems are used for diagnostic purposes or personalized medicine applications. The analysis of human-derived components raises questions about consent, data ownership, and potential discrimination based on biological information. Establishing ethical frameworks that balance innovation with protection of individual rights remains a critical challenge.
Regulatory frameworks must evolve to address these unique challenges. Current regulations often focus on organism-based risk assessments rather than process-based or component-based evaluations more appropriate for cell-free systems. International harmonization of these regulations becomes increasingly important as the technology crosses borders through collaborative research and commercial applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!