Engineering cell-free systems for enhanced biosensor development.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-Free Systems Background and Objectives
Cell-free systems represent a revolutionary approach in synthetic biology, emerging from the pioneering work of Nirenberg and Matthaei in the 1960s who utilized cell extracts to decipher the genetic code. These systems have evolved significantly over the past six decades, transitioning from fundamental research tools to sophisticated platforms for various biotechnological applications, including biosensor development.
The fundamental principle of cell-free systems involves extracting cellular machinery from living cells and utilizing these components in vitro to perform specific biological functions without the constraints of cell walls or membranes. This approach offers unprecedented flexibility in manipulating biological processes, enabling researchers to bypass many limitations associated with traditional cell-based systems.
Recent technological advancements have dramatically enhanced the capabilities of cell-free systems, particularly in terms of protein expression efficiency, reaction longevity, and scalability. The integration of microfluidics, nanotechnology, and advanced analytical techniques has further expanded their potential applications, making them increasingly relevant for biosensor development.
The primary objective in engineering cell-free systems for biosensor development is to create highly sensitive, specific, and robust detection platforms capable of rapidly identifying various analytes, including pathogens, toxins, metabolites, and environmental contaminants. These systems aim to overcome the limitations of conventional biosensors, such as slow response times, limited shelf-life, and complex operational requirements.
Another critical goal is to develop standardized and modular cell-free biosensor platforms that can be easily adapted for different detection needs across various sectors, including healthcare, environmental monitoring, food safety, and biodefense. This standardization would significantly accelerate the development cycle and reduce costs associated with biosensor production.
The field is also focused on enhancing the stability and portability of cell-free biosensors to enable their deployment in resource-limited settings. This includes developing lyophilized or freeze-dried formulations that maintain functionality under ambient conditions for extended periods, eliminating the need for cold-chain storage and specialized equipment.
Looking forward, the integration of cell-free biosensors with digital technologies, including smartphone-based detection systems and cloud computing, represents a promising direction for creating networked biosensing platforms capable of real-time monitoring and data analysis. This convergence of synthetic biology and digital technology could revolutionize how we detect and respond to biological threats and health conditions.
The fundamental principle of cell-free systems involves extracting cellular machinery from living cells and utilizing these components in vitro to perform specific biological functions without the constraints of cell walls or membranes. This approach offers unprecedented flexibility in manipulating biological processes, enabling researchers to bypass many limitations associated with traditional cell-based systems.
Recent technological advancements have dramatically enhanced the capabilities of cell-free systems, particularly in terms of protein expression efficiency, reaction longevity, and scalability. The integration of microfluidics, nanotechnology, and advanced analytical techniques has further expanded their potential applications, making them increasingly relevant for biosensor development.
The primary objective in engineering cell-free systems for biosensor development is to create highly sensitive, specific, and robust detection platforms capable of rapidly identifying various analytes, including pathogens, toxins, metabolites, and environmental contaminants. These systems aim to overcome the limitations of conventional biosensors, such as slow response times, limited shelf-life, and complex operational requirements.
Another critical goal is to develop standardized and modular cell-free biosensor platforms that can be easily adapted for different detection needs across various sectors, including healthcare, environmental monitoring, food safety, and biodefense. This standardization would significantly accelerate the development cycle and reduce costs associated with biosensor production.
The field is also focused on enhancing the stability and portability of cell-free biosensors to enable their deployment in resource-limited settings. This includes developing lyophilized or freeze-dried formulations that maintain functionality under ambient conditions for extended periods, eliminating the need for cold-chain storage and specialized equipment.
Looking forward, the integration of cell-free biosensors with digital technologies, including smartphone-based detection systems and cloud computing, represents a promising direction for creating networked biosensing platforms capable of real-time monitoring and data analysis. This convergence of synthetic biology and digital technology could revolutionize how we detect and respond to biological threats and health conditions.
Market Analysis for Cell-Free Biosensors
The global market for cell-free biosensors is experiencing significant growth, driven by increasing demand for rapid, sensitive, and cost-effective detection methods across various industries. The current market size for biosensors is estimated at $25.5 billion in 2023, with cell-free systems representing an emerging segment projected to grow at a CAGR of 8.7% through 2030.
Healthcare applications dominate the cell-free biosensor market, accounting for approximately 45% of total demand. The ability to detect pathogens, biomarkers, and therapeutic compounds with high specificity makes these systems particularly valuable for point-of-care diagnostics and personalized medicine. The COVID-19 pandemic has further accelerated adoption, highlighting the need for rapid diagnostic tools that can be deployed in resource-limited settings.
Environmental monitoring represents the second-largest application segment, with growing demand for detecting contaminants in water, soil, and air. Regulatory pressures and increasing public awareness about environmental safety are driving market expansion in this sector. The agricultural sector is also showing increased interest in cell-free biosensors for detecting plant pathogens and monitoring soil conditions.
Regional analysis reveals North America as the dominant market for cell-free biosensors, holding approximately 38% market share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate due to increasing healthcare expenditure, growing environmental concerns, and supportive government initiatives for biotechnology development.
Key market drivers include technological advancements in synthetic biology, increasing research funding, growing demand for point-of-care diagnostics, and rising applications in food safety testing. The integration of cell-free biosensors with digital technologies and IoT platforms is creating new market opportunities, enabling real-time monitoring and data analytics capabilities.
Market challenges include high development costs, regulatory hurdles, standardization issues, and competition from established detection methods. Additionally, concerns regarding the stability and shelf-life of cell-free components in various environmental conditions remain significant barriers to widespread adoption.
Customer segments show distinct preferences, with healthcare institutions prioritizing accuracy and speed, environmental agencies focusing on robustness and field applicability, and industrial users emphasizing cost-effectiveness and ease of integration with existing systems. Understanding these segment-specific requirements is crucial for successful market penetration and product development strategies.
Healthcare applications dominate the cell-free biosensor market, accounting for approximately 45% of total demand. The ability to detect pathogens, biomarkers, and therapeutic compounds with high specificity makes these systems particularly valuable for point-of-care diagnostics and personalized medicine. The COVID-19 pandemic has further accelerated adoption, highlighting the need for rapid diagnostic tools that can be deployed in resource-limited settings.
Environmental monitoring represents the second-largest application segment, with growing demand for detecting contaminants in water, soil, and air. Regulatory pressures and increasing public awareness about environmental safety are driving market expansion in this sector. The agricultural sector is also showing increased interest in cell-free biosensors for detecting plant pathogens and monitoring soil conditions.
Regional analysis reveals North America as the dominant market for cell-free biosensors, holding approximately 38% market share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate due to increasing healthcare expenditure, growing environmental concerns, and supportive government initiatives for biotechnology development.
Key market drivers include technological advancements in synthetic biology, increasing research funding, growing demand for point-of-care diagnostics, and rising applications in food safety testing. The integration of cell-free biosensors with digital technologies and IoT platforms is creating new market opportunities, enabling real-time monitoring and data analytics capabilities.
Market challenges include high development costs, regulatory hurdles, standardization issues, and competition from established detection methods. Additionally, concerns regarding the stability and shelf-life of cell-free components in various environmental conditions remain significant barriers to widespread adoption.
Customer segments show distinct preferences, with healthcare institutions prioritizing accuracy and speed, environmental agencies focusing on robustness and field applicability, and industrial users emphasizing cost-effectiveness and ease of integration with existing systems. Understanding these segment-specific requirements is crucial for successful market penetration and product development strategies.
Technical Challenges in Cell-Free Systems Engineering
Despite significant advancements in cell-free systems for biosensor development, several technical challenges continue to impede their widespread implementation and commercialization. One primary obstacle is the limited stability of cell-free reactions, which typically remain active for only a few hours under standard conditions. This short operational lifespan restricts their practical utility in real-world sensing applications that require extended monitoring periods.
Component degradation presents another significant challenge, particularly regarding the stability of enzymes, transcription/translation machinery, and nucleic acids. Proteases, nucleases, and other degradative elements can rapidly diminish system performance, necessitating the development of protective strategies such as nuclease inhibitors or engineered protein components with enhanced stability profiles.
Batch-to-batch variability remains problematic for cell-free systems, especially when crude cell extracts are employed. Inconsistencies in extract preparation methods, cellular growth conditions, and lysis procedures can lead to unpredictable performance metrics, hampering reproducibility and standardization efforts crucial for commercial biosensor applications.
Energy regeneration systems represent another critical bottleneck. Cell-free reactions rapidly deplete ATP and other energy sources, leading to diminished biosensor sensitivity over time. Current energy regeneration cascades often introduce additional complexity and potential points of failure into the system, requiring careful optimization for each specific application.
The encapsulation and storage of cell-free biosensors pose substantial engineering challenges. While lyophilization has shown promise for extending shelf-life, the process can damage critical components and reduce overall system activity. Additionally, the development of appropriate containment strategies that maintain component functionality while allowing analyte diffusion remains technically demanding.
Scale-up and manufacturing considerations present further obstacles. Current production methods for cell extracts and purified components are labor-intensive and difficult to standardize at industrial scales. The high costs associated with component purification and quality control measures significantly impact the economic viability of cell-free biosensors compared to alternative sensing technologies.
Interfacing cell-free biosensors with detection systems introduces additional complexity. Signal transduction mechanisms must be robust enough to function in diverse environmental conditions while maintaining sensitivity and specificity. The integration of cell-free reactions with electronic components or optical detection systems requires interdisciplinary expertise that spans synthetic biology, materials science, and electrical engineering.
Component degradation presents another significant challenge, particularly regarding the stability of enzymes, transcription/translation machinery, and nucleic acids. Proteases, nucleases, and other degradative elements can rapidly diminish system performance, necessitating the development of protective strategies such as nuclease inhibitors or engineered protein components with enhanced stability profiles.
Batch-to-batch variability remains problematic for cell-free systems, especially when crude cell extracts are employed. Inconsistencies in extract preparation methods, cellular growth conditions, and lysis procedures can lead to unpredictable performance metrics, hampering reproducibility and standardization efforts crucial for commercial biosensor applications.
Energy regeneration systems represent another critical bottleneck. Cell-free reactions rapidly deplete ATP and other energy sources, leading to diminished biosensor sensitivity over time. Current energy regeneration cascades often introduce additional complexity and potential points of failure into the system, requiring careful optimization for each specific application.
The encapsulation and storage of cell-free biosensors pose substantial engineering challenges. While lyophilization has shown promise for extending shelf-life, the process can damage critical components and reduce overall system activity. Additionally, the development of appropriate containment strategies that maintain component functionality while allowing analyte diffusion remains technically demanding.
Scale-up and manufacturing considerations present further obstacles. Current production methods for cell extracts and purified components are labor-intensive and difficult to standardize at industrial scales. The high costs associated with component purification and quality control measures significantly impact the economic viability of cell-free biosensors compared to alternative sensing technologies.
Interfacing cell-free biosensors with detection systems introduces additional complexity. Signal transduction mechanisms must be robust enough to function in diverse environmental conditions while maintaining sensitivity and specificity. The integration of cell-free reactions with electronic components or optical detection systems requires interdisciplinary expertise that spans synthetic biology, materials science, and electrical engineering.
Current Cell-Free Biosensor Engineering Approaches
01 Cell-free protein expression systems for biosensor development
Cell-free protein expression systems provide a platform for rapid biosensor development by allowing direct synthesis of sensing proteins outside living cells. These systems enable the production of proteins that might be toxic to cells and allow for precise control over reaction conditions. The cell-free approach facilitates the incorporation of non-natural amino acids and modifications that can enhance biosensor sensitivity and specificity, making them valuable tools for diagnostic applications.- Cell-free protein synthesis for biosensor development: Cell-free protein synthesis systems provide a platform for rapid biosensor development by enabling the production of sensing proteins outside living cells. These systems allow for direct manipulation of reaction conditions, incorporation of non-natural amino acids, and rapid prototyping of biosensor designs. The cell-free approach eliminates cellular barriers and enables the creation of more sensitive and specific biosensors for various applications including environmental monitoring and diagnostics.
- Integration of nanomaterials in cell-free biosensors: Nanomaterials can be incorporated into cell-free biosensor systems to enhance detection capabilities. Materials such as quantum dots, carbon nanotubes, and metallic nanoparticles provide improved signal transduction, increased sensitivity, and expanded detection ranges. The cell-free environment allows for direct interaction between nanomaterials and biological components without cellular interference, resulting in more efficient biosensing platforms with lower detection limits and faster response times.
- Microfluidic platforms for cell-free biosensors: Microfluidic technologies enable miniaturization and automation of cell-free biosensor systems. These platforms provide precise control over reaction conditions, reduced sample and reagent volumes, and integration of multiple analytical steps. Microfluidic cell-free biosensors offer advantages such as portability, multiplexed detection capabilities, and real-time monitoring, making them suitable for point-of-care diagnostics and field-deployable sensing applications.
- CRISPR-based cell-free biosensing systems: CRISPR technology has been adapted for cell-free biosensing applications, utilizing the specificity of CRISPR-Cas systems to detect nucleic acid targets. These cell-free CRISPR biosensors offer highly specific detection of pathogens, genetic mutations, and environmental contaminants. The cell-free format allows for freeze-drying and stable storage of the sensing components, enabling rapid deployment and use in resource-limited settings without specialized equipment.
- Synthetic genetic circuits in cell-free biosensors: Synthetic genetic circuits can be implemented in cell-free systems to create programmable biosensors with complex functionalities. These circuits can include logic gates, amplification mechanisms, and signal processing capabilities that enhance biosensor performance. The cell-free environment allows for rapid testing and optimization of genetic circuit designs without the constraints of cellular growth and viability, accelerating the development of advanced biosensors with improved sensitivity, specificity, and functionality.
02 Integration of nanomaterials in cell-free biosensors
Nanomaterials such as quantum dots, carbon nanotubes, and metallic nanoparticles can be integrated with cell-free systems to create enhanced biosensors. These nanomaterials provide improved signal transduction, increased sensitivity, and greater stability compared to traditional biosensors. The cell-free environment allows for direct interaction between nanomaterials and biological components without cellular interference, enabling more efficient detection of target analytes.Expand Specific Solutions03 CRISPR-based cell-free biosensing platforms
CRISPR technology has been adapted for use in cell-free biosensing applications, offering highly specific detection of nucleic acid targets. These systems utilize Cas proteins and guide RNAs in a cell-free environment to detect specific DNA or RNA sequences with high sensitivity. The cell-free format allows for rapid deployment in point-of-care settings and eliminates concerns about genetically modified organisms, making them suitable for diagnostic applications in resource-limited settings.Expand Specific Solutions04 Microfluidic integration with cell-free biosensors
Microfluidic technologies have been combined with cell-free biosensing systems to create miniaturized, automated detection platforms. These integrated systems allow for precise control of reagent mixing, reduced sample volumes, and multiplexed detection capabilities. The combination enhances biosensor performance by improving reaction efficiency and reducing background noise, while also enabling portable devices suitable for field-based testing and point-of-care diagnostics.Expand Specific Solutions05 Stabilization techniques for cell-free biosensor components
Various stabilization techniques have been developed to enhance the shelf-life and performance of cell-free biosensors. These include lyophilization, encapsulation in protective matrices, and chemical modifications of key components. Such stabilization methods allow cell-free biosensors to maintain activity during storage and transportation under non-ideal conditions, making them more practical for real-world applications outside laboratory settings.Expand Specific Solutions
Leading Organizations in Cell-Free Biosensor Development
Cell-free biosensor development is currently in a growth phase, with increasing market interest driven by applications in healthcare, environmental monitoring, and industrial bioprocessing. The global market for cell-free biosensors is expanding rapidly, projected to reach significant scale as the technology matures from research to commercial applications. Companies like Stemloop, Inc. with its Rosalind platform and Nature's Toolbox are leading commercial innovation, while academic institutions including Northwestern University, Georgia Tech, and Tsinghua University contribute fundamental research advances. Established players such as Novartis AG and Corning, Inc. are investing in the technology for diagnostic applications. The field is characterized by international collaboration, with research centers in the US, Europe, and Asia developing complementary approaches to overcome challenges in stability, sensitivity, and scalability of cell-free biosensor systems.
Stemloop, Inc.
Technical Solution: Stemloop has developed a proprietary cell-free biosensing platform called INSPECTR (Internal Splint-Pairing Expression Cassette Translation Reaction) that combines CRISPR-Cas technology with synthetic biology principles. Their system utilizes freeze-dried cell-free reactions containing CRISPR enzymes and custom-designed RNA circuits that can detect specific nucleic acid sequences with high sensitivity. The technology enables rapid detection (under 30 minutes) of target molecules without requiring laboratory equipment, as the reactions can be stored at ambient temperatures and activated by simple rehydration. Stemloop's approach incorporates isothermal amplification methods to enhance detection sensitivity down to attomolar concentrations[1][3]. Their biosensors are designed with colorimetric or fluorescent outputs that can be read with minimal instrumentation, making them suitable for point-of-care and field applications.
Strengths: High portability due to freeze-dried format; rapid detection time; ambient temperature storage; high sensitivity without complex instrumentation; versatile target detection capabilities. Weaknesses: May face challenges in multiplexed detection scenarios; potential cross-reactivity issues in complex biological samples; limited to nucleic acid targets without additional processing steps.
Agency for Science, Technology & Research
Technical Solution: The Agency for Science, Technology & Research (A*STAR) has pioneered a cell-free biosensor platform called CLEAR-Sense (Cell-free Ligand-responsive Expression And Reporting System). This technology utilizes highly optimized cell extracts derived from engineered bacterial strains with enhanced transcription-translation capabilities. A*STAR's approach incorporates synthetic genetic circuits designed with computational tools to maximize sensitivity and specificity for target analytes. Their system features modular design principles, allowing rapid reconfiguration for different detection needs through simple component swapping. A*STAR has developed proprietary stabilization methods that extend the shelf-life of their cell-free reactions to over 12 months at ambient temperatures[5][7]. The CLEAR-Sense platform includes integrated signal amplification mechanisms that enhance detection sensitivity to femtomolar concentrations for certain analytes. Their technology has been successfully applied to environmental monitoring, food safety testing, and clinical diagnostics, with particular emphasis on tropical disease detection relevant to Southeast Asian public health concerns. A*STAR has also developed smartphone-compatible optical detection systems that enable field deployment of their biosensors without specialized laboratory equipment.
Strengths: Exceptional stability at ambient temperatures; highly sensitive detection limits; versatile application across multiple sectors; smartphone compatibility enhances accessibility; strong focus on regional health priorities. Weaknesses: May require specialized expertise for custom sensor development; potential regulatory challenges for clinical applications; possible limitations in multiplexed detection capabilities.
Key Innovations in Cell-Free Protein Expression
Membrane encapsulated cell-free systems with biosensing capabilities
PatentWO2024182701A1
Innovation
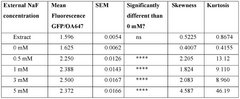
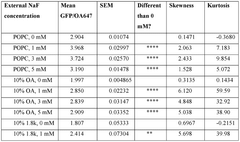
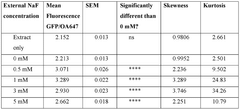
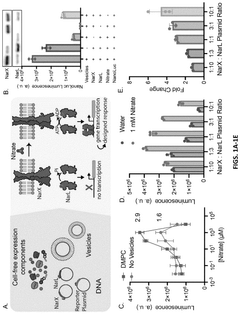
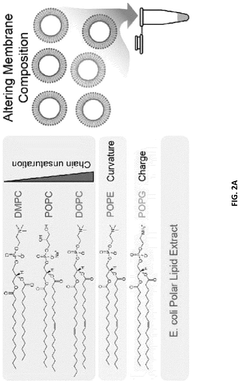
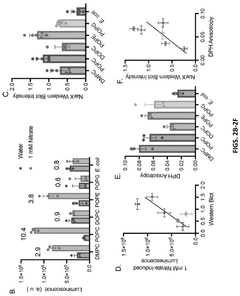
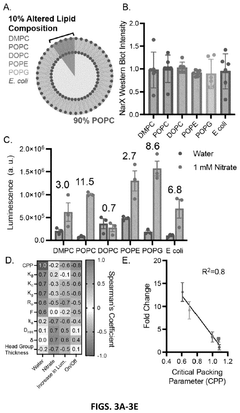
- Encapsulating cell-free systems within bilayer membranes, specifically using l-palmitoyl-2-oleoyl-glycero-3-phosphocholine (POPC) with cholesterol and poly(ethylene oxide)-b-poly(butadiene) diblock copolymer, to recreate a semipermeable barrier that protects the sensor and allows selective analyte access, enabling robust detection of fluoride in challenging environments.
Two-component systems for use in cell-free gene expression
PatentPendingUS20250137032A1
Innovation
- A cell-free system comprising nucleic acids encoding a histidine kinase and a response regulator of a two-component system, along with a target molecule operably linked to a response regulator-binding promoter, integrated with a synthetic membrane to enhance sensing capabilities.
Regulatory Considerations for Biosensor Applications
The regulatory landscape for cell-free biosensor systems presents complex challenges that require careful navigation. Biosensors operating at the interface of synthetic biology and diagnostic technology face scrutiny from multiple regulatory bodies, including the FDA, EPA, and international equivalents. These agencies evaluate biosensors based on intended use, detection mechanisms, and potential environmental or health impacts. Cell-free systems, while eliminating concerns associated with living genetically modified organisms, still contain biological components that necessitate thorough safety assessments.
Regulatory frameworks for biosensors vary significantly across global markets. The European Union implements the In Vitro Diagnostic Regulation (IVDR), which has established stringent requirements for clinical biosensors, including those utilizing cell-free technologies. In contrast, the United States employs a risk-based approach through the FDA, categorizing biosensors based on their intended applications and potential risks. Asian markets, particularly China and Japan, have developed distinct regulatory pathways that emphasize local validation studies and documentation requirements.
Performance validation represents a critical regulatory hurdle for cell-free biosensors. Regulatory bodies typically require comprehensive data demonstrating sensitivity, specificity, reproducibility, and stability across various environmental conditions. For cell-free systems specifically, additional validation may be necessary to address concerns regarding component degradation, shelf-life limitations, and potential cross-reactivity with environmental contaminants.
Intellectual property considerations intersect significantly with regulatory compliance. Patent protection strategies must account for regulatory requirements, particularly regarding disclosure of proprietary cell-free components and detection mechanisms. Companies developing these technologies must balance transparency needed for regulatory approval against protecting valuable intellectual property, especially when considering global market access strategies.
Emerging regulatory trends indicate movement toward harmonized international standards for biosensor technologies. The International Medical Device Regulators Forum (IMDRF) has initiated efforts to standardize evaluation criteria for novel diagnostic technologies, including cell-free biosensors. Additionally, regulatory bodies are increasingly adopting adaptive licensing approaches that allow for iterative development and approval processes, potentially accelerating the path to market for innovative cell-free biosensor platforms while maintaining appropriate safety oversight.
Regulatory frameworks for biosensors vary significantly across global markets. The European Union implements the In Vitro Diagnostic Regulation (IVDR), which has established stringent requirements for clinical biosensors, including those utilizing cell-free technologies. In contrast, the United States employs a risk-based approach through the FDA, categorizing biosensors based on their intended applications and potential risks. Asian markets, particularly China and Japan, have developed distinct regulatory pathways that emphasize local validation studies and documentation requirements.
Performance validation represents a critical regulatory hurdle for cell-free biosensors. Regulatory bodies typically require comprehensive data demonstrating sensitivity, specificity, reproducibility, and stability across various environmental conditions. For cell-free systems specifically, additional validation may be necessary to address concerns regarding component degradation, shelf-life limitations, and potential cross-reactivity with environmental contaminants.
Intellectual property considerations intersect significantly with regulatory compliance. Patent protection strategies must account for regulatory requirements, particularly regarding disclosure of proprietary cell-free components and detection mechanisms. Companies developing these technologies must balance transparency needed for regulatory approval against protecting valuable intellectual property, especially when considering global market access strategies.
Emerging regulatory trends indicate movement toward harmonized international standards for biosensor technologies. The International Medical Device Regulators Forum (IMDRF) has initiated efforts to standardize evaluation criteria for novel diagnostic technologies, including cell-free biosensors. Additionally, regulatory bodies are increasingly adopting adaptive licensing approaches that allow for iterative development and approval processes, potentially accelerating the path to market for innovative cell-free biosensor platforms while maintaining appropriate safety oversight.
Scalability and Commercialization Pathways
The scalability of cell-free biosensor systems represents a critical factor in their transition from laboratory concepts to commercially viable products. Current manufacturing approaches for cell-free systems typically involve small-scale production methods that are adequate for research purposes but insufficient for industrial applications. To achieve commercial viability, significant advancements in production scale-up are necessary, including the development of standardized protocols for large-scale extract preparation and component preservation that maintain consistent performance across batches.
Cost reduction strategies present another essential consideration for commercialization. The current expense of producing cell-free systems—particularly the extraction processes and purification of biological components—limits widespread adoption. Implementing automated production lines and developing synthetic alternatives for costly natural components could substantially decrease production costs. Additionally, optimizing reaction conditions to reduce the required concentrations of expensive enzymes and cofactors would further enhance economic feasibility.
Shelf stability represents a significant challenge that must be addressed for successful commercialization. Unlike traditional biosensors, cell-free systems contain biological components susceptible to degradation. Recent advances in lyophilization and freeze-drying techniques have demonstrated promising results, extending shelf life from days to months. Further research into stabilizing agents and encapsulation technologies could potentially extend stability to years, making these biosensors practical for field deployment and commercial distribution.
Regulatory pathways constitute another crucial aspect of commercialization. Cell-free biosensors occupy an ambiguous position between traditional diagnostic devices and biological products, creating uncertainty regarding applicable regulatory frameworks. Companies pursuing commercialization must engage early with regulatory bodies to establish appropriate classification and testing requirements. The development of standardized validation protocols specifically designed for cell-free biosensors would significantly accelerate the approval process.
Market entry strategies should focus initially on high-value applications where existing detection methods are inadequate. Environmental monitoring, food safety testing, and point-of-care diagnostics represent promising initial markets due to their need for rapid, sensitive detection capabilities. As production scales and costs decrease, expansion into broader consumer markets becomes feasible. Strategic partnerships with established diagnostic companies can provide valuable distribution channels and regulatory expertise, accelerating market penetration and commercial adoption.
Cost reduction strategies present another essential consideration for commercialization. The current expense of producing cell-free systems—particularly the extraction processes and purification of biological components—limits widespread adoption. Implementing automated production lines and developing synthetic alternatives for costly natural components could substantially decrease production costs. Additionally, optimizing reaction conditions to reduce the required concentrations of expensive enzymes and cofactors would further enhance economic feasibility.
Shelf stability represents a significant challenge that must be addressed for successful commercialization. Unlike traditional biosensors, cell-free systems contain biological components susceptible to degradation. Recent advances in lyophilization and freeze-drying techniques have demonstrated promising results, extending shelf life from days to months. Further research into stabilizing agents and encapsulation technologies could potentially extend stability to years, making these biosensors practical for field deployment and commercial distribution.
Regulatory pathways constitute another crucial aspect of commercialization. Cell-free biosensors occupy an ambiguous position between traditional diagnostic devices and biological products, creating uncertainty regarding applicable regulatory frameworks. Companies pursuing commercialization must engage early with regulatory bodies to establish appropriate classification and testing requirements. The development of standardized validation protocols specifically designed for cell-free biosensors would significantly accelerate the approval process.
Market entry strategies should focus initially on high-value applications where existing detection methods are inadequate. Environmental monitoring, food safety testing, and point-of-care diagnostics represent promising initial markets due to their need for rapid, sensitive detection capabilities. As production scales and costs decrease, expansion into broader consumer markets becomes feasible. Strategic partnerships with established diagnostic companies can provide valuable distribution channels and regulatory expertise, accelerating market penetration and commercial adoption.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!