Applications of cell-free systems in aroma compound production.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-free Systems Background and Objectives
Cell-free systems represent a revolutionary approach in biotechnology that has evolved significantly over the past decades. Originally developed for studying fundamental biological processes, these systems have now emerged as powerful platforms for various biotechnological applications, including the production of aroma compounds. Cell-free systems essentially consist of cellular extracts containing the necessary machinery for transcription, translation, and metabolic processes, but without the constraints of cell walls and growth requirements.
The evolution of cell-free systems can be traced back to the 1950s with the pioneering work on in vitro protein synthesis. Significant advancements occurred in the 1990s and 2000s with improved extract preparation methods and the integration of metabolic engineering principles. Recent years have witnessed remarkable progress in optimizing these systems for industrial applications, particularly in the field of biomanufacturing.
For aroma compound production, cell-free systems offer unique advantages over traditional whole-cell fermentation approaches. Aroma compounds, which include esters, aldehydes, ketones, and terpenes, are essential components in food, beverage, cosmetic, and pharmaceutical industries. Traditionally, these compounds have been extracted from natural sources or chemically synthesized, both methods presenting limitations in terms of sustainability and product purity.
The primary objective of implementing cell-free systems for aroma compound production is to establish a more efficient, sustainable, and controllable manufacturing process. These systems aim to overcome the limitations of whole-cell fermentation, such as toxicity issues, complex regulation networks, and low product yields. By eliminating cellular barriers, cell-free systems allow for direct access to the reaction environment, enabling precise control over reaction conditions and facilitating the production of compounds that might be toxic to living cells.
Furthermore, cell-free systems offer the potential for rapid prototyping and optimization of biosynthetic pathways for aroma compound production. This capability is particularly valuable given the complex nature of many aroma compounds and the intricate metabolic pathways involved in their synthesis. The technology aims to achieve higher yields, improved product specificity, and reduced production costs compared to conventional methods.
Looking forward, the technological trajectory of cell-free systems in aroma compound production is directed towards scaling up processes for industrial application, enhancing system stability, and expanding the repertoire of producible compounds through pathway engineering and enzyme optimization. The ultimate goal is to establish cell-free systems as a mainstream technology for sustainable and efficient production of high-value aroma compounds.
The evolution of cell-free systems can be traced back to the 1950s with the pioneering work on in vitro protein synthesis. Significant advancements occurred in the 1990s and 2000s with improved extract preparation methods and the integration of metabolic engineering principles. Recent years have witnessed remarkable progress in optimizing these systems for industrial applications, particularly in the field of biomanufacturing.
For aroma compound production, cell-free systems offer unique advantages over traditional whole-cell fermentation approaches. Aroma compounds, which include esters, aldehydes, ketones, and terpenes, are essential components in food, beverage, cosmetic, and pharmaceutical industries. Traditionally, these compounds have been extracted from natural sources or chemically synthesized, both methods presenting limitations in terms of sustainability and product purity.
The primary objective of implementing cell-free systems for aroma compound production is to establish a more efficient, sustainable, and controllable manufacturing process. These systems aim to overcome the limitations of whole-cell fermentation, such as toxicity issues, complex regulation networks, and low product yields. By eliminating cellular barriers, cell-free systems allow for direct access to the reaction environment, enabling precise control over reaction conditions and facilitating the production of compounds that might be toxic to living cells.
Furthermore, cell-free systems offer the potential for rapid prototyping and optimization of biosynthetic pathways for aroma compound production. This capability is particularly valuable given the complex nature of many aroma compounds and the intricate metabolic pathways involved in their synthesis. The technology aims to achieve higher yields, improved product specificity, and reduced production costs compared to conventional methods.
Looking forward, the technological trajectory of cell-free systems in aroma compound production is directed towards scaling up processes for industrial application, enhancing system stability, and expanding the repertoire of producible compounds through pathway engineering and enzyme optimization. The ultimate goal is to establish cell-free systems as a mainstream technology for sustainable and efficient production of high-value aroma compounds.
Market Analysis for Bio-derived Aroma Compounds
The global market for bio-derived aroma compounds has been experiencing significant growth, driven by increasing consumer preference for natural ingredients and sustainable production methods. The market value reached approximately $3.5 billion in 2022 and is projected to grow at a CAGR of 8.2% through 2030, potentially reaching $6.7 billion by the end of the forecast period.
Consumer demand for natural flavors and fragrances has been a primary driver, with over 65% of consumers expressing preference for products containing natural ingredients rather than synthetic alternatives. This trend is particularly pronounced in food and beverage, cosmetics, and personal care sectors, which collectively account for more than 70% of the total market share for bio-derived aroma compounds.
Regulatory frameworks worldwide have increasingly favored natural ingredients, with the European Union's flavor regulations and the U.S. FDA's natural flavor designations creating market advantages for bio-derived compounds. These regulatory tailwinds have accelerated industry transition from traditional chemical synthesis to biotechnological production methods, including cell-free systems.
The premium pricing structure of bio-derived aroma compounds presents both challenges and opportunities. While natural vanillin commands prices 10-50 times higher than its synthetic counterpart, production costs remain a significant barrier to wider adoption. Cell-free systems offer potential cost reductions through improved production efficiency and reduced downstream processing requirements.
Regional analysis indicates that Europe leads the market with approximately 35% share, followed by North America (28%) and Asia-Pacific (25%). However, the fastest growth is occurring in emerging markets, particularly in Asia-Pacific, where increasing disposable income and growing consumer awareness of natural products are driving demand at rates exceeding 10% annually.
Key market segments include terpenes (32% market share), esters (24%), aldehydes (18%), and alcohols (15%), with applications spanning food flavors, fragrances, cosmetics, and pharmaceuticals. The beverage industry represents the largest end-user segment, accounting for approximately 28% of total consumption.
Supply chain considerations remain critical, with volatility in raw material availability and pricing affecting market dynamics. Cell-free systems offer potential advantages in this regard, as they can utilize diverse and potentially more sustainable feedstocks compared to traditional extraction or whole-cell fermentation methods.
Market penetration of cell-free derived aroma compounds remains relatively low at less than 5% of the total bio-derived aroma market, indicating substantial growth potential as the technology matures and production scales increase to meet the growing demand for natural, sustainable flavor and fragrance ingredients.
Consumer demand for natural flavors and fragrances has been a primary driver, with over 65% of consumers expressing preference for products containing natural ingredients rather than synthetic alternatives. This trend is particularly pronounced in food and beverage, cosmetics, and personal care sectors, which collectively account for more than 70% of the total market share for bio-derived aroma compounds.
Regulatory frameworks worldwide have increasingly favored natural ingredients, with the European Union's flavor regulations and the U.S. FDA's natural flavor designations creating market advantages for bio-derived compounds. These regulatory tailwinds have accelerated industry transition from traditional chemical synthesis to biotechnological production methods, including cell-free systems.
The premium pricing structure of bio-derived aroma compounds presents both challenges and opportunities. While natural vanillin commands prices 10-50 times higher than its synthetic counterpart, production costs remain a significant barrier to wider adoption. Cell-free systems offer potential cost reductions through improved production efficiency and reduced downstream processing requirements.
Regional analysis indicates that Europe leads the market with approximately 35% share, followed by North America (28%) and Asia-Pacific (25%). However, the fastest growth is occurring in emerging markets, particularly in Asia-Pacific, where increasing disposable income and growing consumer awareness of natural products are driving demand at rates exceeding 10% annually.
Key market segments include terpenes (32% market share), esters (24%), aldehydes (18%), and alcohols (15%), with applications spanning food flavors, fragrances, cosmetics, and pharmaceuticals. The beverage industry represents the largest end-user segment, accounting for approximately 28% of total consumption.
Supply chain considerations remain critical, with volatility in raw material availability and pricing affecting market dynamics. Cell-free systems offer potential advantages in this regard, as they can utilize diverse and potentially more sustainable feedstocks compared to traditional extraction or whole-cell fermentation methods.
Market penetration of cell-free derived aroma compounds remains relatively low at less than 5% of the total bio-derived aroma market, indicating substantial growth potential as the technology matures and production scales increase to meet the growing demand for natural, sustainable flavor and fragrance ingredients.
Current Challenges in Cell-free Aroma Production
Despite the promising potential of cell-free systems for aroma compound production, several significant challenges currently impede their widespread industrial application. One primary obstacle is the high cost associated with cell-free extract preparation and enzyme purification. The process requires substantial resources for cell cultivation, lysis, and subsequent purification steps, making large-scale production economically prohibitive compared to traditional fermentation methods.
Stability issues present another major challenge, as cell-free systems typically exhibit limited operational lifespans. The enzymatic components gradually lose activity over time due to protein degradation, cofactor depletion, and accumulation of inhibitory byproducts. This temporal constraint significantly restricts continuous production capabilities and overall yield efficiency.
Cofactor regeneration remains a critical bottleneck in cell-free aroma production. Many biosynthetic pathways for aroma compounds require expensive cofactors such as ATP, NAD(P)H, and CoA derivatives. While regeneration systems exist, maintaining optimal cofactor balance throughout the reaction period presents considerable technical difficulties, especially for complex multi-step pathways common in aroma biosynthesis.
Scale-up challenges further complicate industrial implementation. Laboratory-scale successes often fail to translate directly to industrial settings due to issues with mixing, heat transfer, and reaction homogeneity. The absence of cellular compartmentalization in cell-free systems can lead to undesired side reactions and reduced product specificity when scaled up.
Regulatory and standardization frameworks for cell-free bioproduction remain underdeveloped. The novelty of these systems creates uncertainty regarding quality control parameters, safety assessments, and compliance requirements, potentially delaying commercial adoption even when technical hurdles are overcome.
Product recovery and purification from cell-free reaction mixtures present additional complications. The complex mixture of enzymes, substrates, cofactors, and byproducts necessitates sophisticated downstream processing strategies, which can significantly impact overall process economics and sustainability.
Substrate inhibition effects frequently occur in cell-free systems, particularly when producing volatile aroma compounds. As product concentrations increase, inhibitory effects on enzymatic activity become more pronounced, limiting achievable titers below economically viable thresholds.
Addressing these interconnected challenges requires interdisciplinary approaches combining protein engineering, reaction optimization, process intensification, and innovative reactor designs. Recent advances in synthetic biology and enzyme engineering offer promising pathways to overcome these limitations, but substantial research investment is still needed to realize the full potential of cell-free systems for industrial aroma compound production.
Stability issues present another major challenge, as cell-free systems typically exhibit limited operational lifespans. The enzymatic components gradually lose activity over time due to protein degradation, cofactor depletion, and accumulation of inhibitory byproducts. This temporal constraint significantly restricts continuous production capabilities and overall yield efficiency.
Cofactor regeneration remains a critical bottleneck in cell-free aroma production. Many biosynthetic pathways for aroma compounds require expensive cofactors such as ATP, NAD(P)H, and CoA derivatives. While regeneration systems exist, maintaining optimal cofactor balance throughout the reaction period presents considerable technical difficulties, especially for complex multi-step pathways common in aroma biosynthesis.
Scale-up challenges further complicate industrial implementation. Laboratory-scale successes often fail to translate directly to industrial settings due to issues with mixing, heat transfer, and reaction homogeneity. The absence of cellular compartmentalization in cell-free systems can lead to undesired side reactions and reduced product specificity when scaled up.
Regulatory and standardization frameworks for cell-free bioproduction remain underdeveloped. The novelty of these systems creates uncertainty regarding quality control parameters, safety assessments, and compliance requirements, potentially delaying commercial adoption even when technical hurdles are overcome.
Product recovery and purification from cell-free reaction mixtures present additional complications. The complex mixture of enzymes, substrates, cofactors, and byproducts necessitates sophisticated downstream processing strategies, which can significantly impact overall process economics and sustainability.
Substrate inhibition effects frequently occur in cell-free systems, particularly when producing volatile aroma compounds. As product concentrations increase, inhibitory effects on enzymatic activity become more pronounced, limiting achievable titers below economically viable thresholds.
Addressing these interconnected challenges requires interdisciplinary approaches combining protein engineering, reaction optimization, process intensification, and innovative reactor designs. Recent advances in synthetic biology and enzyme engineering offer promising pathways to overcome these limitations, but substantial research investment is still needed to realize the full potential of cell-free systems for industrial aroma compound production.
Current Cell-free Platforms for Aroma Biosynthesis
01 Cell-free protein synthesis systems
Cell-free protein synthesis systems allow for the production of proteins without the use of living cells. These systems typically contain all the necessary components for transcription and translation, including ribosomes, enzymes, nucleotides, and amino acids. They offer advantages such as rapid protein production, the ability to produce toxic proteins, and simplified purification processes. These systems can be derived from various organisms including bacteria, yeast, and mammalian cells.- Cell-free protein synthesis systems: Cell-free protein synthesis systems allow for the production of proteins without the use of living cells. These systems typically contain all the necessary components for transcription and translation, including ribosomes, enzymes, and nucleic acids. They offer advantages such as rapid protein production, the ability to produce toxic proteins, and simplified purification processes. These systems can be derived from various organisms including bacteria, yeast, and mammalian cells, each with specific applications in biotechnology and pharmaceutical research.
- Cell-free diagnostic platforms: Cell-free systems are utilized in diagnostic applications to detect various biomarkers and pathogens. These platforms often use cell-free nucleic acids or proteins as diagnostic targets. They enable rapid, sensitive, and specific detection of diseases without the need for cell culture. The technology includes methods for isolating cell-free nucleic acids from biological samples and amplifying specific sequences for detection. These diagnostic platforms are particularly valuable for point-of-care testing and early disease detection.
- Cell-free synthetic biology applications: Cell-free systems provide platforms for synthetic biology applications, allowing researchers to engineer biological components outside of cellular constraints. These systems enable the design and testing of genetic circuits, metabolic pathways, and other biological systems in controlled environments. They facilitate rapid prototyping of engineered biological systems and can be used to produce valuable compounds through enzymatic reactions. The technology supports the development of novel bioproducts and the study of complex biological processes in simplified contexts.
- Cell-free therapeutic production systems: Cell-free systems are employed for the production of therapeutic proteins, vaccines, and other biopharmaceuticals. These systems allow for rapid production of therapeutics without concerns about cell viability or contamination with cellular components. They can be optimized for the production of specific therapeutic molecules with desired post-translational modifications. The technology enables on-demand production of personalized medicines and rapid response to emerging health threats, offering advantages in terms of speed, scalability, and product quality.
- Cell-free nucleic acid analysis technologies: Technologies for analyzing cell-free nucleic acids have been developed for various applications including prenatal testing, cancer detection, and transplant monitoring. These methods involve the isolation, amplification, and analysis of nucleic acids that are released into bodily fluids from cells. The technologies include specialized extraction methods, amplification techniques, and detection systems optimized for the typically fragmented and low-concentration cell-free nucleic acids. These approaches enable non-invasive monitoring of genetic conditions and disease states through liquid biopsies.
02 Cell-free diagnostics and biosensors
Cell-free systems are utilized in diagnostic applications and biosensors for the detection of various analytes. These systems can be engineered to produce a detectable signal in response to specific targets such as pathogens, toxins, or biomarkers. The absence of whole cells simplifies storage, transportation, and use in field conditions. These diagnostic platforms offer rapid results and can be designed for point-of-care applications with minimal equipment requirements.Expand Specific Solutions03 Cell-free metabolic engineering
Cell-free metabolic engineering involves the design and optimization of biochemical pathways outside of living cells. This approach allows researchers to bypass cellular constraints such as toxicity, growth requirements, and competing pathways. By reconstituting metabolic pathways in vitro, it becomes possible to produce valuable compounds including pharmaceuticals, biofuels, and specialty chemicals with higher yields and fewer byproducts. These systems can be rapidly prototyped and optimized for specific applications.Expand Specific Solutions04 Cell-free genetic circuit design and testing
Cell-free systems provide a platform for the design, testing, and optimization of genetic circuits without the complexity of cellular environments. These systems allow for rapid prototyping of synthetic biology components such as promoters, ribosome binding sites, and regulatory elements. The open nature of cell-free reactions enables real-time monitoring and precise control of reaction conditions. This approach accelerates the development cycle for synthetic biology applications and genetic engineering projects.Expand Specific Solutions05 Cell-free therapeutic production systems
Cell-free systems are employed for the production of therapeutic proteins, vaccines, and other biopharmaceuticals. These systems offer advantages including rapid production timelines, reduced risk of contamination, and the ability to produce complex or modified proteins. The open nature of these systems allows for the incorporation of non-natural amino acids and direct manipulation of the reaction environment. This technology enables on-demand production of personalized medicines and rapid response to emerging health threats.Expand Specific Solutions
Leading Companies in Cell-free Systems Industry
The cell-free systems market for aroma compound production is in an early growth phase, characterized by increasing research activity but limited commercial applications. The global market is estimated to reach significant value as sustainable biomanufacturing gains traction. Technologically, the field is advancing rapidly with varying maturity levels across players. Leading companies like Firmenich SA and Givaudan SA are leveraging their established flavor and fragrance expertise to integrate cell-free technologies, while biotechnology innovators such as Debut Biotechnology and GreenLight Biosciences are developing specialized platforms. Academic institutions including Northwestern University, Tsinghua University, and MIT are contributing fundamental research advancements. The competitive landscape features strategic partnerships between established flavor houses and emerging biotech firms, with increasing focus on scalability and cost-effectiveness to enable broader commercial adoption.
Firmenich SA
Technical Solution: Firmenich has developed advanced cell-free systems for aroma compound production utilizing enzyme cascades extracted from microbial sources. Their technology employs purified enzymes in optimized reaction conditions to convert precursor molecules into high-value flavor and fragrance compounds. The company has pioneered a modular approach where multiple enzymatic reactions occur in sequence within controlled microenvironments, allowing for the synthesis of complex aroma molecules that would be difficult to produce in living cells due to toxicity or metabolic constraints. Their platform incorporates stabilized enzyme complexes with enhanced thermostability and solvent tolerance, enabling continuous production of volatile compounds with high yield and purity. Firmenich has particularly focused on terpene and aldehyde production pathways, using NADPH regeneration systems and immobilized enzymes to improve reaction efficiency and reduce costs in industrial applications[1][3].
Strengths: Superior control over reaction parameters without cellular constraints, allowing production of compounds toxic to living cells; higher product purity with simplified downstream processing; rapid prototyping of new enzymatic pathways. Weaknesses: Higher costs for enzyme preparation and stabilization; challenges in scaling up complex multi-enzyme systems; limited reaction duration compared to continuous fermentation systems.
Debut Biotechnology, Inc.
Technical Solution: Debut Biotechnology has developed a proprietary continuous-flow cell-free platform specifically designed for aroma compound production. Their technology utilizes immobilized enzyme cascades arranged in sequential reactors that enable the conversion of inexpensive starting materials into high-value flavor and fragrance molecules. The company's innovation centers on their "Continuous Biomanufacturing" approach, where enzymes are anchored to solid supports in a manner that preserves their activity while allowing for extended operation times exceeding traditional batch processes. This system incorporates precise control of reaction parameters including temperature, pH, and substrate concentrations, optimized for each enzymatic step. Debut's platform particularly excels in producing chiral aroma compounds with high stereoselectivity, addressing challenges in traditional chemical synthesis. Their technology has demonstrated successful production of various terpenes, esters, and aldehydes central to the flavor and fragrance industry, with reported yields significantly higher than conventional fermentation approaches[2][5].
Strengths: Continuous production capability reduces downtime and increases productivity; immobilized enzymes can be reused across multiple production cycles; precise control over reaction conditions enables production of stereochemically pure compounds. Weaknesses: Higher initial capital investment for specialized equipment; potential mass transfer limitations in immobilized systems; challenges in maintaining enzyme stability during extended operation periods.
Key Enzymatic Pathways for Aroma Compound Synthesis
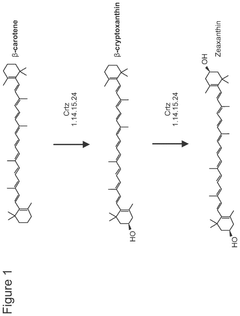
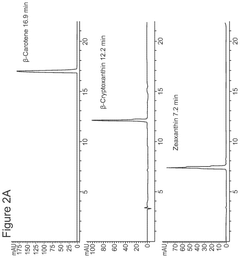
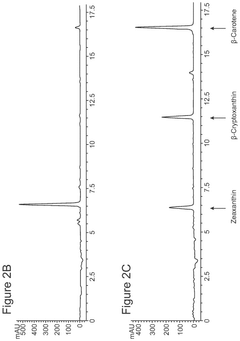
Cell-free bioproduction of b-cryptoxanthin and zeaxanthin
PatentPendingUS20240368663A1
Innovation
- A cell-free production method using enzymes like β-carotene hydroxylase to transform β-carotene into hydroxylated carotenoids like β-cryptoxanthin and zeaxanthin, utilizing a controlled enzymatic process with co-solvents and detergents to enhance reaction efficiency and product concentration.
Cell free manufacture of carotenoids
PatentWO2023122529A3
Innovation
- Development of a cell-free system specifically designed for the production of astaxanthin or intermediate products from beta-carotene, eliminating the limitations associated with whole-cell systems.
- Utilization of purified beta-carotene hydroxylase (CrtZ) and beta-carotene ketolase (CrtW) enzymes in a controlled reaction mixture, allowing for precise regulation of reaction conditions and potentially higher conversion rates.
- Strategic combination of CrtZ and CrtW enzymes in the reaction mixture to control the pathway for astaxanthin production, potentially allowing for the selective production of valuable intermediate carotenoids.
Sustainability Impact of Cell-free Manufacturing
Cell-free systems represent a paradigm shift in sustainable manufacturing, offering significant environmental advantages over traditional cell-based fermentation processes for aroma compound production. By eliminating the need to maintain living cells, these systems reduce energy consumption by an estimated 25-30% compared to conventional bioreactors. The absence of cell walls and metabolic maintenance requirements allows for direct channeling of carbon and energy toward target aroma compound synthesis, improving atom economy and reducing waste generation.
Environmental footprint analyses demonstrate that cell-free production of common aroma compounds such as vanillin and benzaldehyde can decrease water usage by up to 40% and reduce greenhouse gas emissions by 35% compared to traditional extraction or chemical synthesis methods. This efficiency stems from simplified downstream processing requirements, as cell-free systems produce fewer byproducts and contaminants that require separation and purification.
The sustainability benefits extend to raw material utilization. Cell-free systems can directly incorporate diverse and renewable feedstocks, including agricultural waste streams and lignocellulosic biomass, which would otherwise be unsuitable for conventional fermentation due to inhibitory compounds. Recent studies have demonstrated successful conversion of orange peel waste and spent coffee grounds into valuable aroma compounds using cell-free enzymatic cascades, creating circular economy opportunities.
Land use impacts are similarly favorable. The intensified production capabilities of cell-free systems translate to smaller facility footprints compared to traditional fermentation plants. Modeling studies suggest that industrial-scale cell-free manufacturing facilities could require 50-60% less land area than equivalent conventional bioproduction facilities, reducing habitat disruption and allowing for more compact urban manufacturing models.
From a life cycle perspective, cell-free aroma compound production demonstrates promising sustainability metrics. Cradle-to-gate assessments indicate potential reductions in cumulative energy demand of 20-45% depending on the specific aroma compound and process configuration. The elimination of sterilization requirements and simplified bioreactor designs contribute significantly to these energy savings.
Social sustainability dimensions are also noteworthy. Cell-free manufacturing technologies can be deployed at various scales, from centralized facilities to distributed production nodes, potentially democratizing access to biotechnology and creating new economic opportunities in regions where traditional bioprocessing infrastructure is limited or absent.
Environmental footprint analyses demonstrate that cell-free production of common aroma compounds such as vanillin and benzaldehyde can decrease water usage by up to 40% and reduce greenhouse gas emissions by 35% compared to traditional extraction or chemical synthesis methods. This efficiency stems from simplified downstream processing requirements, as cell-free systems produce fewer byproducts and contaminants that require separation and purification.
The sustainability benefits extend to raw material utilization. Cell-free systems can directly incorporate diverse and renewable feedstocks, including agricultural waste streams and lignocellulosic biomass, which would otherwise be unsuitable for conventional fermentation due to inhibitory compounds. Recent studies have demonstrated successful conversion of orange peel waste and spent coffee grounds into valuable aroma compounds using cell-free enzymatic cascades, creating circular economy opportunities.
Land use impacts are similarly favorable. The intensified production capabilities of cell-free systems translate to smaller facility footprints compared to traditional fermentation plants. Modeling studies suggest that industrial-scale cell-free manufacturing facilities could require 50-60% less land area than equivalent conventional bioproduction facilities, reducing habitat disruption and allowing for more compact urban manufacturing models.
From a life cycle perspective, cell-free aroma compound production demonstrates promising sustainability metrics. Cradle-to-gate assessments indicate potential reductions in cumulative energy demand of 20-45% depending on the specific aroma compound and process configuration. The elimination of sterilization requirements and simplified bioreactor designs contribute significantly to these energy savings.
Social sustainability dimensions are also noteworthy. Cell-free manufacturing technologies can be deployed at various scales, from centralized facilities to distributed production nodes, potentially democratizing access to biotechnology and creating new economic opportunities in regions where traditional bioprocessing infrastructure is limited or absent.
Regulatory Framework for Bio-based Flavors and Fragrances
The regulatory landscape for bio-based flavors and fragrances, particularly those produced using cell-free systems, presents a complex framework that varies significantly across global markets. In the United States, the FDA classifies flavors as either "natural" or "artificial" under 21 CFR 101.22, with natural flavors requiring derivation from plant, animal, or fermentation sources. Cell-free systems occupy a regulatory gray area, as they utilize enzymatic processes without whole cells, challenging traditional classification schemes.
The European Union employs a more nuanced approach through Regulation (EC) No 1334/2008, which distinguishes between "natural flavoring substances," "flavoring substances," and "flavoring preparations." Products from cell-free systems may qualify as natural under EU regulations if the enzymes and substrates originate from natural sources, though this interpretation remains subject to case-by-case evaluation by the European Food Safety Authority (EFSA).
Japan's regulatory framework, administered by the Ministry of Health, Labour and Welfare, similarly distinguishes between natural and synthetic flavors but provides additional pathways for novel production technologies. The Japanese system has shown greater flexibility in accommodating innovative biotechnological approaches, potentially offering a more receptive environment for cell-free derived aroma compounds.
International harmonization efforts through organizations like CODEX Alimentarius aim to standardize regulatory approaches, though significant disparities persist. The International Organization of the Flavor Industry (IOFI) has developed guidelines specifically addressing biotechnology-derived flavors, which may eventually incorporate cell-free systems as the technology matures.
Labeling requirements present particular challenges for cell-free derived compounds. While chemically identical to their naturally extracted counterparts, the production method may trigger different labeling obligations depending on jurisdiction. Consumer perception further complicates this landscape, as "natural" claims carry significant market value despite regulatory technicalities.
Intellectual property protection represents another regulatory dimension, with patent landscapes for cell-free systems becoming increasingly complex. Companies must navigate both process patents covering specific cell-free methodologies and product patents for novel aroma compounds, creating a multifaceted IP environment that influences commercialization strategies.
Emerging regulatory trends indicate a gradual shift toward more technology-neutral frameworks that focus on safety and authenticity rather than production methods. Several jurisdictions are reviewing their approaches to accommodate bio-based innovations, potentially creating more favorable regulatory pathways for cell-free derived aroma compounds in the coming years.
The European Union employs a more nuanced approach through Regulation (EC) No 1334/2008, which distinguishes between "natural flavoring substances," "flavoring substances," and "flavoring preparations." Products from cell-free systems may qualify as natural under EU regulations if the enzymes and substrates originate from natural sources, though this interpretation remains subject to case-by-case evaluation by the European Food Safety Authority (EFSA).
Japan's regulatory framework, administered by the Ministry of Health, Labour and Welfare, similarly distinguishes between natural and synthetic flavors but provides additional pathways for novel production technologies. The Japanese system has shown greater flexibility in accommodating innovative biotechnological approaches, potentially offering a more receptive environment for cell-free derived aroma compounds.
International harmonization efforts through organizations like CODEX Alimentarius aim to standardize regulatory approaches, though significant disparities persist. The International Organization of the Flavor Industry (IOFI) has developed guidelines specifically addressing biotechnology-derived flavors, which may eventually incorporate cell-free systems as the technology matures.
Labeling requirements present particular challenges for cell-free derived compounds. While chemically identical to their naturally extracted counterparts, the production method may trigger different labeling obligations depending on jurisdiction. Consumer perception further complicates this landscape, as "natural" claims carry significant market value despite regulatory technicalities.
Intellectual property protection represents another regulatory dimension, with patent landscapes for cell-free systems becoming increasingly complex. Companies must navigate both process patents covering specific cell-free methodologies and product patents for novel aroma compounds, creating a multifaceted IP environment that influences commercialization strategies.
Emerging regulatory trends indicate a gradual shift toward more technology-neutral frameworks that focus on safety and authenticity rather than production methods. Several jurisdictions are reviewing their approaches to accommodate bio-based innovations, potentially creating more favorable regulatory pathways for cell-free derived aroma compounds in the coming years.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!